Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymeric Solutions
2.3. Electrospinning
2.4. Characterization of the Scaffold
2.4.1. Scanning Electron Microscopy—SEM
2.4.2. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy—ATR-FTIR
2.4.3. Wettability
2.4.4. Thermal analyses
2.5. Antimicrobial Activity
2.6. Statistical Analysis
3. Results
3.1. Characterization of the PG and CA Scaffold
3.1.1. Scanning Electron Microscopy—SEM
3.1.2. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy—ATR-FTIR
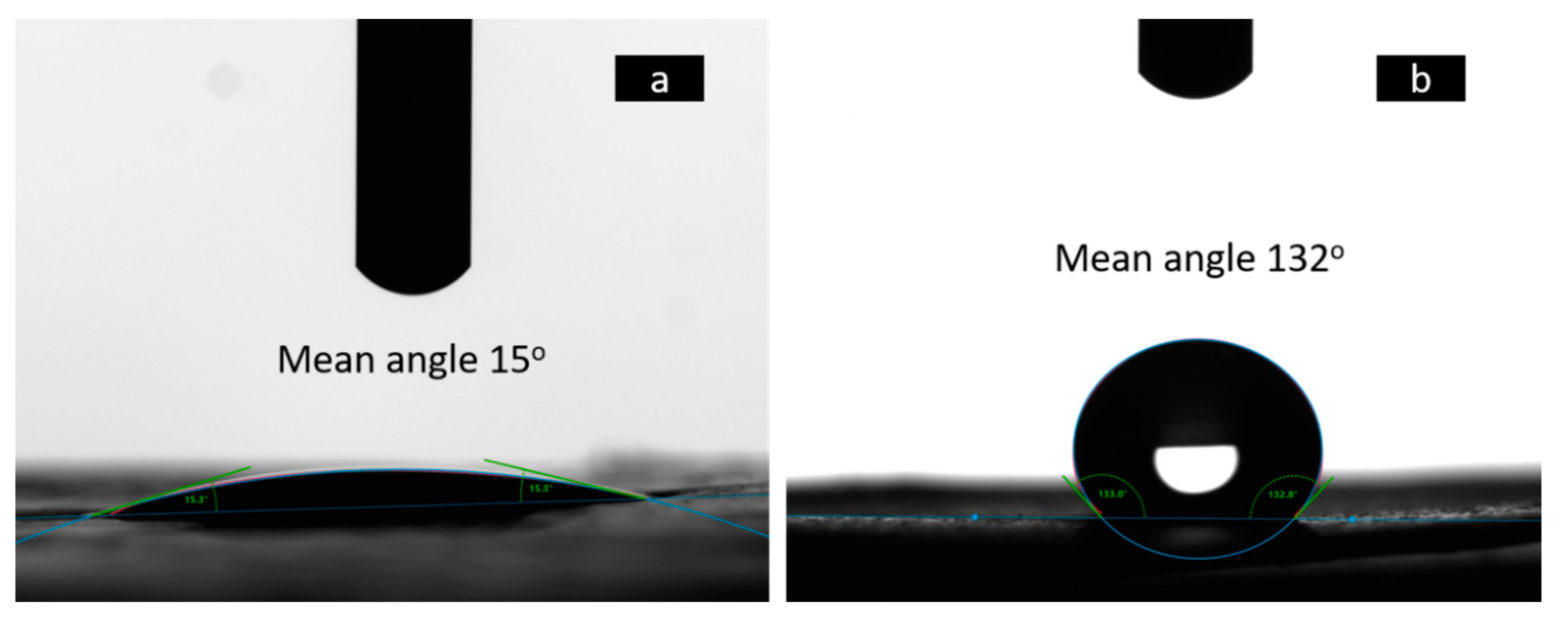
3.1.3. Wettability
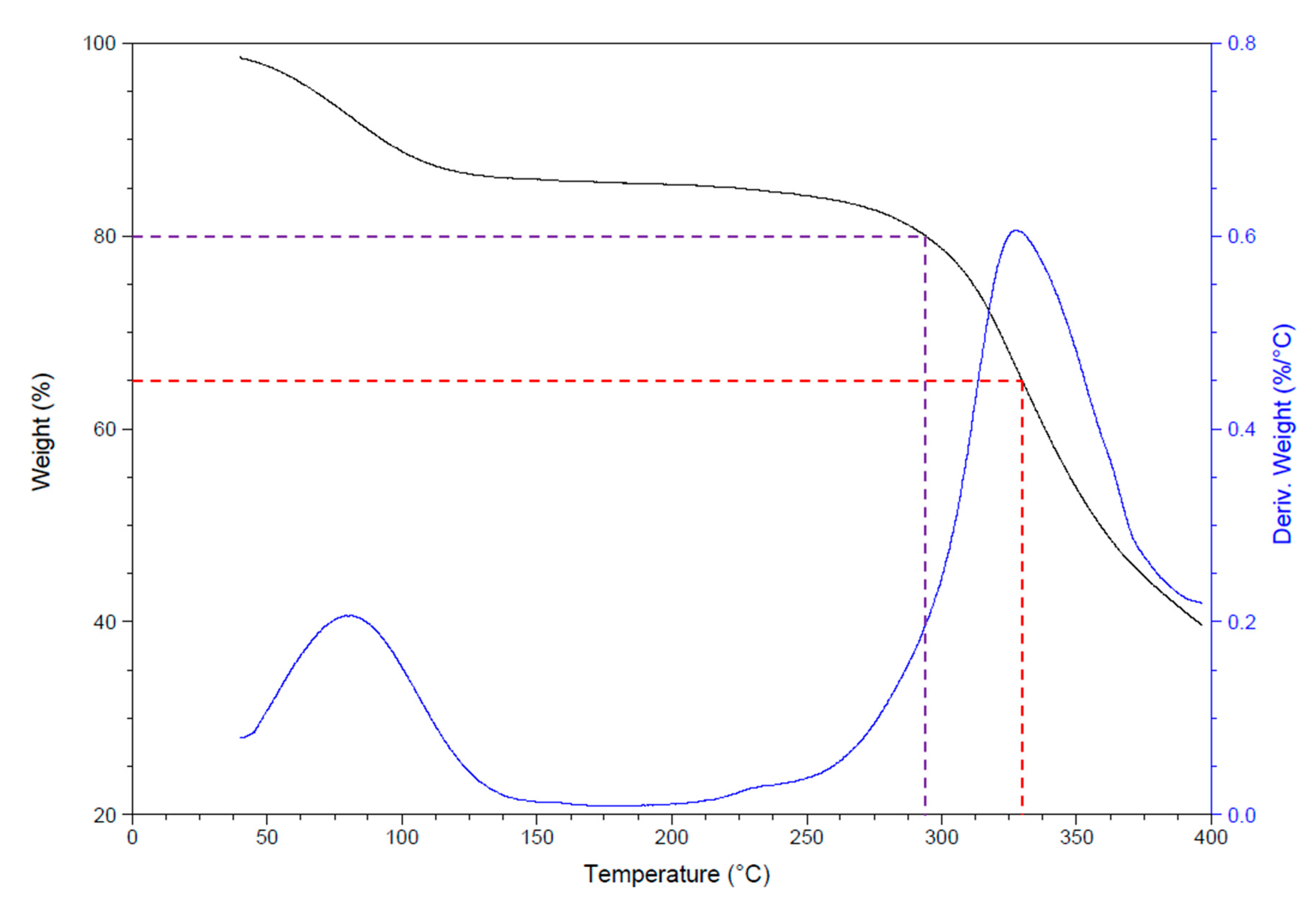
3.1.4. Thermal Analyses
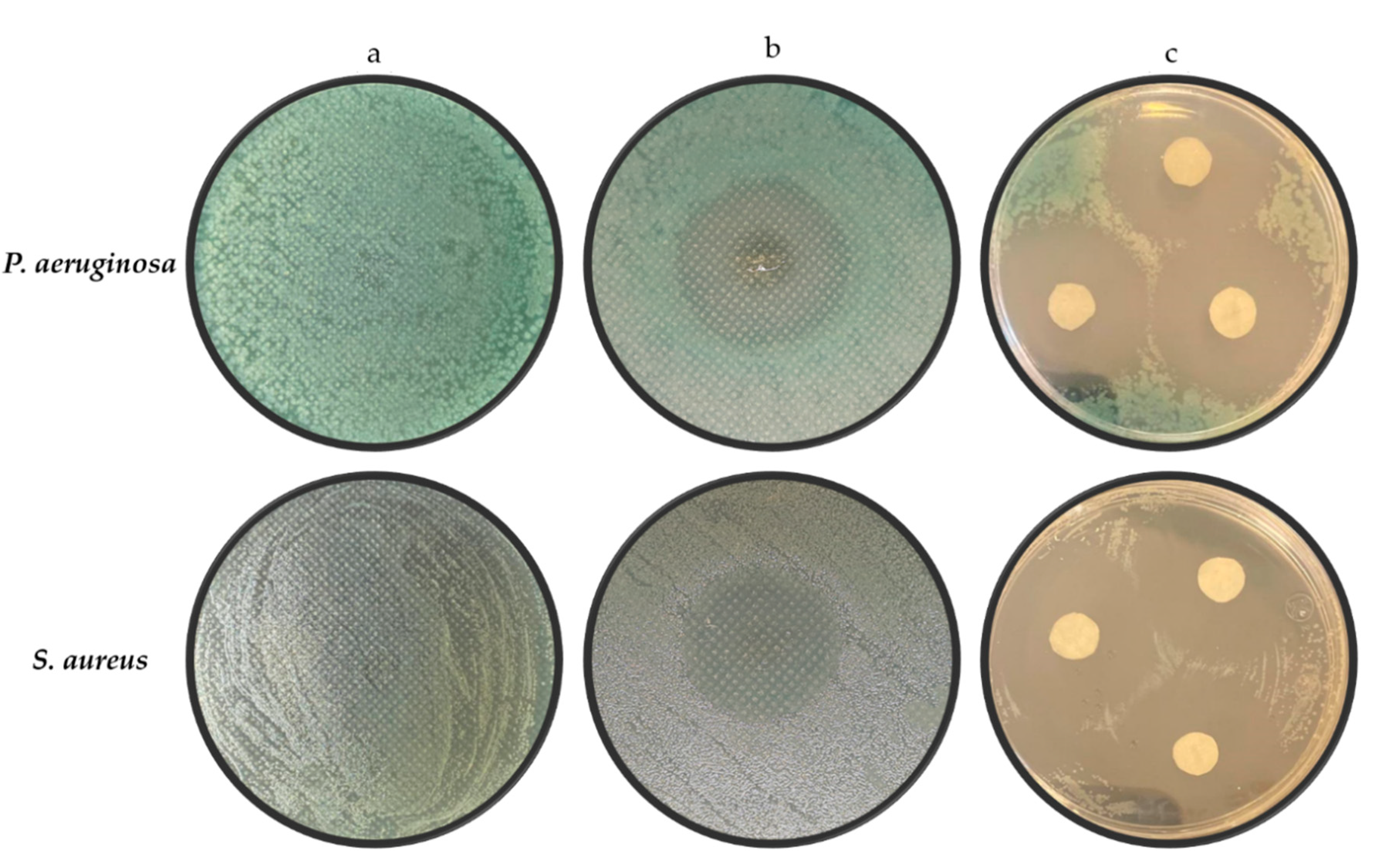
3.2. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qu, X.; Liu, H.; Zhang, C.; Lei, Y.; Lei, M.; Xu, M.; Jin, D.; Li, P.; Yin, M.; Payne, G.F.; et al. Electrofabrication of functional materials: Chloramine-based antimicrobial film for infectious wound treatment. Acta Biomater. 2018, 73, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Villegas, G.M.; Martímez-Hernádez, R.C.; Morales, J.; Olayo, R. Incorporation of fluoroquinolone/beta cyclodextrin inclusion complex from polylactic acid electrospun fibers and modeling of the release behavior. Rev. Mex. Ing. Química 2019, 18, 737–747. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, B.C.; Reinholz, J.; Li, Q.; Wang, J.; Zhang, K.A.I.; Landfester, K.; Crespy, D. Efficient Nanofibrous Membranes for Antibacterial Wound Dressing and UV Protection. ACS Appl. Mater. Interfaces 2016, 8, 29915–29922. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Heunis, T.D.J. Nanofibers offer alternative ways to the treatment of skin infections. J. Biomed. Biotechnol. 2010, 2010, 510682. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Knappe, P.; Bienert, R.; Weidner, S.; Thünemann, A.F. Characterization of poly(N-vinyl-2-pyrrolidone)s with broad size distributions. Polymer (Guildf.) 2010, 51, 1723–1727. [Google Scholar] [CrossRef]
- Costa-Salles, T.H.; Bertachini-Lombello, C.; Akira-D’Ávila, M. Electrospinning of Gelatin/Poly (Vinyl Pyrrolidone) Blends from Water/Acetic Acid Solutions. Mater. Res. 2015, 18, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Saadatkish, N.; Khoshakhlagh, K. Characterization of gelatin/cellulose acetate nanofibrous scaffolds: Prediction and optimization by response surface methodology and artificial neural networks. Polym. Sci. Ser. A 2016, 58, 399–408. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-W.; Park, J.-S.; Han, S.-Y.; Lee, E.-A.; Kwon, G.-J.; Seo, Y.-H.; Gwon, J.-G.; Lee, S.-Y.; Lee, S.-H. Preparation and Characteristics of Wet-Spun Filament Made of Cellulose Nanofibrils with Different Chemical Compositions. Polymers 2020, 12, 949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasim-Anuar, T.A.T.; Ariffin, H.; Norrrahim, M.N.F.; Hassan, M.A.; Andou, Y.; Tsukegi, T.; Nishida, H. Well-dispersed cellulose nanofiber in low density polyethylene nanocomposite by liquid-assisted extrusion. Polymers 2020, 12, 927. [Google Scholar] [CrossRef] [Green Version]
- Efendy, M.G.A.; Pickering, K.L. Composites: Part A Fibre orientation of novel dynamically sheet formed discontinuous natural fibre PLA composites. Compos. Part A 2016, 90, 82–89. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to. Polymers 2018, 10, 517. [Google Scholar] [CrossRef] [Green Version]
- Mosselhy, D.A.; Ge, Y.; Gasik, M.; Nordström, K.; Natri, O.; Hannula, S.P. Silica-gentamicin nanohybrids: Synthesis and antimicrobial action. Materials 2016, 9, 170. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, M.C.; Pérez-Ortega, F.; Felizberti, M.I. Efectos de la presencia de fibras de celulosa y curauá en las propiedades térmicas y mecanicas de eco-compositos de acetato de celulosa. Rev. Mex. Ing. Química 2018, 17, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Abramoff, M.D.; Magalhães, P.J.; Ram, S.J. Biophotonics international. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Catauro, M.; Scolaro, C.; Dal Poggetto, G.; Pacifico, S.; Visco, A. Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation. Polymers 2020, 12, 978. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer (Guildf.) 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, W.; Sun, B.; Li, H.; Wu, T.; Ke, Q.; Huang, C.; EI-Hamshary, H.; Al-Deyab, S.S.; Mo, X. A comparison of nanoscale and multiscale PCL/gelatin scaffolds prepared by disc-electrospinning. Colloids Surf. B Biointerfaces 2016, 146, 632–641. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Liu, H.; Guo, S.; Kumar, D.N.T.; Wang, Q. Preparation of fish gelatin and fish gelatin/poly(l-lactide) nanofibers by electrospinning. Int. J. Biol. Macromol. 2010, 47, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-garcía, E.; García-gonzález, R.; Salazar-schettino, P.M. Características generales del Staphylococcus aureus. Rev. Lat. Patol. Clin. Med. Lab. 2014, 61, 30–32. [Google Scholar]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. Fourier Transform-Mater. Anal. 2012, 3, 45–68. [Google Scholar] [CrossRef] [Green Version]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous Cellulose-Structure and Characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Ibrahim, M.; Nada, A.; Kamal, D. Density functional theory and FTIR spectroscopic study of carboxyl group. Indian J. Pure 2005, 43, 911–917. [Google Scholar]
- Hashim, D.M.; Man, Y.B.C.; Norakasha, R.; Shuhaimi, M.; Salmah, Y.; Syahariza, Z.A. Potential use of Fourier transform infrared spectroscopy for differentiation of bovine and porcine gelatins. Food Chem. 2010, 118, 856–860. [Google Scholar] [CrossRef]
- He, W.; Mosselhy, D.A.; Zheng, Y.; Feng, Q.; Li, X.; Yang, X.; Yue, L.; Hannula, S.P. Effects of silica–gentamicin nanohybrids on osteogenic differentiation of human osteoblast-like SaOS-2 cells. Int. J. Nanomed. 2018, 13, 877–893. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Choi, S.H.; Kang, M.L.; Lee, K.W.; Kim, K.N.; Sung, H.J. Synergistic Adhesiveness of Fibronectin with PHSRN Peptide in Gelatin Mixture Promotes the Therapeutic Potential of Human ES-Derived MSCs. Cell. Mol. Bioeng. 2020, 13, 73–86. [Google Scholar] [CrossRef]
- Grundke, K.; Pöschel, K.; Synytska, A.; Frenzel, R.; Drechsler, A.; Nitschke, M.; Cordeiro, A.L.; Uhlmann, P.; Welzel, P.B. Experimental studies of contact angle hysteresis phenomena on polymer surfaces-Toward the understanding and control of wettability for different applications. Adv. Colloid Interface Sci. 2015, 222, 350–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, X.; Jiang, L.; Qin, J. Flexible generation of gradient electrospinning nanofibers using a microfluidic assisted approach. Langmuir 2012, 28, 10026–10032. [Google Scholar] [CrossRef] [PubMed]
- Prado-Prone, G.; Bazzar, M.; Focarete, M.L.; García-Macedo, J.A.; Perez-Orive, J.; Ibarra, C.; Velasquillo, C.; Silva-Bermudez, P. Single-step, acid-based fabrication of homogeneous gelatin-polycaprolactone fibrillar scaffolds intended for skin tissue engineering. Biomed. Mater. 2020, 15, 035001. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hu, Y.; He, T.; Huang, M.; Zhang, X.; Yuan, J.; Wei, Y.; Dong, X.; Liu, W.; Ko, F.; et al. Electrospun Sandwich-Structure Composite Membranes for Wound Dressing Scaffolds with High Antioxidant and Antibacterial Activity. Macromol. Mater. Eng. 2018, 303, 1–13. [Google Scholar] [CrossRef]
- LaFountaine, J.S.; Prasad, L.K.; Brough, C.; Miller, D.A.; McGinity, J.W.; Williams, R.O. Thermal Processing of PVP- and HPMC-Based Amorphous Solid Dispersions. AAPS PharmSciTech 2016, 17, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Correia, D.M.; Padrão, J.; Rodrigues, L.R.; Dourado, F.; Lanceros-Méndez, S.; Sencadas, V. Thermal and hydrolytic degradation of electrospun fish gelatin membranes. Polym. Test. 2013, 32, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Fabrication and characterization of PCL/gelatin/chitosan ternary nanofibrous composite scaffold for tissue engineering applications. J. Mater. Sci. 2014, 49, 1076–1089. [Google Scholar] [CrossRef]
- Jalaja, K.; James, N.R. Electrospun gelatin nanofibers: A facile cross-linking approach using oxidized sucrose. Int. J. Biol. Macromol. 2015, 73, 270–278. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef]
- Tonda-turo, C.; Ruini, F.; Ceresa, C.; Gentile, P.; Varela, P.; Ferreira, A.M.; Fracchia, L.; Ciardelli, G. Colloids and Surfaces B: Biointerfaces Nanostructured sca ff old with biomimetic and antibacterial properties for wound healing produced by ‘green electrospinning’. Colloids Surf. B Biointerfaces 2018, 172, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, P.; Freitas, J.P.; Gonçalves, T.; Gil, M.H.; Figueiredo, M. Preparation of gentamicin sulfate eluting fiber mats by emulsion and by suspension electrospinning. Mater. Sci. Eng. C 2019, 94, 86–93. [Google Scholar] [CrossRef] [PubMed]
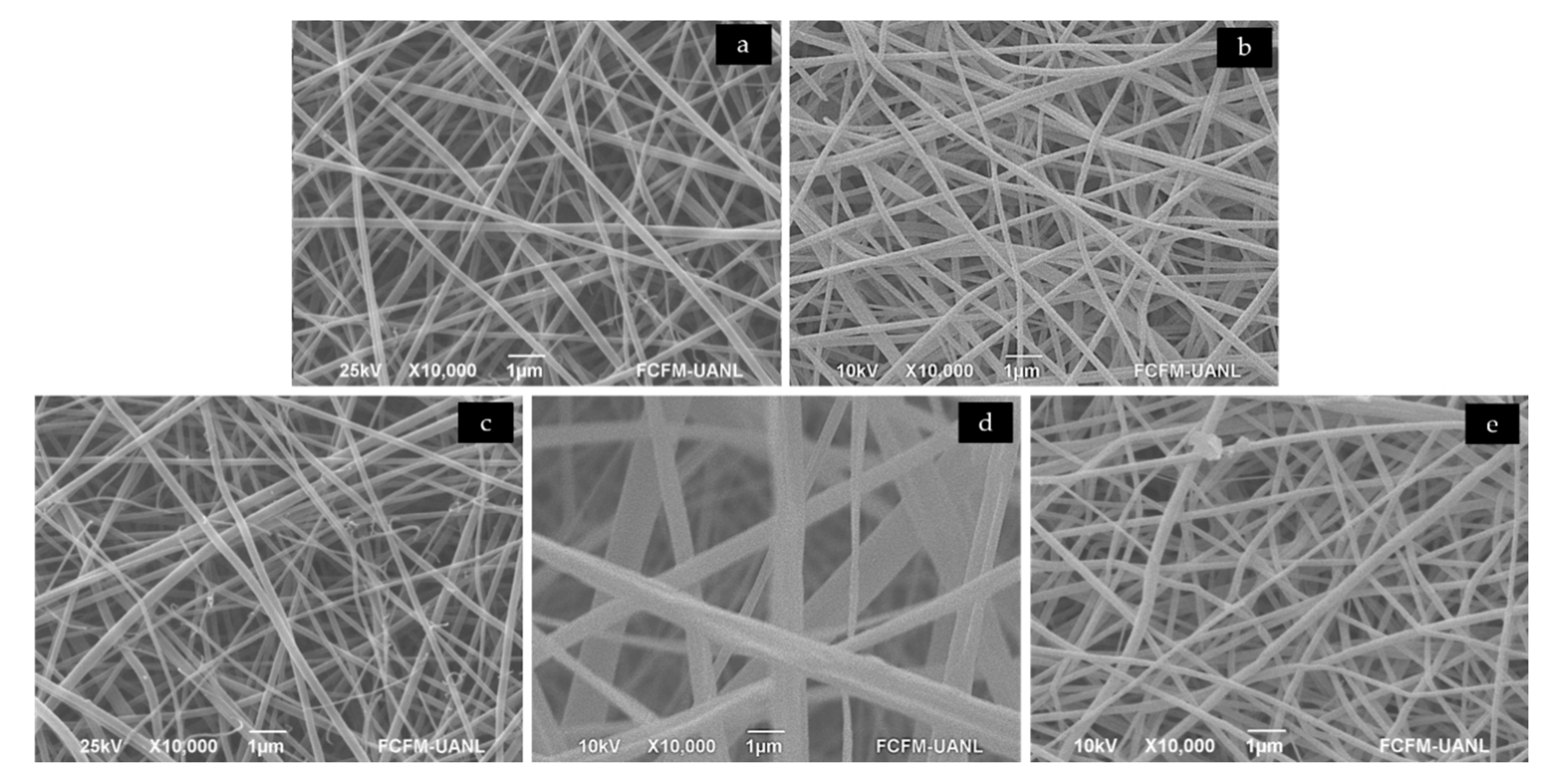

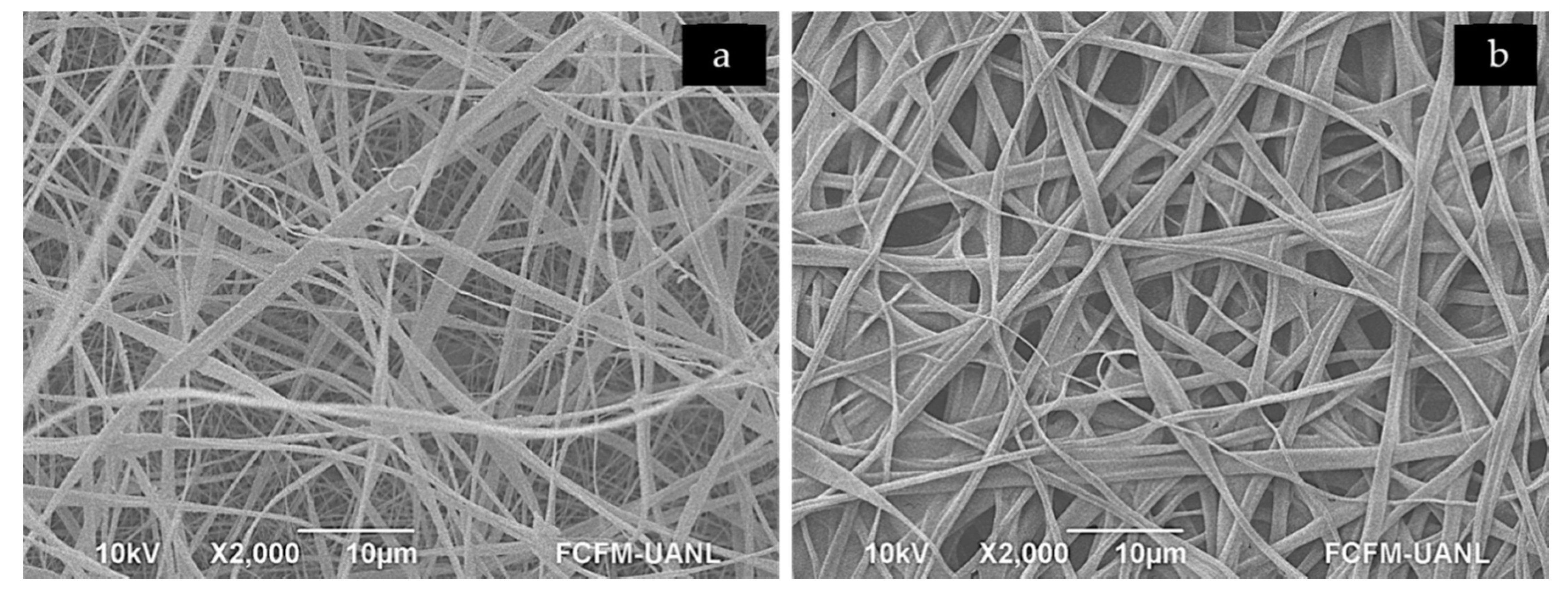

| Structure | PG | PG+gen | CA | Scaffold |
|---|---|---|---|---|
| Fibers | 56 ± 16 * | 124 ± 26 | 138 ± 66 | 307 ± 110 * |
| Pores | 127 ± 47 * | 372 ± 146 | 309 ± 135 | 472 ± 209 * |
| Bacteria | a | b | c |
|---|---|---|---|
| P. aeruginosa | 0 | 24.82 ± 1.10 | 25.17 ± 1.46 |
| S. aureus | 0 | 25.62 ± 0.86 | 25.72 ± 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Calderón, H.D.; Avilés-Arnaut, H.; Galán-Wong, L.J.; Almaguer-Cantú, V.; Laguna-Camacho, J.R.; Calderón-Ramón, C.; Escalante-Martínez, J.E.; Arévalo-Niño, K. Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers 2020, 12, 2311. https://doi.org/10.3390/polym12102311
López-Calderón HD, Avilés-Arnaut H, Galán-Wong LJ, Almaguer-Cantú V, Laguna-Camacho JR, Calderón-Ramón C, Escalante-Martínez JE, Arévalo-Niño K. Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers. 2020; 12(10):2311. https://doi.org/10.3390/polym12102311
Chicago/Turabian StyleLópez-Calderón, Héctor D., Hamlet Avilés-Arnaut, Luis J. Galán-Wong, Verónica Almaguer-Cantú, J. R. Laguna-Camacho, C. Calderón-Ramón, J. E. Escalante-Martínez, and Katiushka Arévalo-Niño. 2020. "Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing" Polymers 12, no. 10: 2311. https://doi.org/10.3390/polym12102311
APA StyleLópez-Calderón, H. D., Avilés-Arnaut, H., Galán-Wong, L. J., Almaguer-Cantú, V., Laguna-Camacho, J. R., Calderón-Ramón, C., Escalante-Martínez, J. E., & Arévalo-Niño, K. (2020). Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers, 12(10), 2311. https://doi.org/10.3390/polym12102311








