Size-Dependent Phagocytic Uptake and Immunogenicity of Gliadin Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Preparation of Gliadin Nanoparticles
2.4. Characterization of Gliadin Nanoparticles
2.4.1. Particle Size Analysis and Zeta Potential
2.4.2. Scanning Electron Microscopy (SEM) Analysis
2.4.3. Encapsulation Efficiency
2.5. Hemolysis Assay
2.6. Polymorphonuclear Cell Uptake
2.7. FITC Tagged IgG Adsorption
2.8. In Vitro Phagocytic Uptake Assay
2.9. In Vivo Immunization of Mice with Gliadin Nanoparticles
2.10. Data Analysis
3. Results
3.1. Preparation of Gliadin Nanoparticles
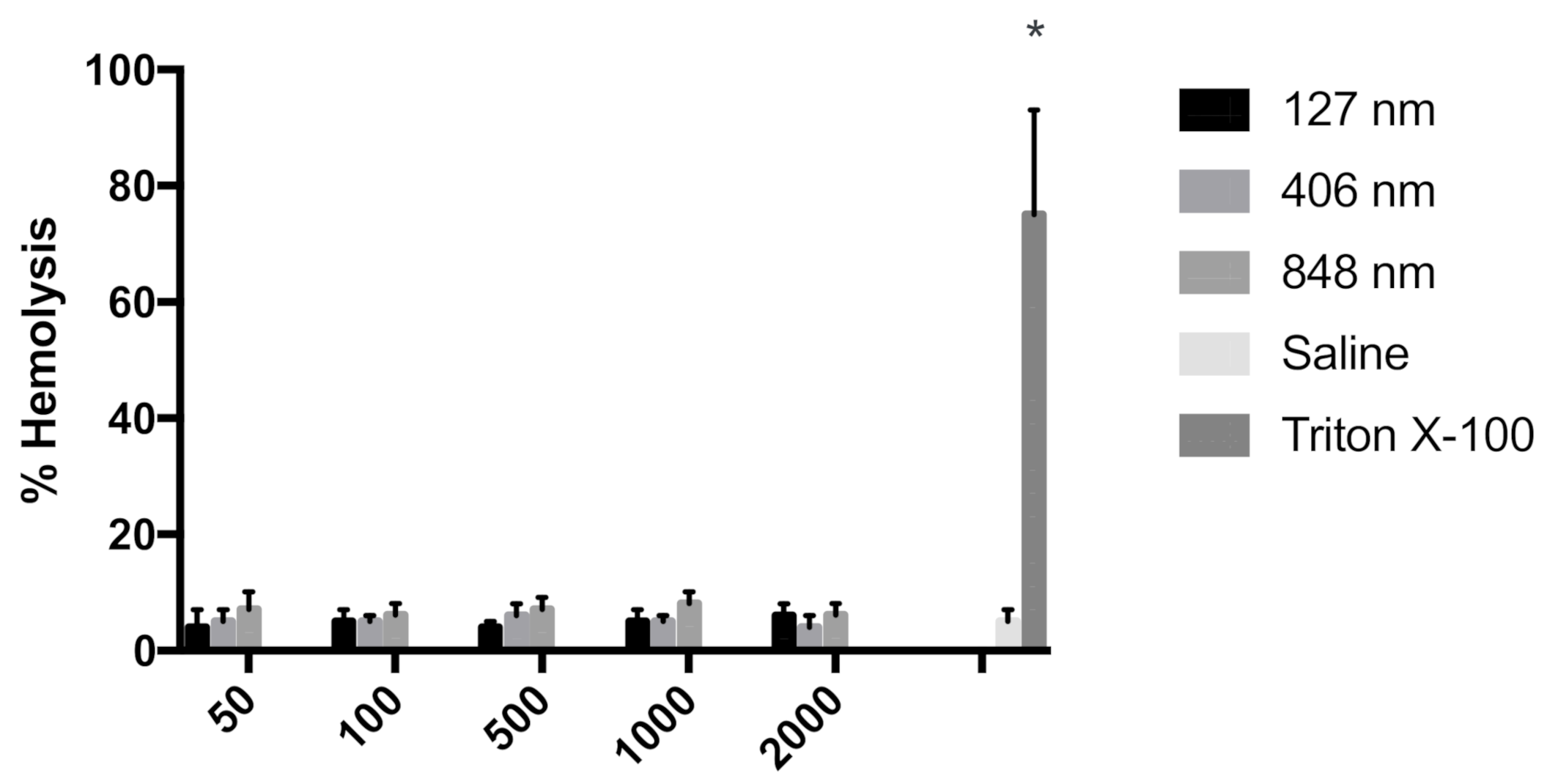
3.2. Hemolysis Assay
3.3. Polymorphonuclear (PMN) Cell Uptake
3.4. FITC Tagged IgG Adsorption
3.5. In Vitro Phagocytic Uptake Assay
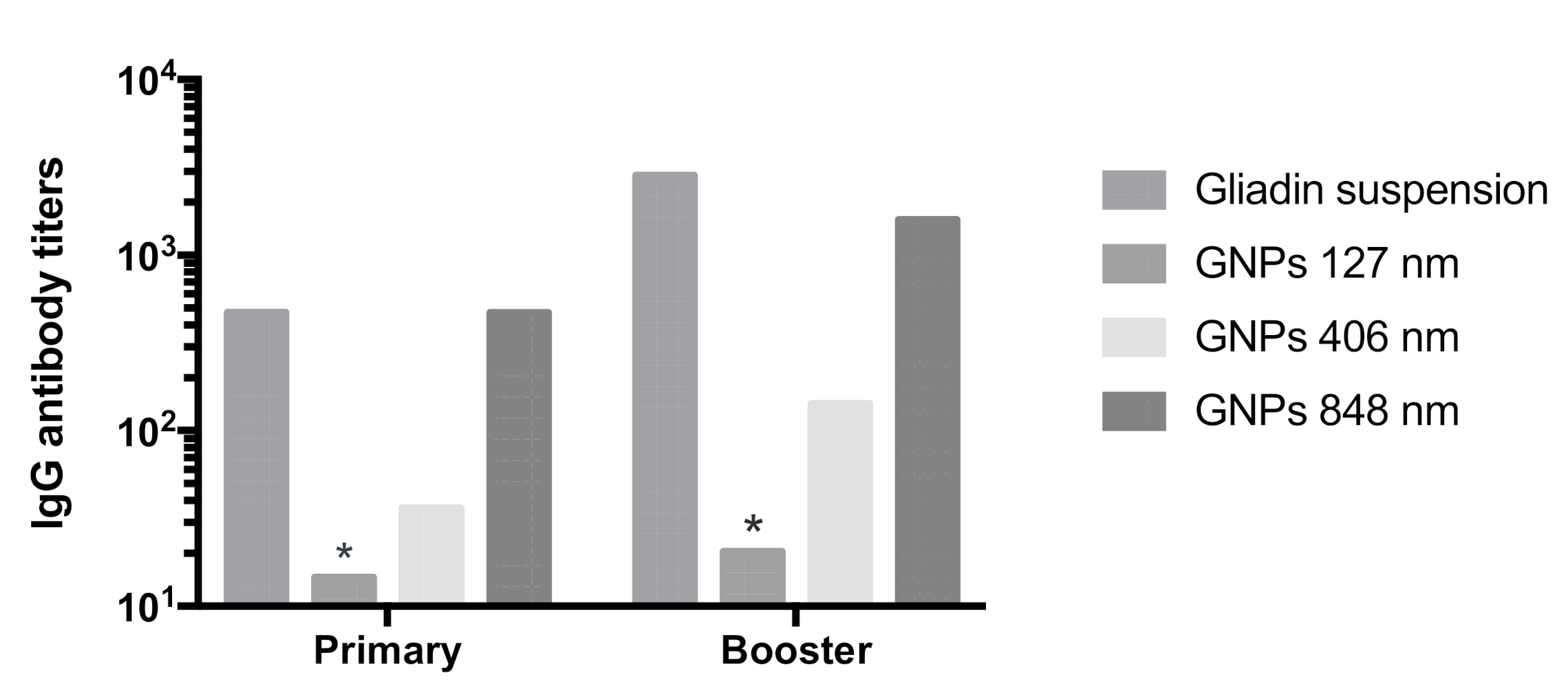
3.6. In Vivo Immunogenicity of Gliadin Nanoparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Couvreur, P.; Vauthier, C. Nanotechnology: Intelligent design to treat complex disease. Pharm. Res. 2006, 23, 1417–1450. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Wang, H.J.; Lin, Z.X.; Liu, X.M.; Sheng, S.Y.; Wang, J.Y. Heparin-loaded zein microsphere film and hemocompatibility. J. Control. Release 2005, 105, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Podaralla, S.; Perumal, O. Influence of formulation factors on the preparation of zein nanoparticles. AAPS PharmSciTech 2012, 13, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, N.; Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.; Mneimneh, A. Updated but not outdated “Gliadin”: A plant protein in advanced pharmaceutical nanotechnologies. Inter. J. Pharm. 2020, 587, 119672. [Google Scholar] [CrossRef] [PubMed]
- Arangoa, M.A.; Campanero, M.A.; Renedo, M.J.; Ponchel, G.; Irache, J.M. Gliadin nanoparticles as carriers for the oral administration of lipophilic drugs. Relationships between bioadhesion and pharmacokinetics. Pharm. Res. 2001, 18, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Sripriyalakshmi, S.; Jose, P.; Ravindran, A.; Anjali, C. Recent trends in drug delivery system using protein nanoparticles. Cell Biochem. Biophys. 2014, 70, 17–26. [Google Scholar] [CrossRef]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2018, 10, 435–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Li, J.-J.; Yu, D.-G.; Williams, G.R.; Yang, J.-H.; Wang, X. Influence of the drug distribution in electrospun gliadin fibers on drug-release behavior. Eur. J. Pharm. Sci. 2017, 106, 422–430. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Ba, Z.; Cui, S.; Wei, J.; Li, H. 3D-printed scaffolds of mesoporous bioglass/gliadin/polycaprolactone ternary composite for enhancement of compressive strength, degradability, cell responses and new bone tissue ingrowth. Int. J. Nanomed. 2018, 13, 5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.-Y.; Zhang, M.; Liu, Z.-P.; Wang, K.; Yu, D.-G. Meletin sustained-release gliadin nanoparticles prepared via solvent surface modification on blending electrospraying. Appl. Surf. Sci. 2018, 434, 1040–1047. [Google Scholar] [CrossRef]
- Umamaheshwari, R.; Ramteke, S.; Jain, N.K. Anti-Helicobacter pylori effect of mucoadhesive nanoparticles bearing amoxicillin in experimental gerbils model. AAPS Pharm. 2004, 5, 60–68. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 1: Preparation and characterization. Food Hydrocoll. 2015, 45, 309–316. [Google Scholar] [CrossRef]
- Wang, X. Assessing the Cytotoxicity of Newly Developed Gliadin Nanoparticles Loading Polymethoxyflavones. Available online: https://rucore.libraries.rutgers.edu/rutgers-lib/51492/ (accessed on 31 October 2020).
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharmaceutics 2008, 5, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Walker, A.; Mitragotri, S. Role of particle size in phagocytosis of polymeric microspheres. Pharm. Res. 2008, 25, 1815–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, U.; Sahoo, S.K.; De Tapas, K.; Ghosh, P.C.; Maitra, A.; Ghosh, P. Biodistribution of fluoresceinated dextran using novel nanoparticles evading reticuloendothelial system. Inter. J. Pharm. 2000, 202, 1–10. [Google Scholar] [CrossRef]
- Owens III, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Inter. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Valenta, D.T.; Altman, Y.; Harvey, S.; Xie, H.; Mitragotri, S.; Smith, J.W. Polymer particle shape independently influences binding and internalization by macrophages. J. Control. Release 2010, 147, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.-P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef]
- Ziv, O.; Avtalion, R.R.; Margel, S. Immunogenicity of bioactive magnetic nanoparticles: Natural and acquired antibodies. J. Biomed. Mater. Res. Part 2008, 85, 1011–1021. [Google Scholar] [CrossRef]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.-Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Mirshahi, T.; Irache, J.; Nicolas, C.; Mirshahi, M.; Faure, J.; Gueguen, J.; Hecquet, C.; Orecchioni, A. Adaptive immune responses of legumin nanoparticles. J. Drug Target. 2002, 10, 625–631. [Google Scholar] [CrossRef]
- Hurtado-López, P.; Murdan, S. An investigation into the adjuvanticity and immunogenicity of zein microspheres being researched as drug and vaccine carriers. J. Pharm. Pharmacol. 2006, 58, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Deevenapalli, M.; Singh, D.; Chourasia, M.K.; Bathula, S.R. Preparation and characterization of paclitaxel-loaded gliadin nanoparticles. J. Biomater. Tissue Eng. 2014, 4, 399–404. [Google Scholar] [CrossRef]
- Lai, L.; Guo, H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Inter. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef]
- Potter, T.M.; Skoczen, S.L.; Rodriguez, J.C.; Neun, B.W.; Ilinskaya, A.N.; Cedrone, E.; Dobrovolskaia, M.A. In Vitro Analysis of Nanoparticle Effects on the Zymosan Uptake by Phagocytic Cells. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Springer: New York, NY, USA, 2018; pp. 125–133. [Google Scholar]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Inter. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shetab Boushehri, M.A.; Dietrich, D.; Lamprecht, A. Nanotechnology as a Platform for the Development of Injectable Parenteral Formulations: A Comprehensive Review of the Know-Hows and State of the Art. Pharmaceutics 2020, 12, 510. [Google Scholar] [CrossRef]
- Domański, D.; Klajnert, B.; Bryszewska, M. Influence of PAMAM dendrimers on human red blood cells. Bioelectrochemistry 2004, 63, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.; Meijer, E.; Paulus, W.; Duncan, R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Lowe, K.C.; Furmidge, B.A.; Thomas, S. Haemolytic properties of pluronic surfactants and effects of purification. Artif. Cells, Blood Substitutes, Biotechnol. 1995, 23, 135–139. [Google Scholar] [CrossRef]
- Fang, C.; Shi, B.; Pei, Y.-Y.; Hong, M.-H.; Wu, J.; Chen, H.-Z. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: Effect of MePEG molecular weight and particle size. Eur. J. Pharm. Sci. 2006, 27, 27–36. [Google Scholar] [CrossRef]
- Simon, S.I.; Schmid-Schönbein, G. Biophysical aspects of microsphere engulfment by human neutrophils. Biophy. J. 1988, 53, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [Green Version]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, V.; Panda, A.K. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 2007, 28, 5344–5357. [Google Scholar] [CrossRef]
- Naim, J.O.; van Oss, C.J. The effect of hydrophilicity-hydrophobicity and solubility on the immunogenicity of some natural and synthetic polymers. Immunol. Investig. 1992, 21, 649–662. [Google Scholar] [CrossRef]



| Sample # | Particle Size (nm) | PI * |
|---|---|---|
| 1. | 848 ± 20 | 0.361 ± 0.05 |
| 2. | 406 ± 11 | 0.243 ± 0.06 |
| 3. | 127 ± 8 | 0.204 ± 0.02 |
| Sample # | Particle Size (nm) | PI * |
|---|---|---|
| 1. | 912 ± 18 | 0.31 ± 0.08 |
| 2. | 495 ± 12 | 0.259 ± 0.01 |
| 3. | 132 ± 7 | 0.232 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, M.S.; Syed, R.; Alshehri, M. Size-Dependent Phagocytic Uptake and Immunogenicity of Gliadin Nanoparticles. Polymers 2020, 12, 2576. https://doi.org/10.3390/polym12112576
Alqahtani MS, Syed R, Alshehri M. Size-Dependent Phagocytic Uptake and Immunogenicity of Gliadin Nanoparticles. Polymers. 2020; 12(11):2576. https://doi.org/10.3390/polym12112576
Chicago/Turabian StyleAlqahtani, Mohammed S., Rabbani Syed, and Meshal Alshehri. 2020. "Size-Dependent Phagocytic Uptake and Immunogenicity of Gliadin Nanoparticles" Polymers 12, no. 11: 2576. https://doi.org/10.3390/polym12112576
APA StyleAlqahtani, M. S., Syed, R., & Alshehri, M. (2020). Size-Dependent Phagocytic Uptake and Immunogenicity of Gliadin Nanoparticles. Polymers, 12(11), 2576. https://doi.org/10.3390/polym12112576






