Enhanced Olefin Transport by SiO2 Particles for Polymer/Ag Metal/Electron Acceptor Composite Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation and Permeance Measurements
2.3. Characterization
3. Results and Discussion
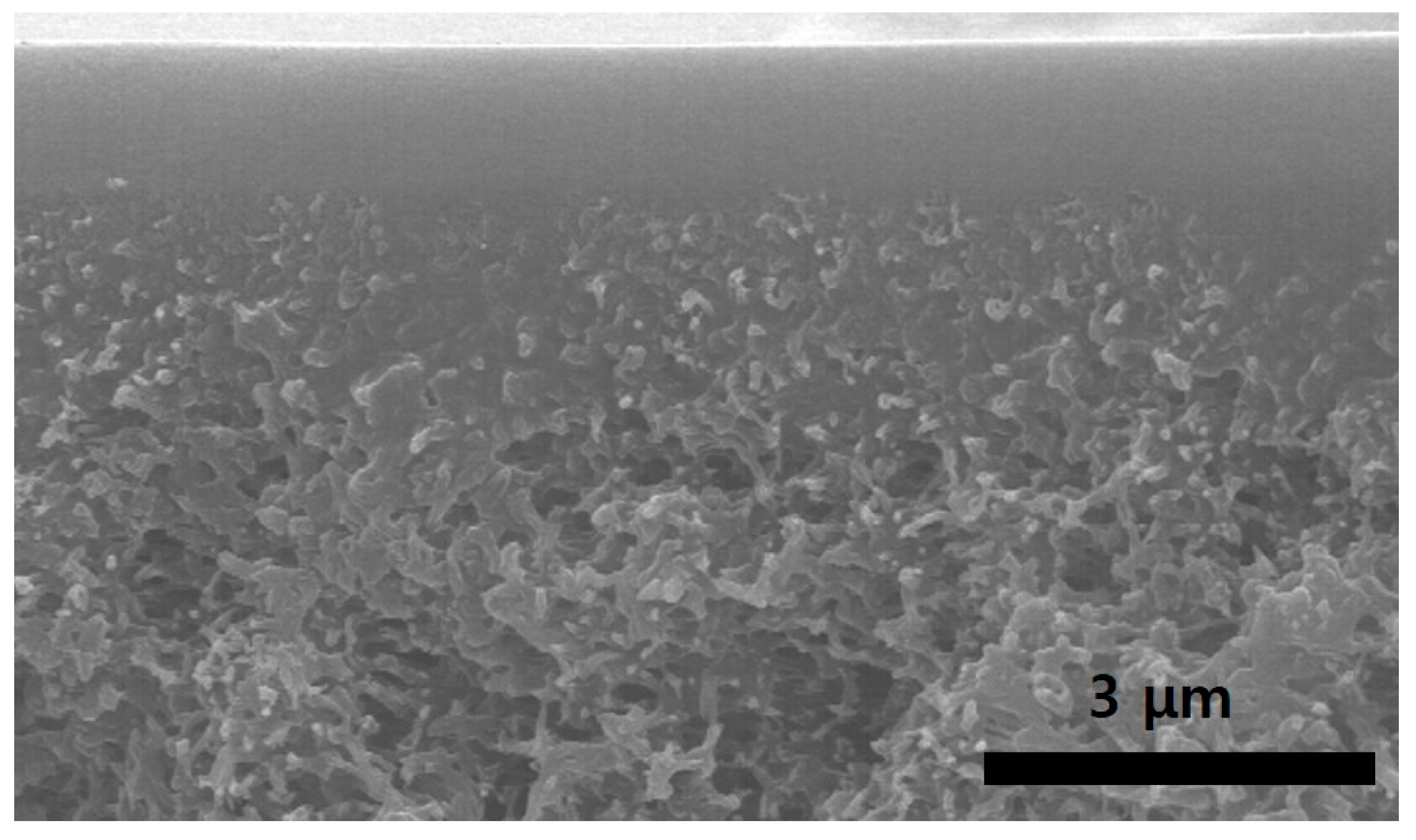
3.1. Coated State of Composite Membranes
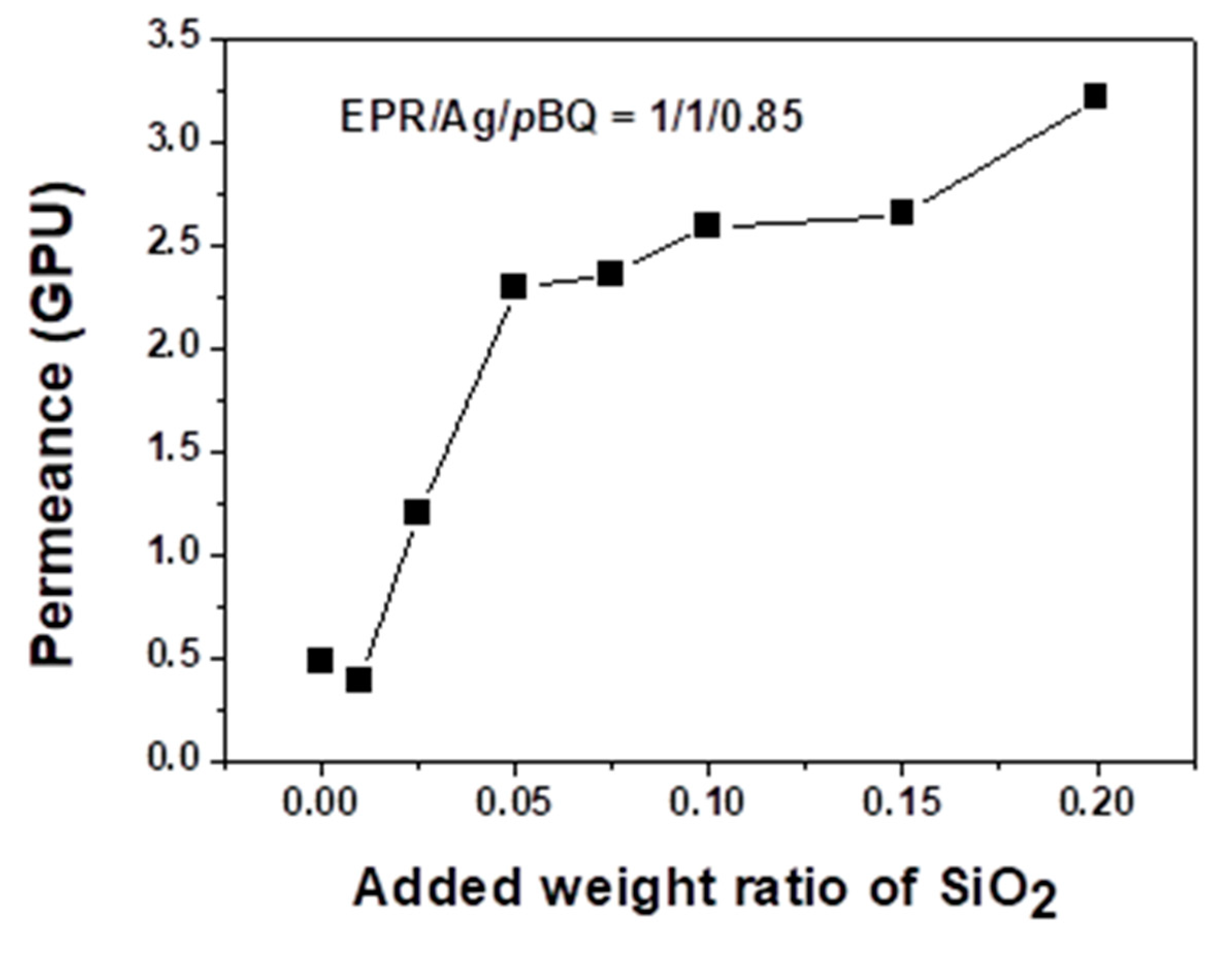
3.2. Separation Performance: Permeance and Selectivity
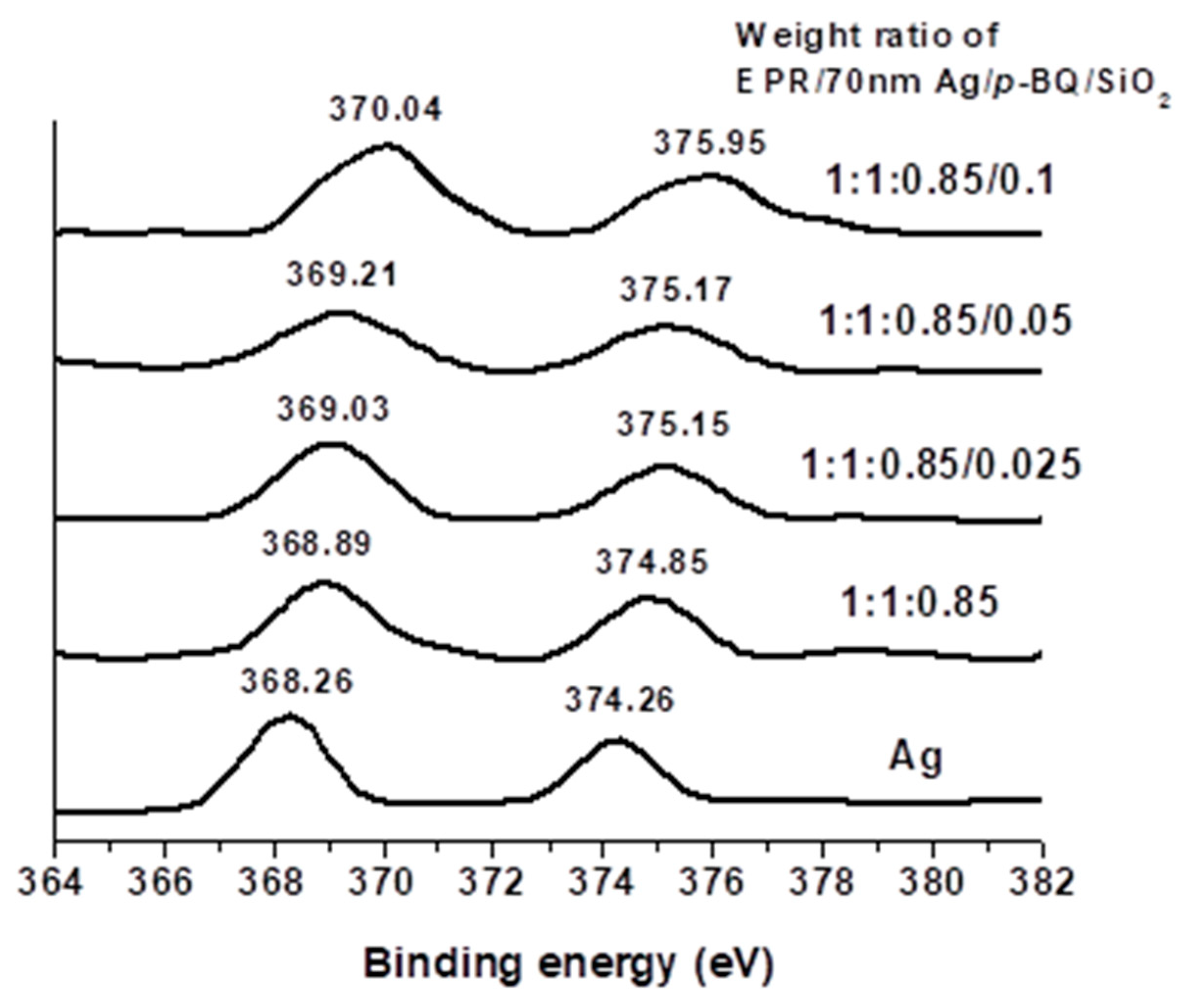
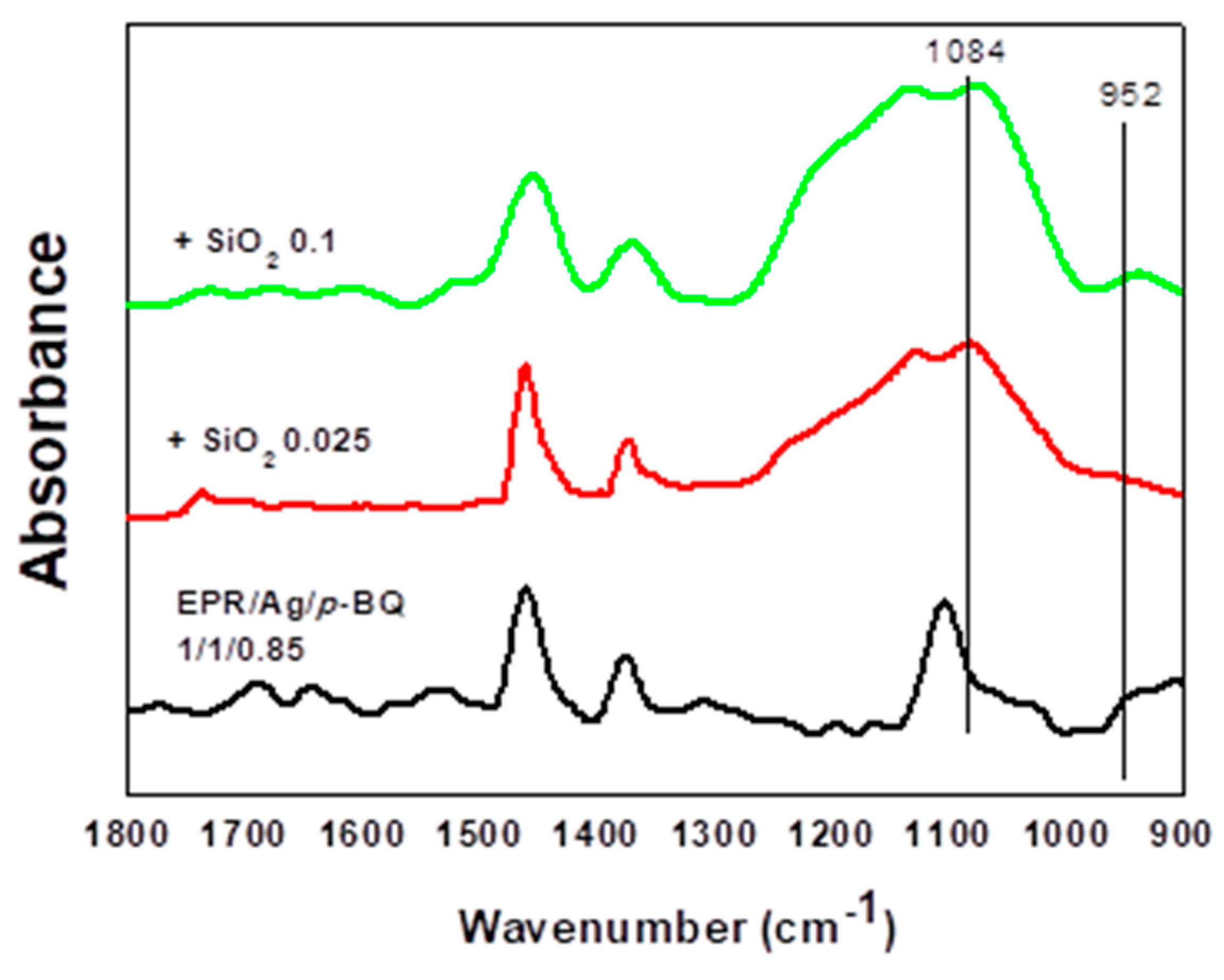
3.3. Binding Energy and FT-IR Spectra
3.4. Glass Transition Temperature
4. Conclusions
Funding
Conflicts of Interest
References
- Haleem, A.; Syaal, S.B.; Ajmal, M.; Ambreen, J.; Rauf, S.; Ali, N.; Muhammad, S.; Shah, A.; Zia, M.A.; Siddiq, M. Silver and palladium nanoparticle embedded poly(n-isopropylacrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hybrid microgel catalyst with pH and temperature dependent catalytic activity. Korean J. Chem. Eng. 2020, 37, 614. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Sorption, Transport, and Structural Evidence for Enhanced Free Volume in Poly(4-methyl-2-pentyne)/Fumed Silica Nanocomposite Membranes. Chem. Mater. 2003, 15, 109. [Google Scholar] [CrossRef]
- Tan, W.-L.; Tan, H.-F.; Ahmad, N.A.; Hamzah, N.; Ahmad, A.L.; Leo, C.P. Carbon capture by alkaline absorbent using octadecyltrichlorosilane modified PVDF/TiO2 membrane. Korean J. Chem. Eng. 2020, 37, 505. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, Reverse-Selective Nanocomposite Membranes. Science 2002, 296, 519. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Maharajan, A.; Dikshit, P.K.; Kim, B.S. Rapid and reusable detection of hydrogen peroxide using polyurethane scaffold incorporated with cerium oxide nanoparticles. Korean J. Chem. Eng. 2019, 36, 2143. [Google Scholar] [CrossRef]
- Garba, Z.N.; Xiao, W.; Zhou, W.; Lawan, I.; Jiang, Y.; Zhang, M.; Yuan, Z. Process optimization and synthesis of lanthanum-cobalt perovskite type nanoparticles (LaCoO3) prepared by modified proteic method: Application of response surface methodology. Korean J. Chem. Eng. 2019, 36, 1826. [Google Scholar] [CrossRef]
- Khalid, F.; Tabish, M.; Bora, K.A.I. Novel poly(vinyl alcohol) nanofiltration membrane modified with dopamine coated anatase TiO2 core shell nanoparticles. J. Water Process Eng. 2020, 37, 101486. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Cao, X.; Sun, W. Comparing the effects of different types of inorganic nanoparticles on 17β-estradiol adsorption by graphene oxide. Environ. Res. 2020, 187, 109656. [Google Scholar] [CrossRef]
- Čubová, K.; Čuba, V. Synthesis of inorganic nanoparticles by ionizing radiation—A review. Radiat. Phys. Chem. 2020, 169, 108774. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.; Choi, K.; Lee, J.; Kang, I.; Ahn, C.; Cho, C.-H. Fabrication and Ionic Current Rectification Characteristics of Biomimetic Aluminum Oxide Membrane. Membr. J. 2020, 30, 181–189. [Google Scholar] [CrossRef]
- Oh, H.; Patel, R. Recent Development in Metal Oxides for Carbon Dioxide Capture and Storage. Membr. J. 2020, 30, 97–110. [Google Scholar] [CrossRef]
- Jang, H.; Kim, J. Effect of Inorganic Particles on Organic Fouling in Pressurized Membrane Filtration. Membr. J. 2020, 30, 131–137. [Google Scholar] [CrossRef]
- Jeoung, Y.; Seok, D.; Lee, S.; Shin, W.H.; Sohn, H. Polymer/Inorganic Nanohybrid Membrane on Lithium Metal Electrode: Effective Control of Surficial Growth of Lithium Layer and Its Improved Electrochemical Performance. Membr. J. 2020, 30, 30–37. [Google Scholar] [CrossRef]
- Su, S.J.; Kuramoto, N. Processable polyaniline–titanium dioxide nanocomposites: Effect of titanium dioxide on the conductivity. Synth. Met. 2000, 114, 147. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Fang, D. An ultralight silica-modified ZrO2–SiO2 aerogel composite with ultra-low thermal conductivity and enhanced mechanical strength. Scr. Mater. 2018, 143, 113. [Google Scholar] [CrossRef]
- Liang, W.; Wang, L.; Zhu, H.; Pan, Y.; Zhu, Z.; Sun, H.; Ma, C.; Li, A. Enhanced thermal conductivity of phase change material nanocomposites based on MnO2 nanowires and nanotubes for energy storage. Sol. Energ. Mater. Solar Cells 2018, 180, 158. [Google Scholar] [CrossRef]
- Chae, I.S.; Kang, S.W.; Park, J.Y.; Lee, Y.G.; Lee, J.H.; Won, J.; Kang, Y.S. Surface energy level tuning of silver nanoparticle for facilitated olefin transport. Angew. Chem. Int. Ed. 2011, 123, 3038. [Google Scholar] [CrossRef]
- Hong, G.H.; Song, D.; Chae, I.S.; Oh, J.H.; Kang, S.W. Highly permeable poly(ethylene oxide) with silver nanoparticles for facilitated olefin transport. RSC Adv. 2014, 4, 4905. [Google Scholar] [CrossRef]
- Kim, M.; Kang, S.W. PEBAX-1657/Ag nanoparticles/7,7,8,8-tetracyanoquinodimethane complex for highly permeable composite membranes with long-term stability. Sci. Rep. 2019, 9, 4266. [Google Scholar] [CrossRef]
- Kang, S.W. Long-Term Stable 1-butyl-3-methylimidazolium Hexafluorophosphate/Ag Metal Composite Membranes for Facilitated Olefin Transport. Membranes 2020, 10, 191. [Google Scholar] [CrossRef]
- Kang, Y.S.; Kang, S.W.; Kim, H.; Kim, J.H.; Won, J.; Kim, C.K.; Char, K. Interactions of the Partially Polarized Surface of Silver Nanoparticles by p-Benzoquinone with Olefin and Its Implication to Facilitated Olefin Transport. Adv. Mater. 2007, 19, 475. [Google Scholar] [CrossRef]


© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.W. Enhanced Olefin Transport by SiO2 Particles for Polymer/Ag Metal/Electron Acceptor Composite Membranes. Polymers 2020, 12, 2316. https://doi.org/10.3390/polym12102316
Kang SW. Enhanced Olefin Transport by SiO2 Particles for Polymer/Ag Metal/Electron Acceptor Composite Membranes. Polymers. 2020; 12(10):2316. https://doi.org/10.3390/polym12102316
Chicago/Turabian StyleKang, Sang Wook. 2020. "Enhanced Olefin Transport by SiO2 Particles for Polymer/Ag Metal/Electron Acceptor Composite Membranes" Polymers 12, no. 10: 2316. https://doi.org/10.3390/polym12102316
APA StyleKang, S. W. (2020). Enhanced Olefin Transport by SiO2 Particles for Polymer/Ag Metal/Electron Acceptor Composite Membranes. Polymers, 12(10), 2316. https://doi.org/10.3390/polym12102316



