Dielectric Elastomers UV-Cured from Poly(dimethylsiloxane) Solution in Vinyl Acetate
Abstract
:1. Introduction
2. Methods
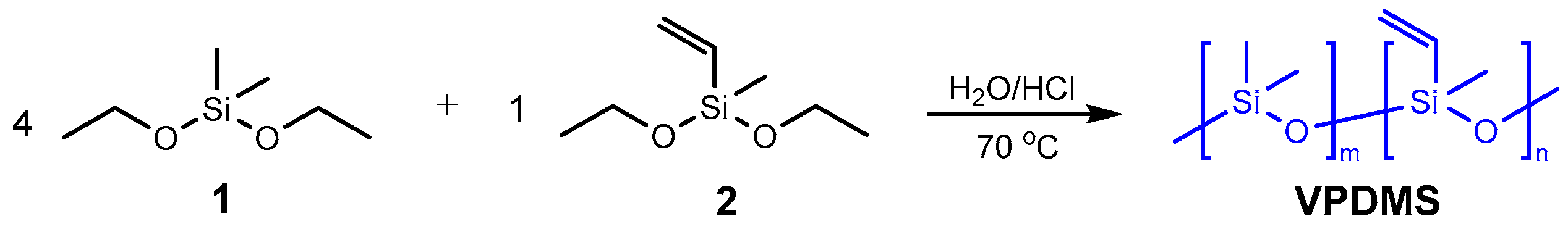
2.1. Synthesis of Poly(dimethylsiloxane-co-methylvinylsiloxane) (VPDMS)
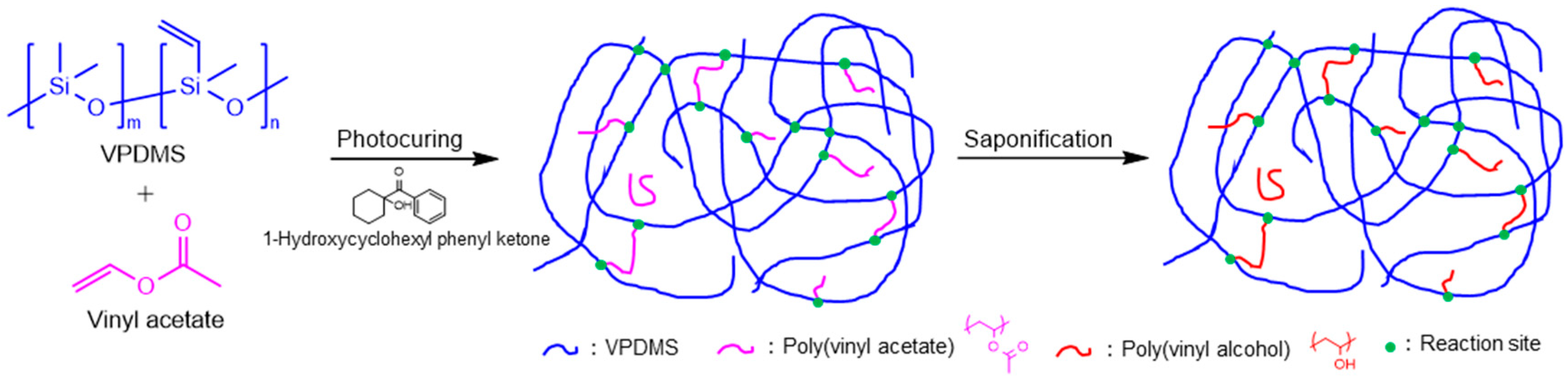
2.2. Preparation of VPDMS Solutions in Vinyl Acetate and Film Fabrication
2.3. Saponification of UV-Cured Film
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; McCarthy, T.J. Rediscovering silicones: Molecular smooth, low surface energy, unfilled, UV/Vis-transparent, extremely crosslinked, thermally stable, hard, elastic PDMS. Langmuir 2010, 26, 18585–18590. [Google Scholar] [CrossRef] [PubMed]
- Speier, J.L.; Webster, J.A.; Barnes, G.H. The addition of silicone hydrides to olefinic double bonds. Part II. The use of group VIII metal catalysts. J. Am. Chem. Soc. 1957, 79, 974–979. [Google Scholar] [CrossRef]
- Hirota, K.; Hara, S.; Wada, H.; Shimojima, A.; Kuroda, K. Fabrication of uniaxially aligned silica nanogrooves with sub-5 nm periodicity on centimeter-scale Si substrate using poly(dimethylsiloxane) stamps. ACS Nano 2019, 13, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, S.; Hu, F.; Yang, G.; Li, J.; Tian, H.; Peng, N. An LED-driven AuNPs-PDMS microfluidic chip and integrated device for the detection of digital loop-mediated isothermal DNS amplification. Micromachines 2020, 11, 177. [Google Scholar] [CrossRef] [Green Version]
- Tsougeni, K.; Tserepi, A.; Gogolides, E. Photosensitive poly(dimethylsiloxane) materials for microfluidic applications. Microelectron. Eng. 2007, 84, 1104–1108. [Google Scholar] [CrossRef]
- Kang, H.; Zhao, C.; Huang, J.; Ho, D.H.; Megra, Y.T.; Suk, J.W.; Sun, J.; Wang, Z.L.; Sun, Q.; Cho, J.H. Fingerprint-inspired conducting hierarchical wrinkles for energy-harvesting E-skin. Adv. Funct. Mater. 2019, 29, 1903580. [Google Scholar] [CrossRef]
- Wang, D.; Sheng, B.; Peng, L.; Huang, Y.; Ni, Z. Flexible and optical fiber sensors composited by graphene and PDMS for motion detection. Polymers 2019, 11, 1433. [Google Scholar] [CrossRef] [Green Version]
- Mun, S.; Yun, S.; Nam, S.; Park, S.K.; Park, S.; Park, B.J.; Lim, J.M.; Kyung, K.-U. Electro-active polymer based soft tactile interface for wearable devices. IEEE Trans. Haptics 2018, 11, 15–21. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities-properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Emah, J.B.; George, N.J.; Akpan, U.B. Interfacial surface modification via nanoimprinting to increase open-circuit voltage of organic solar cells. J. Electron. Mater. 2017, 46, 4989–4998. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Park, B.J.; Choi, M.J.; Kim, D.W.; Yoon, J.W.; Shin, E.J.; Yun, S.; Park, S. Facile functionalization of poly(dimethylsiloxane) elastomer by varying content of hydridosilyl groups in a crosslinker. Polymers 2019, 11, 1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Caspari, P.; Opris, D.M.; Nüesch, F.A.; Ham, S.; Kim, J.-H.; Kim, S.-R.; Ju, B.-K.; Choi, W.K. Electrical energy generated by silicone elastomers filled with nanospring-carbon-nanotubes. J. Mater. Chem. C 2019, 7, 3535–3542. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Alexandru, M.; Nüesch, F.A.; Hofmann, H.; Borgschulte, A.; Opris, D.M. Highly stretchable dielectric elastomer composites containing high volume fractions of silver nanoparticles. J. Mater. Chem. A 2015, 3, 14675–14685. [Google Scholar] [CrossRef]
- Dirany, M.; Dies, L.; Restagno, F.; Léger, L.; Poulard, C.; Miquelard-Garnier, G. Chemical modification of PDMS surface without impacting the viscoelasticity: Model systems for a better understanding of elastomer/elastomer adhesion and friction. Colloid Surf. A-Physicochem. Eng. Asp. 2015, 468, 174–183. [Google Scholar] [CrossRef]
- Goswami, K.; Skov, A.L.; Daugaard, A.E. UV-cured, platinum-free, soft poly(dimethylsiloxane) networks. Chem. Eur. J. 2014, 20, 9230–9233. [Google Scholar] [CrossRef]
- Obata, K.; Slobin, S.; Schonewille, A.; Hohnholz, A.; Unger, C.; Koch, J.; Suttmann, O.; Overmeyer, L. UV laser direct writing of 2D/3D structures using photo-curable polydimethylsiloxane. Appl. A-Mater. Sci. Process. 2017, 123, 495. [Google Scholar] [CrossRef]
- Abd-El-Messieh, S.L.; Mohamed, M.G.; Mazrouaa, A.M.; Soliman, A. Dielectric investigation of some normal alcohols and diols dispersed in some polymeric matrices. J. Appl. Polym. Sci. 2002, 85, 271–281. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Grulke, E.A. Solubility parameter values. In Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley & Sons: New York, NY, USA, 1999; Volume 2, pp. 675–714. [Google Scholar]
- Van Krevelen, D.W.; Hoftyzer, P.J. Practical evaluation of the [η]-M relationship. Ⅲ. Estimation of the exponent a. J. Appl. Polym. Sci. 1967, 11, 2189–2200. [Google Scholar] [CrossRef]
- Photoinitiators for UV Curing. Available online: https://people.rit.edu/deeemc/reference_13/Imprint/Photoinitiators%20for%20UV%20curing.pdf/ (accessed on 8 August 2020).
- Lee, T.Y.; Roper, T.M.; Jonsson, E.S.; Kudyakov, I.; Viswanathan, K.; Nason, C.; Guymon, C.A.; Hoyle, C.E. The kinetics of vinyl acrylate photopolymerization. Polymer 2003, 44, 2859–2865. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Park, S.K.; Kwark, Y.-J.; Moon, J.; Joo, C.W.; Yu, B.; Lee, J.-I. Finely formed, kinetically modulated wrinkle structures in UV-crosslinkable liquid prepolymers. Macromol. Rapid Commun. 2015, 36, 2006–2011. [Google Scholar] [CrossRef] [PubMed]
- Deanin, R.D.; Manion, M.A. Compatibilization of polymer blends. In Polymer Blends and Alloys; Shonaike, G.O., Simon, G.P., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 1–22. [Google Scholar]
- Seferis, J.C. Refractive indices of polymers. In Polymer Handbook, 4th ed.; Brandrup, J., Immergut, E.H., Grulke, E.A., Eds.; John Wiley & Sons: New York, NY, USA, 1999; Volume 2, pp. 571–582. [Google Scholar]
- Lee, S.G.; Kim, J.P.; Kwon, I.C.; Park, K.H.; Noh, S.K.; Han, S.S.; Lyoo, W.S. Heterogeneous surface saponification of suspension polymerized monodisperse poly(vinyl acetate) microspheres using various ions. J. Polym. Sci. Pol. Chem. 2006, 44, 3567–3576. [Google Scholar] [CrossRef]
- Yang, S.B.; Yoo, S.H.; Lee, J.S.; Kim, J.W.; Yeum, J.H. Surface properties of a novel poly(vinyl alcohol) film prepared by heterogeneous saponification of poly(vinyl acetate) film. Polymers 2017, 9, 493. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-J.; Kwon, T.-H.; Im, H.; Moon, D.-I.; Baek, D.J.; Seol, M.-L.; Duarte, J.P.; Choi, Y.-K. A polydimethylsiloxane (PDMS) sponge for the selective absorption of oil from water. ACS Appl. Mater. Interfaces 2011, 3, 4552–4556. [Google Scholar] [CrossRef]
- Dai, L.; Yu, S. Effect of degree of saponification on structural and property change of poly(vinyl alcohol) fibers. Polym. Adv. Technol. 2003, 14, 449–457. [Google Scholar] [CrossRef]
- Pelrine, R.; Kornbluh, R.; Pei, Q.; Joseph, J. High-speed electrically actuated elastomers with strain greater than 100%. Science 2000, 287, 836–839. [Google Scholar] [CrossRef]
- White, B.T.; Long, T.E. Advances in polymeric materials for electromechanical devices. Macromol. Rapid Commun. 2019, 40, 1800521. [Google Scholar] [CrossRef]
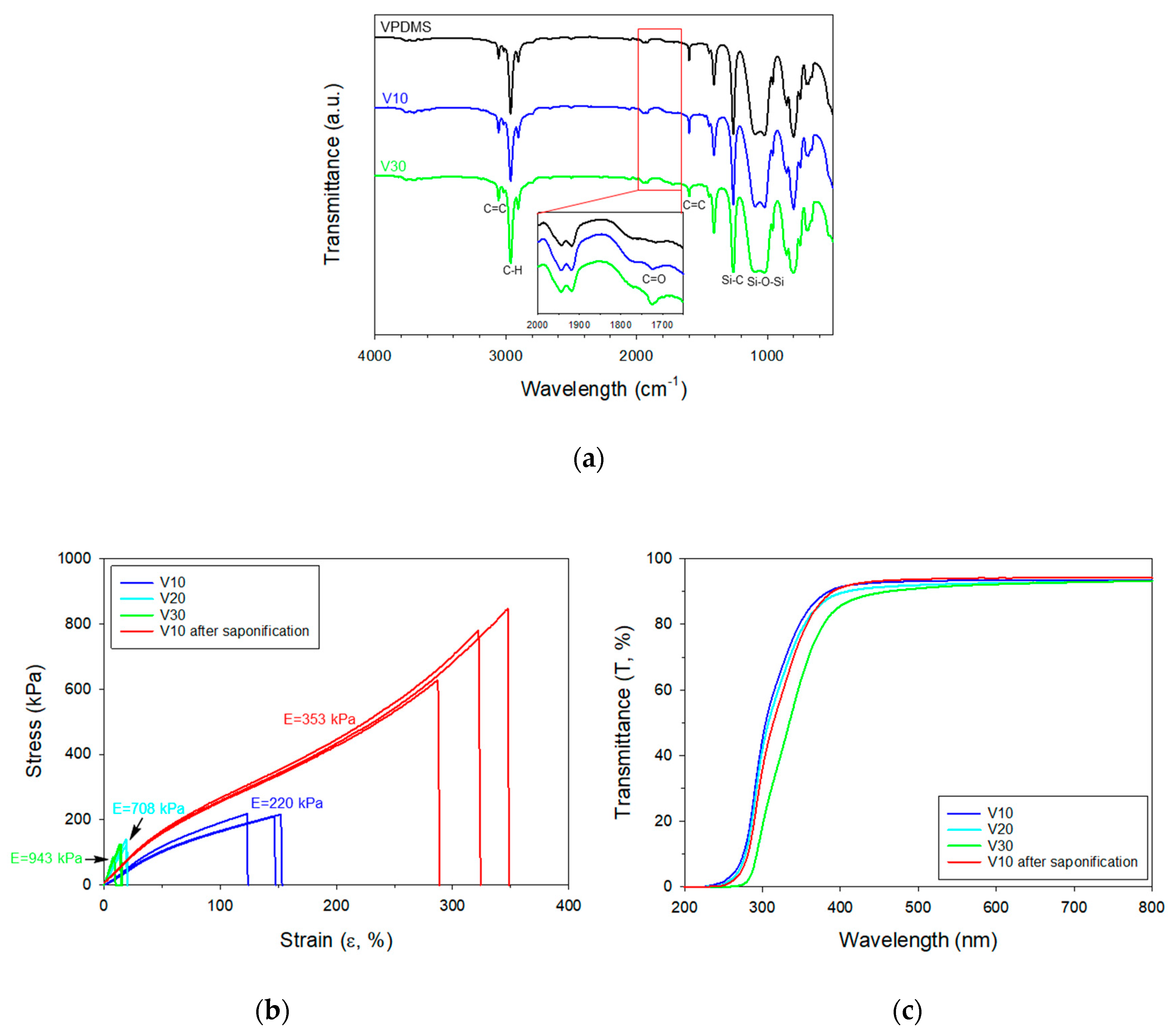

| Name of Polymer Solution 2 | VPDMS (g) | Vac 3 (g) | Initial Modulus (kPa) | Maximum Stress (kPa) | Maximum Strain (%) |
|---|---|---|---|---|---|
| V10 | 4.02 | 0.55 | 220 ± 19.0 | 215 ± 4.48 | 141 ± 15 |
| 353 ± 25.7 4 | 751 ± 112 4 | 320 ± 30 4 | |||
| V20 | 5.07 | 1.27 | 708 ± 63.8 | 119 ± 22.9 | 17.7 ± 2.9 |
| V30 | 3.99 | 1.71 | 943 ± 200 | 111 ± 22.2 | 12.1 ± 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.K.; Choi, M.; Kim, D.W.; Park, B.J.; Shin, E.J.; Park, S.; Yun, S. Dielectric Elastomers UV-Cured from Poly(dimethylsiloxane) Solution in Vinyl Acetate. Polymers 2020, 12, 2660. https://doi.org/10.3390/polym12112660
Park SK, Choi M, Kim DW, Park BJ, Shin EJ, Park S, Yun S. Dielectric Elastomers UV-Cured from Poly(dimethylsiloxane) Solution in Vinyl Acetate. Polymers. 2020; 12(11):2660. https://doi.org/10.3390/polym12112660
Chicago/Turabian StylePark, Seung Koo, Meejeong Choi, Dong Wook Kim, Bong Je Park, Eun Jin Shin, Suntak Park, and Sungryul Yun. 2020. "Dielectric Elastomers UV-Cured from Poly(dimethylsiloxane) Solution in Vinyl Acetate" Polymers 12, no. 11: 2660. https://doi.org/10.3390/polym12112660
APA StylePark, S. K., Choi, M., Kim, D. W., Park, B. J., Shin, E. J., Park, S., & Yun, S. (2020). Dielectric Elastomers UV-Cured from Poly(dimethylsiloxane) Solution in Vinyl Acetate. Polymers, 12(11), 2660. https://doi.org/10.3390/polym12112660







