Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
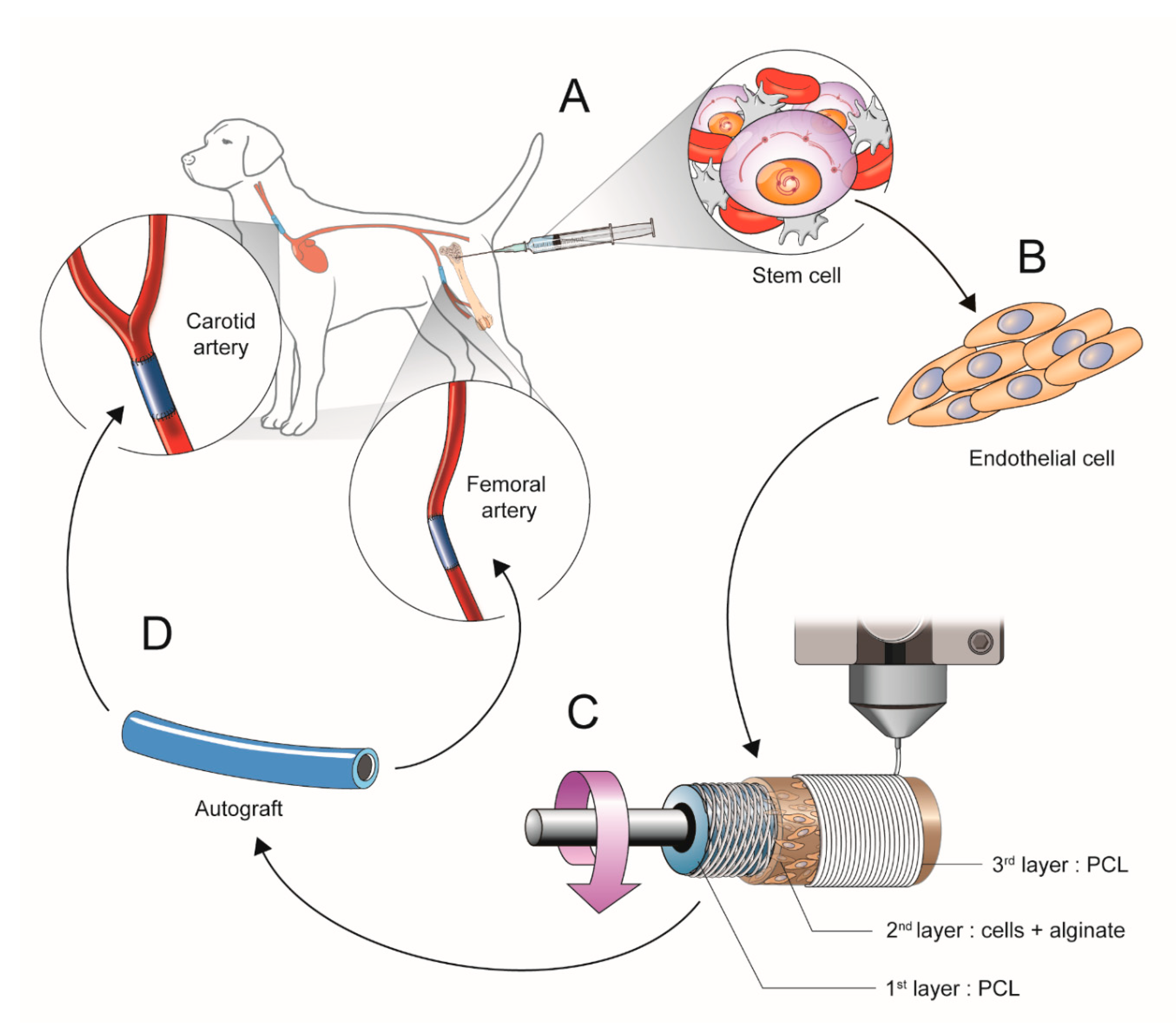
2.1. Scheme of Experimental Study Design
2.1.1. Isolation and Culture of Mesenchymal Stem Cells (b-MSCs) from Canine Bone Marrow
2.1.2. Cell Differentiation into Cell Resembling Endothelial Cells
2.1.3. Manufacturing the Artificial Vessels Using a 3D Bio-Printer
2.1.4. Surgical Implantation of the 3D Printed Vascular Grafts
2.2. Western Blot to Confirm Cell Differentiation
2.3. Scanning Electron Microscopy (SEM), Pre-Operation and Post-Operation.
2.4. Histological Assessment
2.5. Statistical Analysis
3. Results
3.1. Validation of the b-MSCs Differentiation and Artificial Vessels
3.2. In Vivo Studies of 3D Bio-Printed Artificial Vessels
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McBane, J.; Sharifpoor, S.; Labow, R.S.; Ruel, M.; Suuronen, E.J.; Santerre, J.P. Tissue engineering a small diameter vessel substitute: Engineering constructs with select biomaterials and cells. Curr. Vasc. Pharmacol. 2012, 10, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Sellaro, T.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Shachar, M.; Leor, J.; Cohen, S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002, 80, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Z.; Wei, Z.; Liu, C.; Qiao, T.; Ran, F.; Bai, Y.; Jiang, X.; Ding, Y. Development and Validation of Small-diameter Vascular Tissue From a Decellularized Scaffold Coated With Heparin and Vascular Endothelial Growth Factor. Artif. Organs 2009, 33, 230–239. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.-H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Gombotz, W. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Kong, H. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Gu, F.; Amsden, B.; Neufeld, R.J. Sustained delivery of vascular endothelial growth factor with alginate beads. J. Control. Release 2004, 96, 463–472. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, A.I.; Abelseth, E.; Chen, X. Indirect 3D bioprinting and characterization of alginate scaffolds for potential nerve tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 93, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Yoon, J.-K.; Lee, J.B.; Shin, Y.M.; Lee, K.-W.; Bae, S.-W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N.; et al. Experimental Tracheal Replacement Using 3-dimensional Bioprinted Artificial Trachea with Autologous Epithelial Cells and Chondrocytes. Sci. Rep. 2019, 9, 2103. [Google Scholar] [CrossRef]
- Dreher, R.; Starly, B. Biofabrication of Multimaterial Three-Dimensional Constructs Embedded With Patterned Alginate Strands Encapsulating PC12 Neural Cell Lines. J. Nanotechnol. Eng. Med. 2015, 6, 021004. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.-H.; Lee, J.; Youn, Y.-N. The Effect of Pulsatile Flow on bMSC-Derived Endothelial-Like Cells in a Small-Sized Artificial Vessel Made by 3-Dimensional Bioprinting. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Negoescu, A.; Lorimier, P.; Labat-Moleur, F.; Drouet, C.; Robert, C.; Guillermet, C.; Brambilla, C.; Brambilla, E. In situ apoptotic cell labeling by the TUNEL method: Improvement and evaluation on cell preparations. J. Histochem. Cytochem. 1996, 44, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Stefanadis, C.; Toutouzas, K.; Stefanadi, E.; Lazaris, A.; Patsouris, E.; Kipshidze, N. Inhibition of plaque neovascularization and intimal hyperplasia by specific targeting vascular endothelial growth factor with bevacizumab-eluting stent: An experimental study. Atherosclerosis 2007, 195, 269–276. [Google Scholar] [CrossRef]
- Lim, S.Y.; Jeong, J.-O.; Hong, S.J.; Lim, D.-S.; Moon, J.Y.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Kang, J.C. Inflammation and delayed endothelization with overlapping drug-eluting stents in a porcine model of in-stent restenosis. Circ. J. 2008, 72, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Touchard, A.G.; Schwartz, R.S. Preclinical Restenosis Models: Challenges and Successes. Toxicol. Pathol. 2006, 34, 11–18. [Google Scholar] [CrossRef]
- Itoh, M.; Nakayama, K.; Noguchi, R.; Kamohara, K.; Furukawa, K.; Uchihashi, K.; Toda, S.; Oyama, J.I.; Node, K.; Morita, S. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PLoS ONE 2015, 10, e0136681. [Google Scholar]
- Haasters, F.; Prall, W.C.; Anz, D.; Bourquin, C.; Pautke, C.; Endres, S.; Mutschler, W.; Docheva, D.; Schieker, M. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. J. Anat. 2009, 214, 759–767. [Google Scholar] [CrossRef]
- De Valence, S.; Tille, J.-C.; Chaâbane, C.; Gurny, R.; Bochaton-Piallat, M.-L.; Walpoth, B.; Möller, M. Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur. J. Pharm. Biopharm. 2013, 85, 78–86. [Google Scholar] [CrossRef]
- Shi, J.; Chen, S.; Wang, L.; Zhang, X.; Gao, J.; Jiang, L.; Tang, D.; Zhang, L.; Midgley, A.C.; Kong, D.; et al. Rapid endothelialization and controlled smooth muscle regeneration by electrospun heparin-loaded polycaprolactone/gelatin hybrid vascular grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 2040–2049. [Google Scholar] [CrossRef] [PubMed]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Abelson, B.; Babbar, P.; Damaser, M.S. Harnessing the mesenchymal stem cell secretome for regenerative urology. Nat. Rev. Urol. 2019, 16, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Sanchez-Bustamante, C.D.; Ehler, E.; Hoerstrup, S.P.; Djonov, V.; Ittner, L.M.; Fussenegger, M. VEGF profiling and angiogenesis in human microtissues. J. Biotechnol. 2005, 118, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Ehler, E.; Nielsen, L.; Schlatter, S.; Perriard, J.-C.; Fussenegger, M. Design of Artificial Myocardial Microtissues. Tissue Eng. 2004, 10, 201–214. [Google Scholar] [CrossRef]
- Matsumura, G.; Hibino, N.; Ikada, Y.; Kurosawa, H.; Shin’Oka, T. Successful application of tissue engineered vascular autografts: Clinical experience. Biomaterials 2003, 24, 2303–2308. [Google Scholar] [CrossRef]



| Animal No. | Group | Position | Result |
|---|---|---|---|
| 1 | no cell (control) | left carotid artery (LCA) | damaged during dissection |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | none implanted | ||
| right femoral artery (RFA) | none implanted | ||
| 2 | no cell (control) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | none implanted | ||
| right femoral artery (RFA) | none implanted | ||
| 3 | no cell (control) | left carotid artery (LCA) | patent |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | patnet | ||
| 4 | no cell (control) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | occlusion | ||
| 5 | cell (cell-derived) | left carotid artery (LCA) | patent |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | none implanted | ||
| right femoral artery (RFA) | none implanted | ||
| 6 | cell (cell-derived) | left carotid artery (LCA) | patent |
| right carotid artery (RCA) | patent | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | patnet | ||
| 7 | cell (cell-derived) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | patnet | ||
| 8 | cell (cell-derived) | left carotid artery (LCA) | occlusion |
| right carotid artery (RCA) | occlusion | ||
| left femoral artery (LFA) | patent | ||
| right femoral artery (RFA) | occlusion |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.H.; Kim, J.-H.; Lee, J.H.; Kim, D.-H.; Youn, Y.-N. Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers 2020, 12, 538. https://doi.org/10.3390/polym12030538
Jang EH, Kim J-H, Lee JH, Kim D-H, Youn Y-N. Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells. Polymers. 2020; 12(3):538. https://doi.org/10.3390/polym12030538
Chicago/Turabian StyleJang, Eui Hwa, Jung-Hwan Kim, Jun Hee Lee, Dae-Hyun Kim, and Young-Nam Youn. 2020. "Enhanced Biocompatibility of Multi-Layered, 3D Bio-Printed Artificial Vessels Composed of Autologous Mesenchymal Stem Cells" Polymers 12, no. 3: 538. https://doi.org/10.3390/polym12030538





