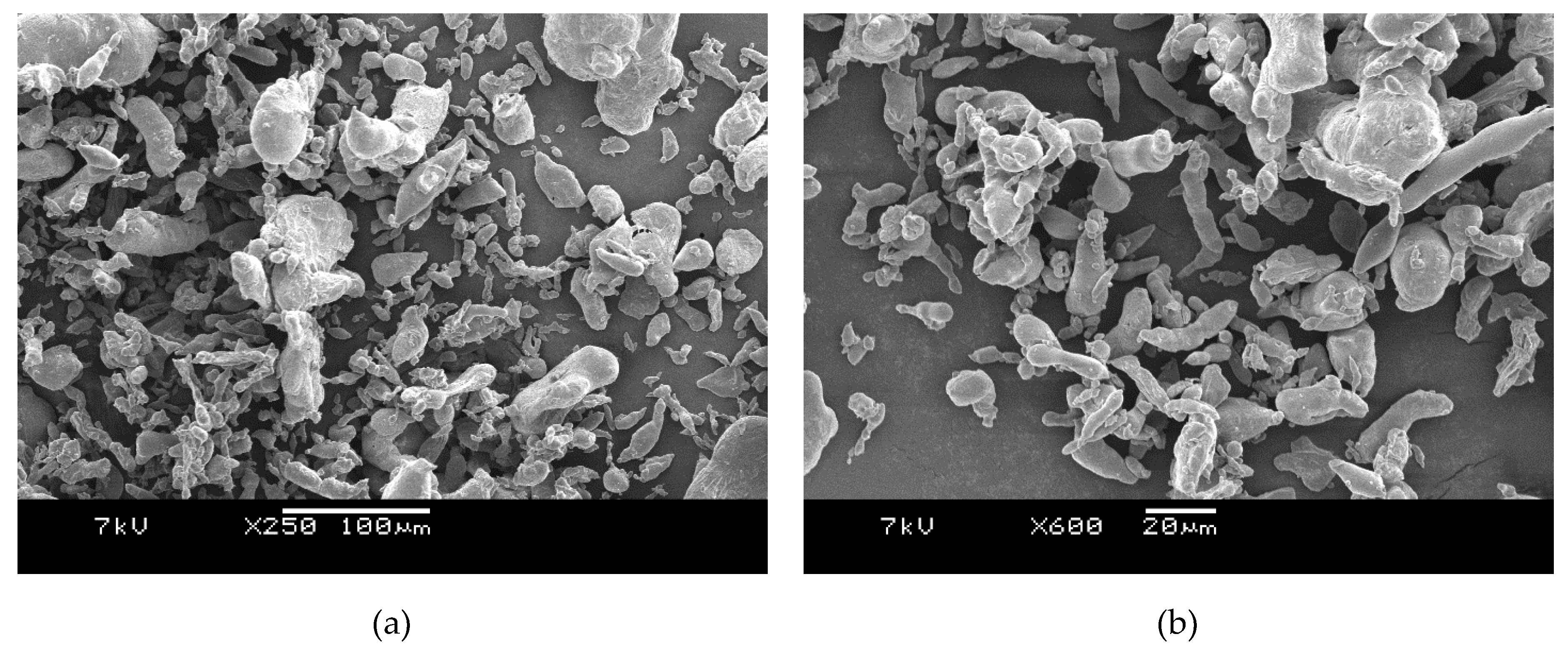
3.4.1. PET Blended with Al 1 Particles
Table 6 shows the tensile and flexural properties of the amorphous PET composites with the Al 1 particles. The tensile modulus increased continuously with increased Al loading. The value of tensile modulus for amorphous PET was 1.60 GPa, while that of the Al 1/PET composites filled with 15 vol. % Al micro particles reached 2.07 GPa. The tensile strength remained invariant at ~60 MPa at all loadings of micro Al. That is, unlike most cases in the literature, there was no major drop in the tensile strength with filler content. Osman and Mariatti [
24] showed that in polypropylene filled with Al particles, the tensile strength dropped monotonically; by 30% vol. of Al, the tensile strength of the Al-PP composite had halved. Similar drops in strength are reported in other metal-plastic pairs [
20].
Table 6 shows the elongation-to-break of amorphous PET at room temperature (with a cross head speed of 50 mm/min) was 96%. The elongation-to-break increased to 136% at 5 vol. % of Al 1 but the variation was high. At higher loadings of Al 1 like 10 vol. % and 15 vol. %, the elongation decreased abruptly to 13–16%. The abrupt drop in elongation-to-break is commonly observed in filler composites. Surprisingly, the notched Izod impact resistance increased at these concentrations despite the decrease in elongation-to-break, as will be discussed shortly.
Table 6 shows also the flexural modulus values increased as the Al 1 content increased. Al 1/PET composites filled with 15 vol. % Al 1 particles showed the highest value of flexural modulus (3.24 GPa), which was about 24% higher than the flexural modulus of the amorphous PET (2.47 GPa). The incorporation of the Al 1 particles into the PET matrix led to no statistically significant change in the flexural strength of the Al 1/PET-composites; it was about 90 MPa. Tensile bars with 20 vol. % and 30 vol. % of Al 1 could not be moulded without defects, hence they were not usable for the tensile and flexural tests. Thus,
Table 6 shows the tensile properties only up to an Al 1 content of 15 vol. %. Note with Izod bars, we could mould with up to 30 vol. % of Al 1.
Table 7 shows the notched Izod impact resistance of the amorphous PET and the composites made with Al 1 particles. Note that in the literature, this is often called ‘notched Izod impact strength’, but we prefer to call it a ‘notched Izod impact resistance’. Strength is based on a force, whereas the Izod impact involves an energy and the value is given in J/m or J/m
2.
Table 7 shows that the addition of Al 1 to the amorphous PET samples resulted in a significant enhancement of the notched Izod impact resistance. However, the standard deviations in
Table 7 are higher than for the tensile and flexural tests (see
Table 6), hence some care has to be taken in the interpretation.
In notch-sensitive materials, the impact results tend to show more variation than the tensile modulus. Ideally, if impact toughening occurs, the magnitude of the effect should be large enough to be meaningful and the measurement should be precise enough to be trustable. Hence, a two sample (or unpaired)
t-test for the Izod impact resistance values for the micro-Al composites was conducted, to see if the mean values of impact resistance in
Table 7 for two compared compositions of Al-PET were significantly different (see
Table 8). The two sample
t-test determines whether or not the means of two independent populations from two normal distributions are equal or whether they differ according to a significance level. We make the assumption the Izod test results are normally distributed for each composition (population) and while their true mean values may differ, their true standard deviations are the same. For the unpaired
t-test, we used the null hypothesis H
null: μ
Al x% − μ
Al y% = 0, that is, there is no difference in the means, and the alternative hypothesis applied was H
a: μ
Al x% − μ
Al y% ≠ 0, where μ is the mean Izod impact values for compositions with x% Al and y% Al. A significance level of 0.05 was chosen for the comparison of the means. This indicates that there is a 5% risk of rejecting the null hypothesis when it is true (or there is a 5% chance of wrongly concluding that there is a difference between Izod impact values of x% Al and y% Al, when there is no actual difference between the two populations).
Inspection of
Table 7 shows that the impact resistances of all the Al 1–amorphous PET compositions are higher than the amorphous PET; that is, the Al 1 particles act as a toughening agent for amorphous PET. This is reflected in the minimum, median and maximum values also, which are higher than the corresponding values for the amorphous PET. Due to the higher variation in impact results, the
t-test analysis in
Table 8 must also be considered for interpreting the results. The general conclusion from
Table 7 and
Table 8 is (1) the impact strength increases up to 15 vol. % of Al 1, reaching a maximum of 51.6 J/m which is more than double the value of amorphous PET (2) between 15 and 20 vol. %, there is a levelling or no real difference (3) the 30 vol. % shows a decline versus the 15 vol. %, but its impact value does not fall below the level of the 10 vol. % Al 1. The SEM fracture surfaces in
Figure 6d,e suggest there is a transition to a more brittle fracture after 15 vol. % of Al 1. The standard deviations in
Table 7 for 20 and 30 vol. % have become very large and in doing the
t-test, there is the assumption that the two normal distributions for each population are the same or close (the ratio of the higher to the lower standard deviation should be at least < 2 for applicability of the test). The higher variation in
Table 7 after 15 vol. % is due to the higher tendency for agglomeration. However, even at high loadings, the values of the 20 vol. % and 30 vol. % Al 1-amorphous PET do not decrease below the base amorphous PET. Thus, we can say with a high degree of confidence that Al 1 particles cause impact toughening of amorphous PET.
Even with the poor quality of the powder, it is remarkable that unlike most cases in the literature, where addition of metal particles decreased the impact resistance [
20], with amorphous PET, the impact resistance more than doubled when Al was added. Taşdemır and Gülsoy [
31] showed that in HDPE, polypropylene, and polystyrene filled with 5, 10 and 15 vol. % iron powder, the notched Izod impact resistance decreased in all three cases, and there was a 50% reduction with HDPE containing 10 vol. % of Fe.
3.4.2. PET Blended with Al 2 Powder
The nano Al 2 powder was incorporated up to only 5 vol. %.
Table 9 shows the tensile modulus and tensile strength of the Al 2/amorphous PET composites were almost invariant (~1.60 GPa for tensile modulus, and ~56 MPa for tensile strength) with the volume percentage (of Al 2 particles. There is not much literature on the mechanical properties of metal-filled PET, so the best we are able to compare is with graphene-amorphous PET composites from recent work. Shabafrooz et al. [
5] reached a tensile modulus of ~1.8 GPa with 2 wt. % of graphene in amorphous PET and this could be raised to 2.4 GPa with graphene that had been surface-modified with trimellitic anhydride. The level of modulus attained is not very different from the Al-PETs in
Table 6, but due to the current cost of graphene (even with 2% loading), and the surface treatment procedure needed [
5] to create bonding to the PET, the graphene-PET composite would be less worthwhile than the Al-PET.
The flexural moduli and strengths of the nano-Al 2/amorphous PET composites were also invariant with the Al content in the range studied. However, the elongation-to-break somewhat surprisingly showed an increase at 1 and 3 vol. % (
Table 9). It is well known that amorphous PET is easily drawn 5–6×
above its Tg, typically at 90–120 °C, by a necking process; it is the basis of PET fibre and filament production, and the production speed is well over 1000 m/min. Less well known is that amorphous PET can neck and draw even at room temperature to 500% if low cross head speeds, such as 2 mm/min, are used. In the current case, the amorphous PET extended up to 96% (average value with high standard deviation) at room temperature at the cross head speed used in the tensile test (50 mm/min). Surprisingly, the 1 vol. % and 3 vol. % Al 2-amorphous PET tensile bars actually extended over 400% at room temperature at 50 mm/min, and the variation was low (see standard deviations in
Table 9). At 5 vol. % Al 2, there was a drop in extensibility, with increased variability compared with the 1 and 3 vol. % loading.
Figure 10 shows a tensile bar of a 3 vol. % Al 2-PET drawn to high draw ratios of ~5:1 at 100 °C and heat set at 170 °C under tension. Two unfilled PET bars, drawn at 100 °C and heat set at 170 °C under tension, are also shown. Note that drawn bars of pure PET are transparent (
Figure 10), although after drawing and heat setting, they transform from amorphous to semi-crystalline. Amorphous PET will be transparent always; semi-crystalline PET will be opaque if thermally crystallized but transparent if formed by stretching of amorphous PET (for example PET bottles are transparent). The unimpaired drawability of compositions of amorphous PET with up to 3 vol. % of the nano Al 2 would be suitable for making Al-PET filaments by hot stretching. In most of the other studies on metal-polymer composites, the oriented form is not considered, but as PET is the principal synthetic fibre of the textile industry, this was useful to establish, as conductive filaments, or fabrics with reduced static build up may be possible with Al. PET with genuinely nano-sized Al should be melt-spinnable without blockage of the spinnerets, and the filaments would be hot drawable like pure PET, up to some percentage of Al.
The notched Izod impact resistances of the Al 2/amorphous PET composites are listed in
Table 10. As with the Al 1/PET composites, the addition of nano aluminum particles produced a major enhancement in the composites’ impact resistance. With the Al 2, an impact resistance of 51 J/m was achieved with 5 vol. % Al and this is more than double the impact value of the amorphous pure PET.
Table 11 uses the
t-test to analyse the mean values of notched Izod for the amorphous PETs with Al 2 particles. All compositions showed an increase in impact resistance compared with amorphous PET, and with 5% Al 2, the impact resistance was more than double of amorphous PET. The standard deviation of the 5% composition was about half that of amorphous PET (
Table 10), hence even without the
t-test, the increase is obvious. Compositions 1% and 3 vol. % of Al 2 were not statistically different from each other. Often with nano particles, effects found at higher loadings with micron sized particles can be replicated with lower loadings if agglomeration does not take place; this is seen here with the notched Izod impact. Doubling of the impact resistance occurred with micron sized Al 1 at 15 vol. % but this effect was seen at 5% with Al 2 (compare
Table 10 and
Table 11 with
Table 7 and
Table 8). However, this was surprising since the SEM micrographs had indicated the presence of some micron sized Al particles in the starting nano Al 2 powder (see
Figure 1b). Perhaps after melt mixing, some of the micron-sized aggregates in the nano Al 2 powder were broken back into the nano particles. If toughening of amorphous PET is needed, low loadings of nano Al 2 appear to be good, as the density is not increased greatly. Our main reservation with nano Al 2 was the difficulty in handling it: despite using a respirator mask, it could be felt in the lungs, hence it is hazardous to handle it.
In contrast to Al-amorphous PET, PET composites with graphite, graphene and CNTs show embrittlement at very low addition levels (even at <1 wt %). Alshammary et al.’s [
1] work showed that addition of 15% graphite to PET was needed to observe an increase in electrical conductivity; there was a 75% increase in tensile modulus, but the elongation-to-break decreased from 6% in the PET to 1.5% in the composite. Rodríguez-Uicab et al. [
4] had a PET with a modulus of 1.33 GPa, a strength of 30.5 MPa, an elongation-to-break of 25%, and a toughness (calculated from the area under the stress-strain curve) of 5.98 J/m
3. With the addition of just 0.1% multi walled CNTs by melt compounding, Rodríguez-Uicab reported the modulus increased from 1.33 GPa to 1.5 MPa, the strength was 29.7 MPa, but the elongation-to-break decreased from 25% to 2.41%, and the toughness decreased from 5.98 J/m
3 to 0.47 J/m
3.; that is, for a very small increase in modulus, a large sacrifice in toughness was made. Aoyama et al. [
6] made composites from untreated and treated graphenes in PET. They reported a PET with a tensile modulus of ~1.6 GPa, and an elongation-to-break of ~420%. One of Aoyama’s graphenes at 4% loading gave a small increase in modulus (from 1.6 to 1.8 GPa), with an elongation-to-break of ~90%. Another graphene after surface treatment, at a 2% loading gave a modulus of 2.3 GPa but with an elongation-to-break < 5% [
6]. Again, from a mechanical point of view, it translates to a small increase in modulus with a large sacrifice in toughness, made at great expense. Aoyama’s values of modulus and elongation-to-break of the graphene-PET composites [
6] may be contrasted with the values in
Table 6 and
Table 9 for the Al 1-amorphous PET and Al 2-amorphous PET composites, where comparable moduli were obtained but the lowest elongation value was 13.7%; further,
Table 7 and
Table 10 show all the Al-PET compositions gave increases in the impact resistance over the base PET.
One notable feature is the high discrepancy in elongation-to-break reported for the unfilled PETs. In Alshammary et al., it is 6%, Rodríguez-Uicab et al. cite 25%, but Aoyama et al. [
6] had ~420%. We had 96% in
Table 6 and
Table 9. The reason for this discrepancy could be due to ‘physical ageing’ that takes place in all amorphous polymers [
32], and due to the tensile testing rate. Physical ageing leads to a densification and embrittlement with time (which shows also in reduced elongation-to-break). The elongation-to-break of freshly made amorphous PET is ~500% at an extension rate of 2–5 mm/min, but after several months, it decreases. The elongation-to-break also depends on testing rate, with high elongation rates leading to low breakage strains. The physical ageing rate and loss of ductility in amorphous materials depends on storage time and the temperature; the closer the storage temperature is to the
Tg, the faster the ageing [
29,
32]. In our work, we made the measurements within 3 months, and as explained the DSC curves in
Figure 8 and
Figure 9 did not show the sign of physical ageing at the
Tg, hence the amorphous PET bars (with and without Al) can be compared In the other works cited, many do not even specify whether their PET is amorphous or semi-crystalline [
1,
3,
4,
5,
6] and do not consider the physical ageing phenomenon in amorphous PET, and these have to be deduced indirectly from their DSC curves, or from their sample preparation method. In Rodríguez-Uicab et al.’s [
4] work on CNT-PET composites, the DSC suggests the PET in the as-prepared samples was semi-crystalline rather than amorphous, hence the elongation-to-break will be naturally different and cannot be compared with cases where the PET is amorphous. Despite this uncertainty about comparing different works on filled PET, inspection of other polymers with CNTs [
33,
34] show in most cases, they make the composite brittle even at loadings like 1%.
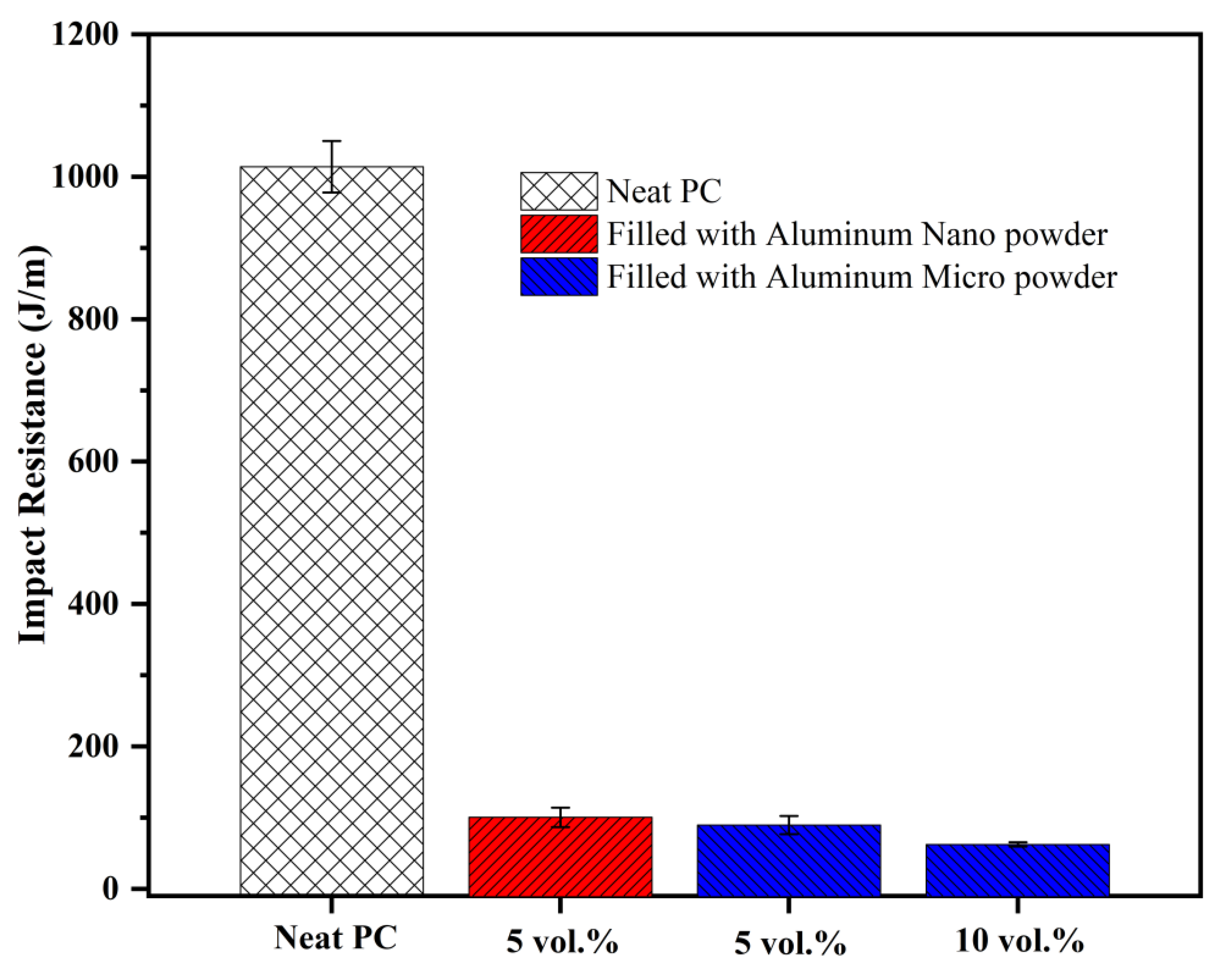
It is worth having a mental picture of the range of intrinsic impact resistances of unfilled plastics. This range spans the most brittle thermoplastics like amorphous polystyrene with a notched Izod impact resistance of ~12 J/m to the toughest plastic, amorphous polycarbonate, at ~1000 J/m. Polycarbonate with a Tg of 150°C is an exception, as most thermoplastics with high Tg fall in the range 25–70 J/m for notched Izod impact resistance. Thermoplastics with low Tg have higher impact strength; thus, PP homopolymer has a notched Izod impact resistance of ~80 J/m while impact PP copolymers with ethylene comonomer reach up to ~170 J/m, but this is associated with a lowered Tg and modulus. PET has a higher Tg, and higher modulus and strength than polyolefins. If unnotched, PET has a high impact resistance, but it is a little on the brittle side when the material is notched (notched Izod impact resistance of ~22 J/m). Even with such a value, amorphous PET is a usable material, for example in blood tubes employed in healthcare. For demanding engineering applications, high Tg polymers are toughened with rubber particles, but this involves a trade-off with sacrificing modulus and strength.