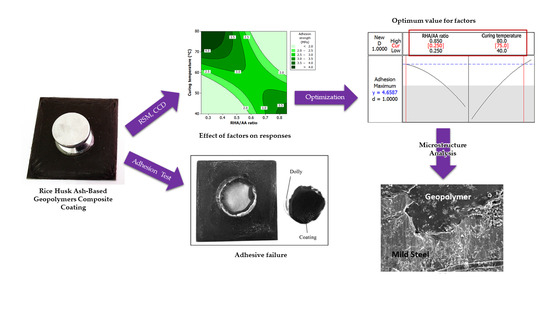
Optimization of Adhesion Strength and Microstructure Properties by Using Response Surface Methodology in Enhancing the Rice Husk Ash-Based Geopolymer Composite Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Factors and Levels of the Design of Experiment (DOE)
2.2. Design of Experiments
2.3. Raw Materials and Sample Preparation
2.4. Microstructure Test
2.5. Adhesion Test
2.6. Microstructure of Rice Husk Ash (RHA)
3. Results and Discussion
3.1. Statistical Analysis of Adhesion Strength
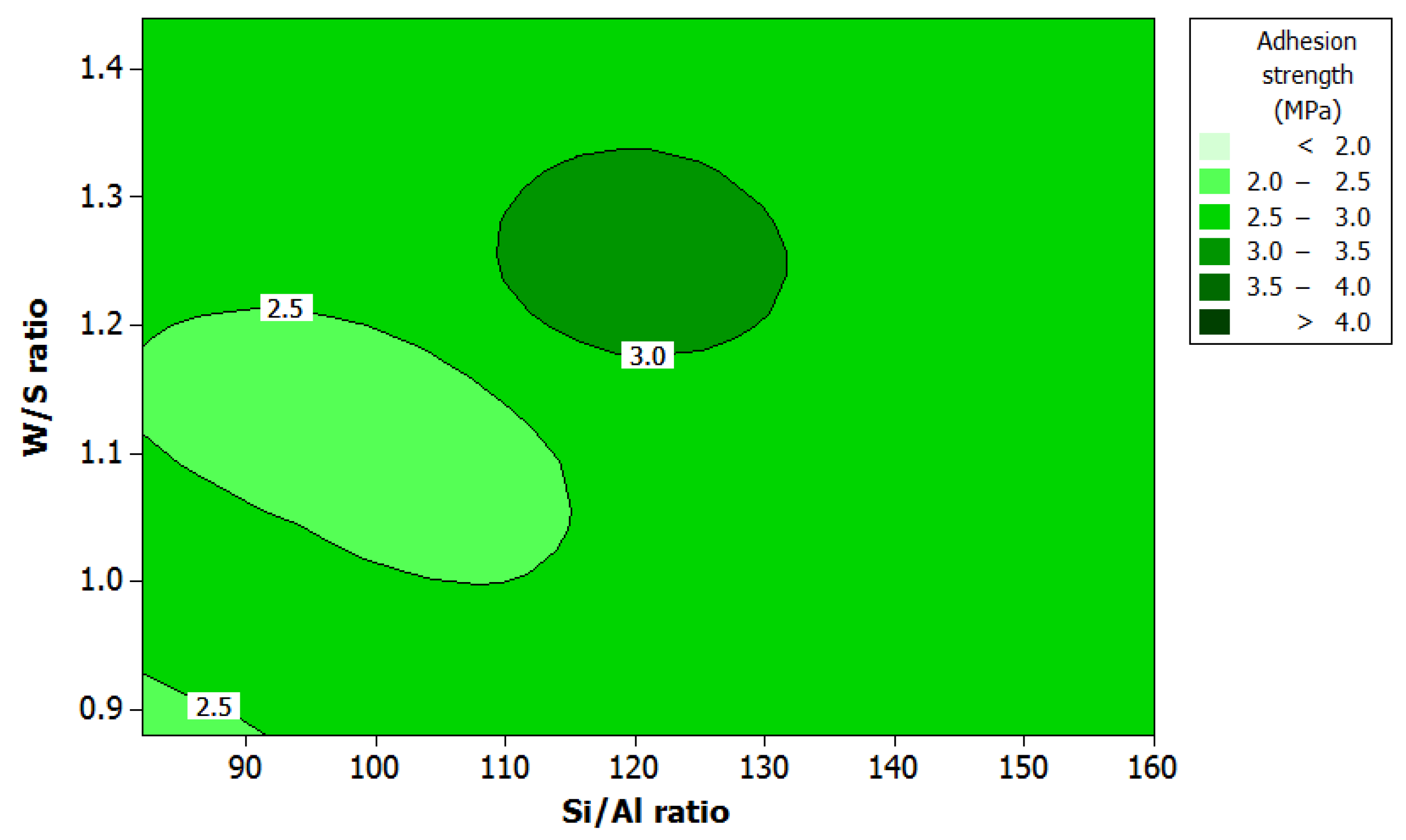
3.2. Effect of Factors on Adhesion Strength
3.3. Optimization of the Responses
3.4. Experimental Validation
3.5. Coating Adhesion Behavior and Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Hull, T.; Colligon, J.; Hill, A. Measurement of thin film adhesion. Vacuum 1987, 37, 327–330. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; van Riessen, A. Thermal properties of spray-coated geopolymer-type compositions. J. Therm. Anal. Calorim. 2012, 107, 287–292. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Fire retardant performance of rice husk ash-based geopolymer coated mild steel-A factorial design and microstructure analysis. In Proceedings of Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2016; pp. 48–54. [Google Scholar]
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of rice husk ash-based geopolymers coating composite for enhancement in flexural properties and microstructure using response surface methodology. Coatings 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Chen, W.; Wang, J.; Fang, C.; Zhang, B.; Liu, F. Coupling effects of recycled aggregate and GGBS/metakaolin on physicochemical properties of geopolymer concrete. Constr. Build. Mater. 2019, 226, 345–359. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Molino, A.; Roviello, G.; Colangelo, F.; Molino, B.; Cioffi, R. Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Constr. Build. Mater. 2017, 133, 14–26. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferrandiz-Mas, V.; Messina, F.; Ferone, C.; Tarallo, O.; Cioffi, R.; Cheeseman, C.R. Mechanical and thermal properties of lightweight geopolymer composites. Cem. Concr. Compos. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Davidovits, J. From ancient concrete to geopolymers. Art Metiers Mag 1993, 180, 8–16. [Google Scholar]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Van Riessen, A. Preparation of metakaolin based geopolymer coatings on metal substrates as thermal barriers. Appl. Clay Sci. 2009, 46, 265–270. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Van Riessen, A. Fly ash based geopolymer thin coatings on metal substrates and its thermal evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef]
- Yusof, A.M.; Nizam, N.A.; Abd Rashid, N.A. Hydrothermal conversion of rice husk ash to faujasite-types and NaA-type of zeolites. J. Porous Mater. 2010, 17, 39–47. [Google Scholar] [CrossRef]
- Khan, M.I.; Azizli, K.; Sufian, S.; Man, Z. Effect of Na/AI and Si/AI ratios on adhesion strength of geopolymers as coating material. Appl. Mech. Mater. 2014. [Google Scholar] [CrossRef]
- Khan, M.I.; Azizli, K.; Sufian, S.; Man, Z. Sodium silicate-free geopolymers as coating materials: Effects of Na/Al and water/solid ratios on adhesion strength. Ceram. Int. 2015, 41, 2794–2805. [Google Scholar] [CrossRef]
- Sulaiman, A.; Silva, F.V. High pressure processing, thermal processing and freezing of ‘Camarosa’strawberry for the inactivation of polyphenoloxidase and control of browning. Food Control 2013, 33, 424–428. [Google Scholar] [CrossRef]
- Sulaiman, A.; Soo, M.J.; Yoon, M.M.; Farid, M.; Silva, F.V. Modeling the polyphenoloxidase inactivation kinetics in pear, apple and strawberry purees after high pressure processing. J. Food Eng. 2015, 147, 89–94. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Naqvi, M.; Kok Keong, L.; Almesfer, M.K.; Al-Qodah, Z.; Naqvi, S.R.; Elboughdiri, N. Production and characterization of controlled release urea using biopolymer and geopolymer as coating materials. Polymers 2020, 12, 400. [Google Scholar] [CrossRef] [Green Version]
- Lian, Y.-S.; Sun, J.-Y.; Zhao, Z.-H.; Li, S.-Z.; Zheng, Z.-L. A refined theory for characterizing adhesion of elastic coatings on rigid substrates based on pressurized blister test methods: Closed-form solution and energy release rate. Polymers 2020, 12, 1788. [Google Scholar] [CrossRef] [PubMed]
- Sherif, G.; Chukov, D.I.; Tcherdyntsev, V.V.; Torokhov, V.G.; Zherebtsov, D.D. Effect of glass fibers thermal treatment on the mechanical and thermal behavior of polysulfone based composites. Polymers 2020, 12, 902. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Ren, S.; Lu, X. Application of eco-friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Czitrom, V. One-factor-at-a-time versus designed experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar]
- Box, G.E.; Wilson, K.B. On the experimental attainment of optimum conditions. In Breakthroughs in Statistics; Springer: Berlin/Heidelberg, Germany, 1992; pp. 270–310. [Google Scholar]
- Czyrski, A.; Jarzębski, H. Response surface methodology as a useful tool for evaluation of the recovery of the fluoroquinolones from plasma—The study on applicability of box-behnken design, central composite design and doehlert design. Processes 2020, 8, 473. [Google Scholar] [CrossRef]
- Flaifel, M.H. An approach towards optimization appraisal of thermal conductivity of magnetic thermoplastic elastomeric nanocomposites using response surface methodology. Polymers 2020, 12, 2030. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Roslan, S.A.; Sapuan, S.; Rasid, Z.A.; Mohd Nor, A.F.; Md Daud, M.Y.; Dolah, R.; Mohamed Yusoff, M.Z. Mercerization optimization of bamboo (bambusa vulgaris) fiber-reinforced epoxy composite structures using a box–behnken design. Polymers 2020, 12, 1367. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Dong, L.; Li, Z.; Chen, L.; He, X.; Gong, J.; Zhang, J.; Li, Q. Design and optimization of flexible polypyrrole/bacterial cellulose conductive nanocomposites using response surface methodology. Polymers 2019, 11, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochemistry 2011, 18, 1279–1286. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kohyama, N. Quantitative analysis of tridymite and cristobalite crystallized in rice husk ash by heating. Ind. Health 2004, 42, 277–285. [Google Scholar] [CrossRef]
- Chaudhary, D.S.; Jollands, M.C. Characterization of rice hull ash. J. Appl. Polym. Sci. 2004, 93, 1–8. [Google Scholar] [CrossRef]
- Chaudhary, D.S.; Jollands, M.C.; Cser, F. Recycling rice hull ash: A filler material for polymeric composites? Adv. Polym. Technol. 2004, 23, 147–155. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S. Effect of source materials on geopolymerization. Ind. Eng. Chem. Res. 2003, 42, 1698–1706. [Google Scholar] [CrossRef]
- Hardjito, D.; Rangan, V. Development and Properties of Low-Calcium Fly Ash-Based Geopolymer Concrete; Research Report GC 1; Curtin University of Technology: Perth, Australia, 2005. [Google Scholar]
- Chindaprasirt, P.; Chotetanorm, C.; Rukzon, S. Use of palm oil fuel ash to improve chloride and corrosion resistance of high-strength and high-workability concrete. J. Mater. Civ. Eng. 2010, 23, 499–503. [Google Scholar] [CrossRef]
- Thuc, C.N.H.; Thuc, H.H. Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar]
- Sindhunata, J.S.J.; Lukey, G.C.; Xu, H. Effect of curing temperature and silicate concentration on fly-ash-based geopolymerization. Ind. Eng. Chem. Res. 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Yong, S.; Feng, D.; Lukey, G.; Van Deventer, J. Chemical characterisation of the steel–geopolymeric gel interface. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 411–423. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K.; Rangan, V.B. Early age properties of low-calcium fly ash geopolymer concrete suitable for ambient curing. Procedia Eng. 2015, 125, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, E.D.; Bernal, S.A.; Provis, J.L.; Gehman, J.D.; Monzó, J.M.; Payá, J.; Borrachero, M.V. Geopolymers based on spent catalyst residue from a fluid catalytic cracking (FCC) process. Fuel 2013, 109, 493–502. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Liu, J. Peeling of an Extensible Soft Microbeam to Solid: Large Deformation Analysis. Int. J. Appl. Mech. 2018, 10, 1850065. [Google Scholar] [CrossRef]
- Grillet, A.M.; Wyatt, N.B.; Gloe, L.M. Polymer gel rheology and adhesion. Rheology 2012, 3, 59–80. [Google Scholar]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]











| Number of Factors | Levels | Total Number of Experimental Runs | |
|---|---|---|---|
| Full Factorial Design | RSM | ||
| 4 | 5 | 625 | 31 |
| 5 | 5 | 3125 | 54 |
| 6 | 5 | 15,625 | 90 |
| 7 | 5 | 78,125 | 160 |
| Factor | Unit | Notation | Levels | ||||
|---|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |||
| RHA/AA ratio | - | V1 | 0.25 | 0.40 | 0.55 | 0.70 | 0.85 |
| Curing temperature | °C | V2 | 40 | 50 | 60 | 70 | 80 |
| Sample | Coded Factor | Uncoded Factor | ||
|---|---|---|---|---|
| V1 | V2 | V1 | V2 | |
| S1 | 0 | 0 | 0.55 | 60 |
| S2 | 2 | 0 | 0.85 | 60 |
| S3 | 0 | 0 | 0.55 | 60 |
| S4 | 0 | 0 | 0.55 | 60 |
| S5 | −1 | 1 | 0.40 | 70 |
| S6 | −1 | −1 | 0.40 | 50 |
| S7 | 0 | 2 | 0.55 | 80 |
| S8 | 1 | −1 | 0.70 | 50 |
| S9 | −2 | 0 | 0.25 | 60 |
| S10 | 1 | 1 | 0.70 | 70 |
| S11 | 0 | 0 | 0.55 | 60 |
| S12 | 0 | 0 | 0.55 | 60 |
| S13 | 0 | −2 | 0.55 | 40 |
| S14 | 0 | −2 | 0.55 | 40 |
| S15 | −1 | −1 | 0.40 | 50 |
| S16 | 1 | 1 | 0.70 | 70 |
| S17 | −1 | 1 | 0.40 | 70 |
| S18 | 0 | 2 | 0.55 | 80 |
| S19 | 0 | 0 | 0.55 | 60 |
| S20 | 0 | 0 | 0.55 | 60 |
| S21 | 0 | 0 | 0.55 | 60 |
| S22 | 2 | 0 | 0.85 | 60 |
| S23 | 0 | 0 | 0.55 | 60 |
| S24 | −2 | 0 | 0.25 | 60 |
| S25 | 1 | −1 | 0.70 | 50 |
| S26 | 0 | 0 | 0.55 | 60 |
| Element | SiO2 | PdO | Al2O3 | Fe2O3 | CaO | Others | LOI |
|---|---|---|---|---|---|---|---|
| Mass% | 87.4 | 5.0 | 3.0 | 1.49 | 1.40 | 1.063 | 0.647 |
| Sample | RHA/AA Ratio (V1) | Curing Temperature (V2) | RHA/AA Ratio (V1) | Curing Temperature (V2) | Adhesion Strength (MPa) |
|---|---|---|---|---|---|
| S1 | 0 | 0 | 0.55 | 60 | 2.0 |
| S2 | 2 | 0 | 0.85 | 60 | 2.4 |
| S3 | 0 | 0 | 0.55 | 60 | 2.1 |
| S4 | 0 | 0 | 0.55 | 60 | 1.9 |
| S5 | −1 | 1 | 0.40 | 70 | 4.2 |
| S6 | −1 | −1 | 0.40 | 50 | 2.1 |
| S7 | 0 | 2 | 0.55 | 80 | 3.0 |
| S8 | 1 | −1 | 0.70 | 50 | 3.4 |
| S9 | −2 | 0 | 0.25 | 60 | 2.7 |
| S10 | 1 | 1 | 0.70 | 70 | 2.2 |
| S11 | 0 | 0 | 0.55 | 60 | 2.1 |
| S12 | 0 | 0 | 0.55 | 60 | 2.0 |
| S13 | 0 | −2 | 0.55 | 40 | 2.2 |
| S14 | 0 | −2 | 0.55 | 40 | 2.2 |
| S15 | −1 | −1 | 0.40 | 50 | 2.0 |
| S16 | 1 | 1 | 0.70 | 70 | 2.3 |
| S17 | −1 | 1 | 0.40 | 70 | 4.4 |
| S18 | 0 | 2 | 0.55 | 80 | 3.0 |
| S19 | 0 | 0 | 0.55 | 60 | 1.8 |
| S20 | 0 | 0 | 0.55 | 60 | 1.9 |
| S21 | 0 | 0 | 0.55 | 60 | 1.8 |
| S22 | 2 | 0 | 0.85 | 60 | 2.3 |
| S23 | 0 | 0 | 0.55 | 60 | 1.8 |
| S24 | −2 | 0 | 0.25 | 60 | 2.6 |
| S25 | 1 | −1 | 0.70 | 50 | 3.6 |
| S26 | 0 | 0 | 0.55 | 60 | 1.8 |
| Term | Notation | Coefficient | Std. Error of Coefficient | p |
|---|---|---|---|---|
| Constant | 2.9733 | 0.06176 | 0.000 | |
| Block 1 | 0.5017 | 0.07756 | 0.000 | |
| Block 2 | −1.0533 | 0.06523 | 0.000 | |
| RHA/AA ratio | V1 | −0.1083 | 0.03317 | 0.004 |
| Curing temperature | V2 | 0.2333 | 0.05245 | 0.000 |
| RHA/AA ratio ∗ RHA/AA ratio | V1 ∗ V1 | −0.2500 | 0.03047 | 0.000 |
| Curing temperature ∗ Curing temperature RHA/AA ratio ∗ Curing Temperature | V2 ∗ V2 V1 ∗ V2 | −0.2250 −0.8750 | 0.03047 0.04063 | 0.000 0.000 |
| Sample | Adhesion Strength (MPa) | ||
|---|---|---|---|
| Experimental Value | Predicted Value | Error (%) | |
| SV1 | 4.6 | 4.7 | 2.13 |
| SV2 | 4.8 | 4.7 | 2.13 |
| SV3 | 4.1 | 4.7 | 12.77 |
| Error | 5.68 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd Basri, M.S.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of Adhesion Strength and Microstructure Properties by Using Response Surface Methodology in Enhancing the Rice Husk Ash-Based Geopolymer Composite Coating. Polymers 2020, 12, 2709. https://doi.org/10.3390/polym12112709
Mohd Basri MS, Mustapha F, Mazlan N, Ishak MR. Optimization of Adhesion Strength and Microstructure Properties by Using Response Surface Methodology in Enhancing the Rice Husk Ash-Based Geopolymer Composite Coating. Polymers. 2020; 12(11):2709. https://doi.org/10.3390/polym12112709
Chicago/Turabian StyleMohd Basri, Mohd Salahuddin, Faizal Mustapha, Norkhairunnisa Mazlan, and Mohd Ridzwan Ishak. 2020. "Optimization of Adhesion Strength and Microstructure Properties by Using Response Surface Methodology in Enhancing the Rice Husk Ash-Based Geopolymer Composite Coating" Polymers 12, no. 11: 2709. https://doi.org/10.3390/polym12112709
APA StyleMohd Basri, M. S., Mustapha, F., Mazlan, N., & Ishak, M. R. (2020). Optimization of Adhesion Strength and Microstructure Properties by Using Response Surface Methodology in Enhancing the Rice Husk Ash-Based Geopolymer Composite Coating. Polymers, 12(11), 2709. https://doi.org/10.3390/polym12112709