Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure for the Synthesis of HMBD
2.3. Procedure for the Synthesis s of HMEO
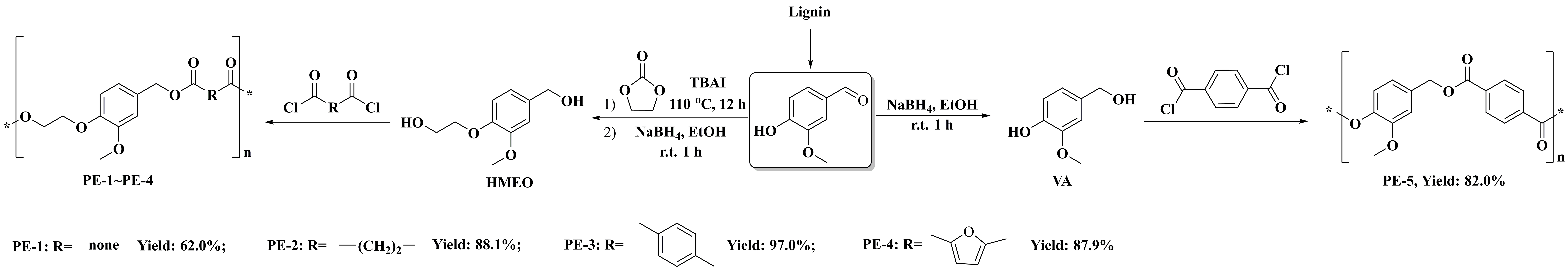
2.4. Procedure to Prepare PEs
2.5. Procedure to Prepare PUs
2.6. Molecular, Thermal and Structural Characterization
3. Results and Discussion
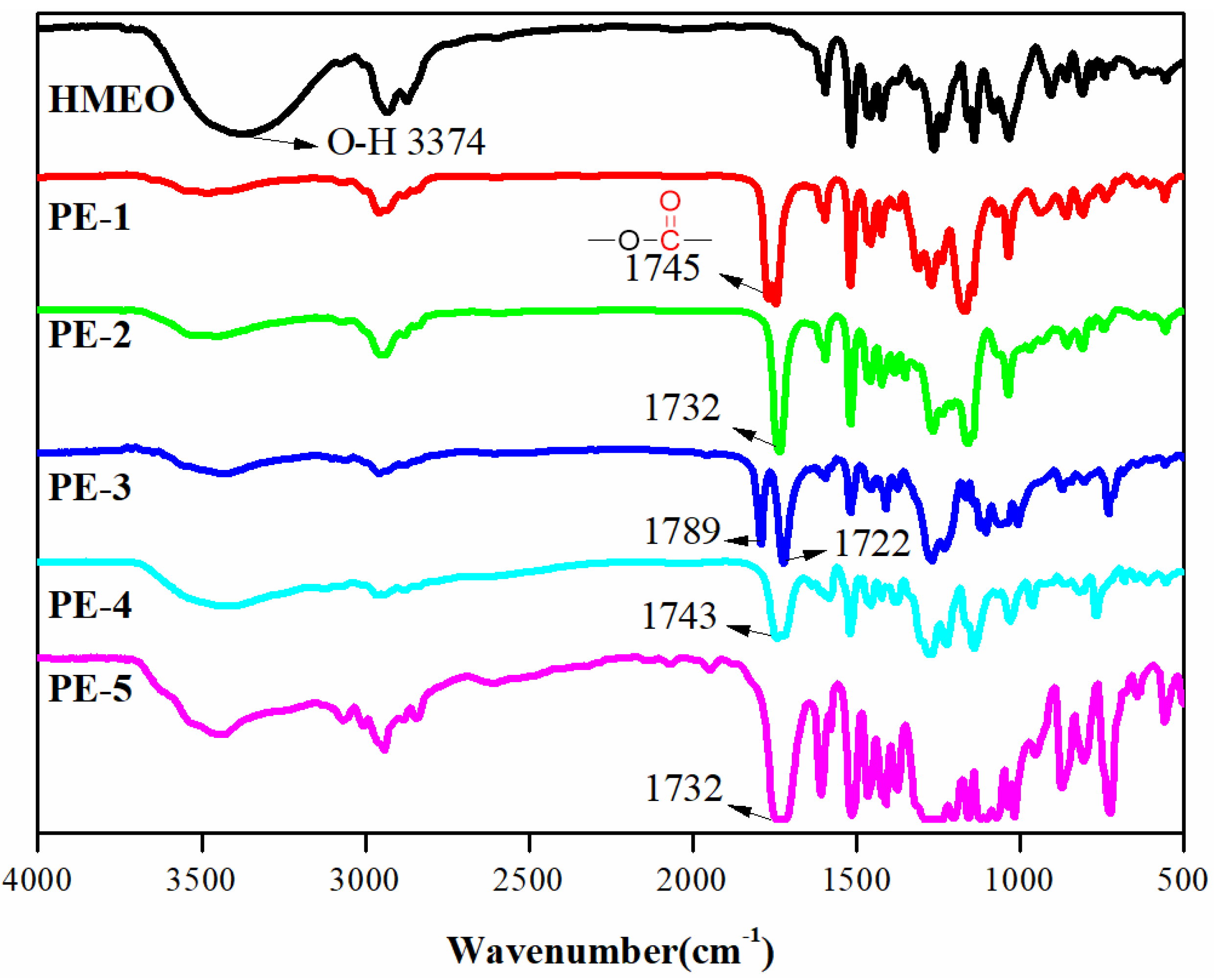
3.1. Structural Analysis of Monomers
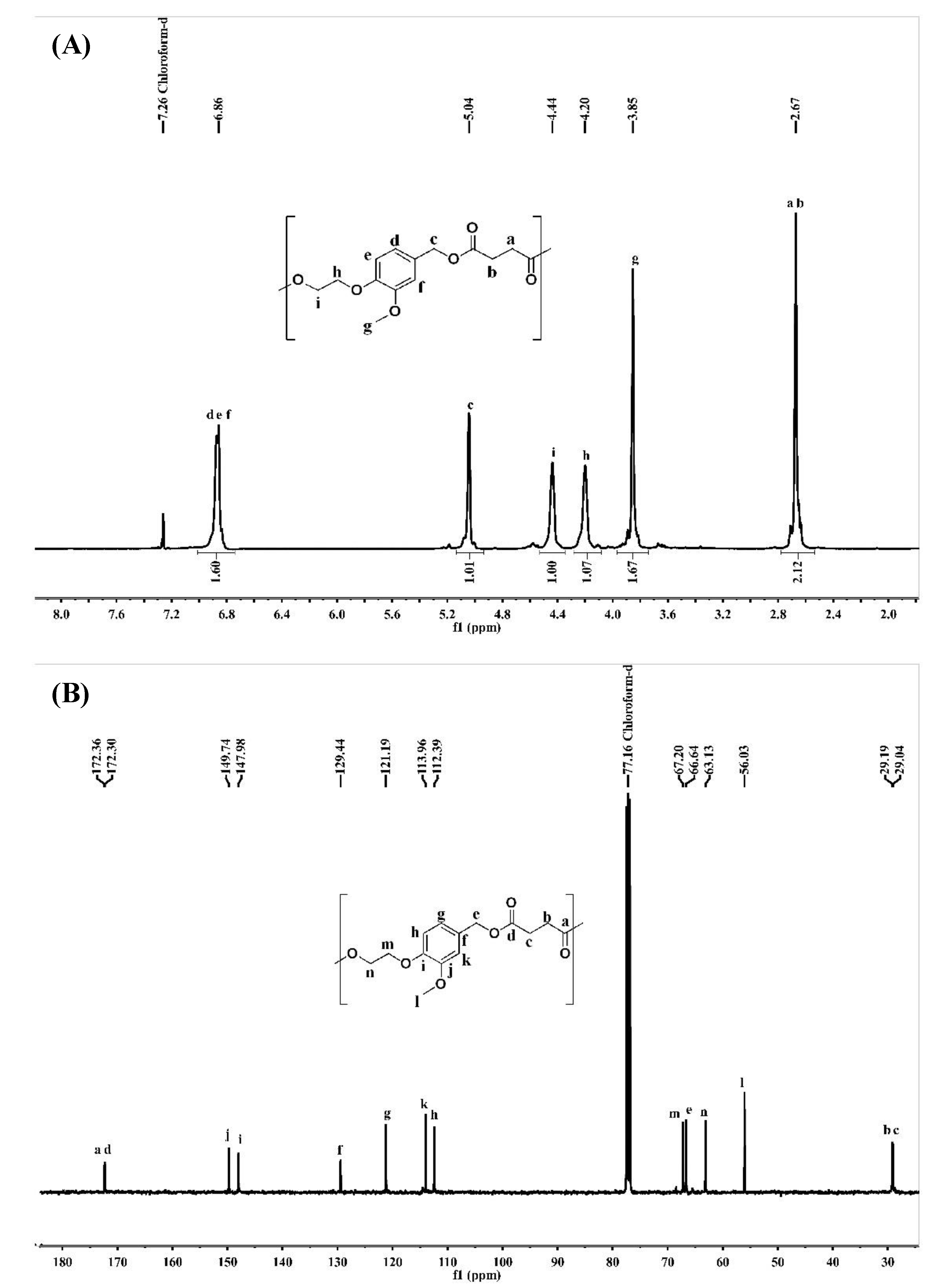
3.2. Structural Analysis of PEs
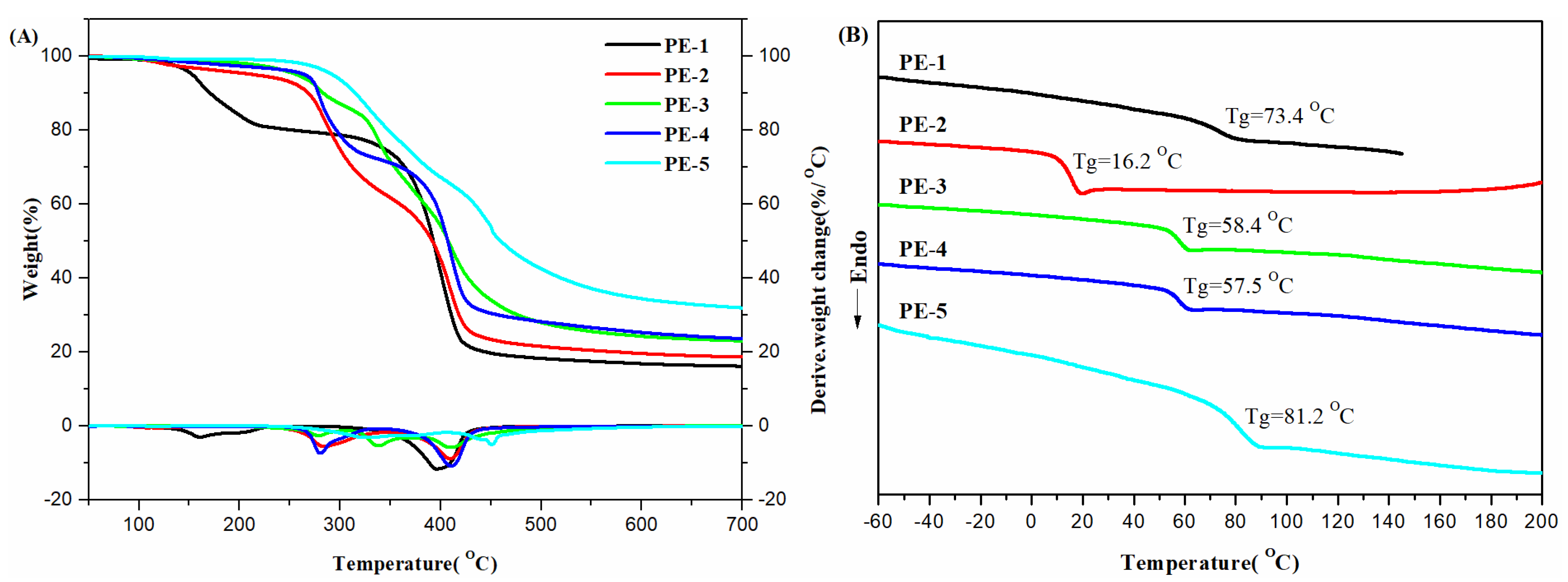
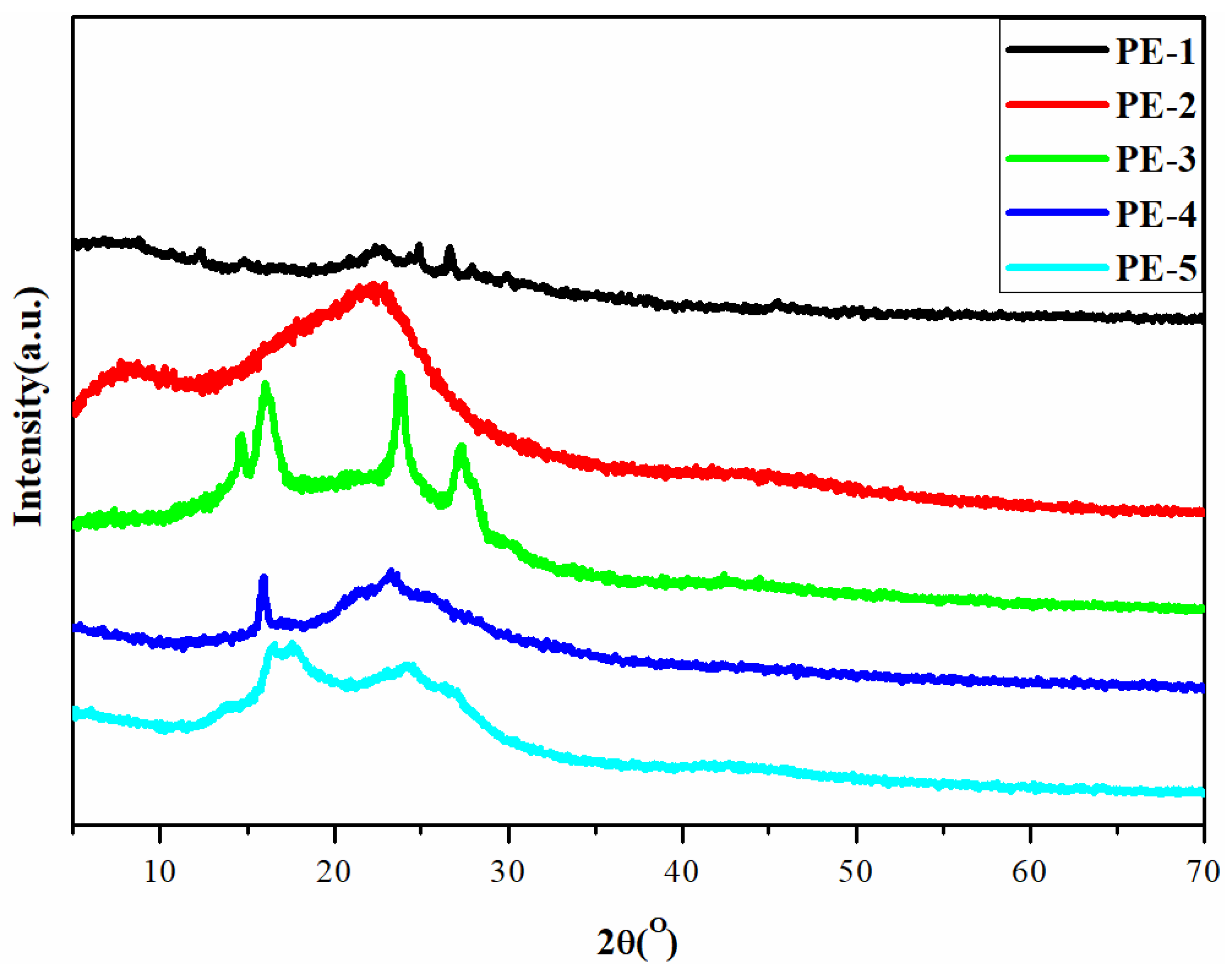
3.3. Molecular, Thermal and XRD Analysis of PEs
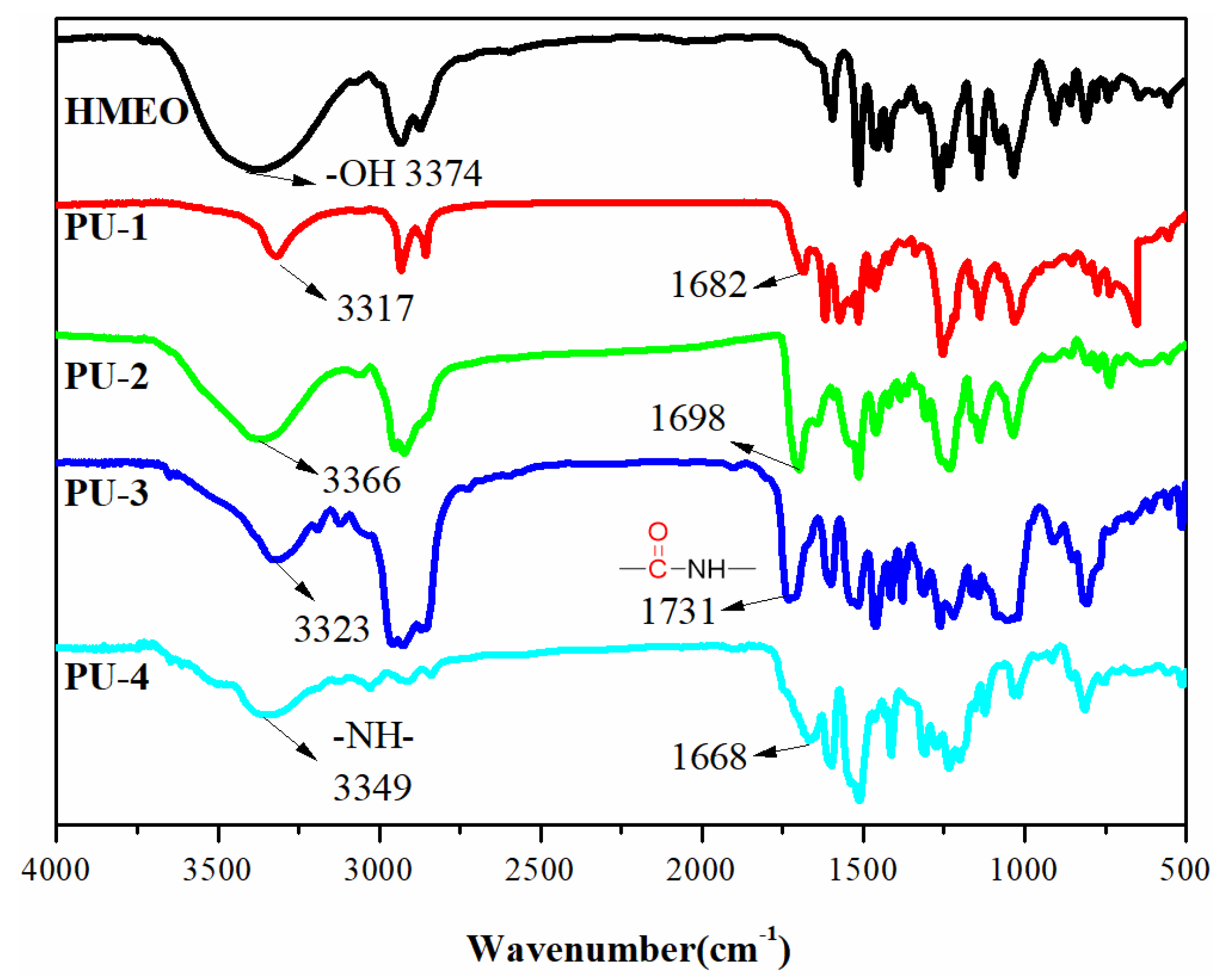
3.4. Structural Analysis of PUs
3.5. Molecular, Thermal and XRD analysis of PUs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, C. Surface modification and selective flotation of waste plastics for effective recycling—A review. Sep. Purif. Technol. 2019, 226, 75–94. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Szumelda, T.; Paone, E.; Mauriello, F. Catalytic Transfer Hydrogenolysis as an Effective Tool for the Reductive Upgrading of Cellulose. Hemicellulose, Lignin, and Their Derived Molecules. Catalysts 2018, 8, 313. [Google Scholar] [CrossRef]
- Kosinkova, J.; Doshi, A.; Maire, J.; Ristovski, Z.; Brown, R.; Rainey, T.J. Measuring the regional availability of biomass for biofuels and the potential for microalgae. Renew. Sustain. Energy Rev. 2015, 49, 1271–1285. [Google Scholar] [CrossRef]
- Xu, X.-L.; Chen, H.H. Examining the efficiency of biomass energy: Evidence from the Chinese recycling industry. Energy Policy 2018, 119, 77–86. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, B.N.; Taraban’ko, V.E.; Kuznetsova, S.A. New catalytic methods for obtaining cellulose and other chemical products from vegetable biomass. Kinet. Catal. 2008, 49, 517–526. [Google Scholar] [CrossRef]
- Verdia, P.; Brandt, A.; Hallett, J.P.; Ray, M.J.; Welton, T. Fractionation of lignocellulosic biomass with the ionic liquid 1-butylimidazolium hydrogen sulfate. Green Chem. 2014, 16, 1617–1627. [Google Scholar] [CrossRef]
- Dai, C.; Tao, J.; Zhao, M.; Jiang, B. Energetic Efficiency and Environmental. Economic, Societal Benefits of Microdiesel From Biowastes. Energy Explor. Exploit. 2007, 25, 219–226. [Google Scholar] [CrossRef]
- Priefert, H.; Rabenhorst, J.A. Steinbüchel, Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Gallage, N.J.; Moller, B.L. Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2015, 4, 35–46. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin, a key-intermediate of biobased polymers. Eur. Polym. J. 2015, 68, 488–502. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Mialon, L.; Pemba, A.G.; Miller, S.A. Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid. Green Chem. 2010, 12, 1704–1706. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Groshens, T.J.; Harvey, B.G. Synthesis of renewable bisphenols from creosol. ChemSusChem 2012, 5, 206–210. [Google Scholar] [CrossRef]
- Bai, D.; Chen, Q.; Chai, Y.; Ren, T.; Huang, C.; Ingram, I.D.V.; North, M.; Zheng, Q.; Xie, H. Vanillin derived a carbonate dialdehyde and a carbonate diol: Novel platform monomers for sustainable polymers synthesis. RSC Adv. 2018, 8, 34297–34303. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. Renewable (semi)aromatic polyesters from symmetrical vanillin-based dimers. Polym. Chem. 2015, 6, 6058–6066. [Google Scholar] [CrossRef]
- Firdaus, M.; Meier, M.A.R. Renewable co-polymers derived from vanillin and fatty acid derivatives. Eur. Polym. J. 2013, 49, 156–166. [Google Scholar] [CrossRef]
- Yuan, W.C.; Ma, S.Q.; Wang, S.; Li, Q.; Wang, B.B.; Xu, X.W.; Huang, K.F.; Chen, J.; You, S.S.; Zhu, J. Synthesis of fully bio-based diepoxy monomer with dicyclo diacetal for high-performance. readily degradable thermosets. Eur. Polym. J. 2019, 117, 200–207. [Google Scholar] [CrossRef]
- Tao, Y.Q.; Zhou, J.F.; Fang, L.X.; Wang, Y.Q.; Chen, X.Y.; Chen, X.R.; Hou, J.R.; Sun, J.; Fang, Q. Fluoro-containing Polysiloxane Thermoset with Good Thermostability and Acid Resistance Based on the Renewable Multifunctional Vanillin. ACS Sustain. Chem. Eng. 2019, 7, 7304–7311. [Google Scholar] [CrossRef]
- Liu, F.; Cao, H.; Mao, Q.; Song, P.; Yang, H. Effects of monomer structure on the morphology of polymer networks and the electro-optical properties of polymer-dispersed liquid crystal films. Liq. Cryst. 2012, 39, 419–424. [Google Scholar] [CrossRef]
- Elouali, F.Z.; Maschke, U. Effects of Monomer Structure on Morphology and Electro-Optical Properties of Polymer/Liquid Crystal Systems. Mol. Cryst. Liq. Cryst. 2011, 543, 107/[873]–116/[882]. [Google Scholar] [CrossRef]
- Li, W.; Cao, Y.; Cao, H.; Kashima, M.; Kong, L.; Yang, H. Effects of the structures of polymerizable monomers on the electro-optical properties of UV cured polymer dispersed liquid crystal films. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1369–1375. [Google Scholar] [CrossRef]
- Mialon, L.; Vanderhenst, R.; Pemba, A.G.; Miller, S.A. Polyalkylenehydroxybenzoates (PAHBs): Biorenewable aromatic/aliphatic polyesters from lignin. Macromol. Rapid Commun. 2011, 32, 1386–1392. [Google Scholar] [CrossRef]
- Chen, Q.; Ren, T.; Chai, Y.; Guo, Y.; Ingram, I.D.V.; North, M.; Xie, H.; Kent Zhao, Z. Preparation of Novel Aromatic-Aliphatic Poly(ketone ester)s through Condensation of Biomass-Derived Monomers. ChemCatChem 2018, 10, 5377–5381. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Esmati, S.; Tahmasebi, S.; Gholamhosseini-Nazari, M. Bisferrocene-containing ionic liquid supported on silica coated Fe3O4: A novel nanomagnetic catalyst for the synthesis of dihydropyrano[2,3-c]coumarin derivatives. J. Organomet. Chem. 2018, 870, 38–50. [Google Scholar] [CrossRef]
- Lee, I.; Yang, J.; Lee, J.H.; Choe, Y.S. Synthesis and evaluation of 1-(4-F-18 fluoroethyl)-7-(4′-methyl) curcumin with improved brain permeability for beta-amyloid plaque imaging. Bioorg. Med. Chem. Lett. 2011, 21, 5765–5769. [Google Scholar] [CrossRef]
- Hoshi, Y.; Xu, Y.; Ober, C.K. Photo-cleavable anti-fouling polymer brushes: A simple and versatile platform for multicomponent protein patterning. Polymer 2013, 54, 1762–1767. [Google Scholar] [CrossRef]
- Kitamura, T.; Inoue, Y.; Maeda, T.; Oyamada, J. Convenient synthesis of ethylene carbonates from carbon dioxide and 1,2-diols at atmospheric pressure of carbon dioxide. Synth. Commun. 2015, 46, 39–45. [Google Scholar] [CrossRef]
- García, K.E.; Navarro, R.; Ramírez-Hernández, A.; Marcos-Fernández, Á. New routes to difunctional macroglycols using ethylene carbonate: Reaction with bis-(2-hydroxyethyl) terephthalate and degradation of poly(ethylene terephthalate). Polym. Degrad. Stab. 2017, 144, 195–206. [Google Scholar] [CrossRef]
- Comerford, J.W.; Ingram, I.D.V.; North, M.; Wu, X. Sustainable metal-based catalysts for the synthesis of cyclic carbonates containing five-membered rings. Green Chem. 2015, 46, 1966–1987. [Google Scholar] [CrossRef]
- Lippits, M.J.; Nieuwenhuys, B.E. Direct conversion of ethanol into ethylene oxide on copper and silver nanoparticles Effect of addition of CeOx and Li2O. Catal. Today 2010, 154, 127–132. [Google Scholar] [CrossRef]
- Oulame, M.Z.; Pion, F.; Allauddin, S.; Raju, K.V.S.N.; Ducrot, P.-H.; Allais, F. Renewable alternating aliphatic-aromatic poly(ester-urethane)s prepared from ferulic acid and bio-based diols. Eur. Polym. J. 2015, 63, 186–193. [Google Scholar] [CrossRef]
- Morales, M.; Ataman, M.; Badr, S.; Linster, S.; Kourlimpinis, I.; Papadokonstantakis, S.; Hatzimanikatis, V.; Hungerbuhler, K. Sustainability assessment of succinic acid production technologies from biomass using metabolic engineering. Energy Environ. Sci 2016, 9, 2794–2805. [Google Scholar] [CrossRef]
- Yan, Y.; Siegwart, D.J. Scalable synthesis and derivation of functional polyesters bearing ene and epoxide side chains. Polym. Chem. 2014, 5, 1362–1371. [Google Scholar] [CrossRef]
- Sousa, A.F.; Coelho, J.F.J.; Silvestre, A.J.D. Renewable-based poly((ether)ester)s from 2,5-furandicarboxylic acid. Polymer 2016, 98, 129–135. [Google Scholar] [CrossRef]
- Hu, L.; He, A.Y.; Liu, X.Y.; Xia, J.; Xu, J.X.; Zhou, S.Y.; Xu, J.M. Biocatalytic Transformation of 5-Hydroxymethylfurfural into High-Value Derivatives: Recent Advances and Future Aspects. ACS Sustain. Chem. Eng. 2018, 6, 15915–15935. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, X.Q.; Zhu, J. From Furan to High Quality Bio-based Poly(ethylene furandicarboxylate). Chin. J. Polym. Sci. 2018, 36, 720–727. [Google Scholar] [CrossRef]
- Gandini, A.; Coelho, D.; Gomes, M.; Reis, B.; Silvestre, A. Materials from renewable resources based on furan monomers and furan chemistry: Work in progress. J. Mater. Chem. 2009, 19, 8656–8664. [Google Scholar] [CrossRef]
- De Vries, J.G. Green Syntheses of Heterocycles of Industrial Importance. 5-Hydroxymethylfurfural as a Platform Chemical. Adv. Heterocycl. Chem. 2017, 121, 247–293. [Google Scholar]
- Mou, Z.; Chen, E.Y.X. Polyesters and Poly(ester-urethane)s from Biobased Difuranic Polyols. ACS Sustain. Chem. Eng. 2016, 4, 7118–7129. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Siracusa, V.; Munari, A. Poly(Neopentyl Glycol Furanoate): A Member of the Furan-Based Polyester Family with Smart Barrier Performances for Sustainable Food Packaging Applications. Materials 2017, 10, 1028. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and Characterization of Poly(2,5-furan dicarboxylate)s Based on a Variety of Diols. J. Polym. Sci. Pol. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Mou, Z.; Feng, S.; Chen, E.Y.X. Bio-based difuranic polyol monomers and their derived linear and cross-linked polyurethanes. Polym. Chem. 2016, 7, 1593–1602. [Google Scholar] [CrossRef]
- Jiang, N.; Li, G.F.; Zhang, B.H.; Zhu, D.X.; Su, Z.M.; Bryce, M.R. Aggregation-Induced Long-Lived Phosphorescence in Nonconjugated Polyurethane Derivatives at 77 K. Macromolecules 2018, 51, 4178–4184. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Devroede, J.; Plasschaert, K.; Jonckheere, L.; Haucourt, N.; Du Prez, F.E. Providing polyurethane foams with functionality: A kinetic comparison of different “click” and coupling reaction pathways. Polym. Chem. 2013, 4, 1546–1556. [Google Scholar] [CrossRef]
- Garcia, D.E.; Glasser, W.G.; Pizzi, A.; Paczkowski, S.; Laborie, M.P. Hydroxypropyl tannin from Pinus pinaster bark as polyol source in urethane chemistry. Eur. Polym. J. 2015, 67, 152–165. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Preparation of bio-based rigid polyurethane foam using hydrolytically depolymerized Kraft lignin via direct replacement or oxypropylation. Eur. Polym. J. 2015, 68, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, K.; Peng, C.; Xie, H.; Zhao, Z.K.; Bao, M. Preparation of lignin/glycerol-based bis(cyclic carbonate) for the synthesis of polyurethanes. Green Chem. 2015, 17, 4546–4551. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Ingram, I.D.V.; Lie, Y.; North, M. Renewable Self-Blowing Non-Isocyanate Polyurethane Foams from Lysine and Sorbitol. Eur. J. Org. Chem. 2018, 2018, 4265–4271. [Google Scholar] [CrossRef]
- Hemingway, J.; Eskandari, M.; Rajcan, I. Genetic and Environmental Effects on Fatty Acid Composition in Soybeans with Potential Use in the Automotive Industry. Crop Sci. 2015, 55, 658–668. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Buffa, F.; Abraham, G.A. Effect of the hard segment chemistry and structure on the thermal and mechanical properties of novel biomedical segmented poly(esterurethanes). J. Mater. Sci. Mater. Med. 2009, 20, 145–155. [Google Scholar] [CrossRef]





| Sample | Monomer | Acyl Chloride | Yield (%) | Mn (g mol−1) | PDI (Mw/Mn) | Tg (DSC) (°C) | Td (5%) (°C) | Td (50%) (°C) |
|---|---|---|---|---|---|---|---|---|
| PE-1 | HMEO | OC | 62.0 | 22,000 | 1.06 | 73.4 | 153 | 393 |
| PE-2 | HMEO | SC | 88.1 | 36,000 | 1.14 | 16.2 | 214 | 392 |
| PE-3 | HMEO | TC | 97.0 | 17,000 | 1.05 | 58.4 | 257 | 408 |
| PE-4 | HMEO | FC | 87.9 | 19,000 | 1.10 | 57.5 | 264 | 407 |
| PE-5 | VA | TC | 82.0 | 21,000 | 1.11 | 81.2 | 293 | 460 |
| Sample | Monomer | Diisocyanate | Yield (%) | Mn (g mol−1) | PDI (Mw/Mn) | Tg (DSC) (°C) | Td (5%) (°C) | Td (50%) (°C) |
|---|---|---|---|---|---|---|---|---|
| PU-1 | HMEO | Hexamethylenediisocyanate (HDI) | 66.0 | 39,000 | 1.23 | 11.6 | 259 | 351 |
| PU-2 | HMEO | Isophoronediisocyanate (IPDI) | 81.9 | 29,000 | 1.11 | 66.8 | 252 | 325 |
| PU-3 | HMEO | Diphenylmethanediisocyanate (MDI) | 92.8 | 40,000 | 1.11 | 80.4 | 229 | 373 |
| PU-4 | VA | MDI | 81.3 | 21,000 | 1.10 | - | 280 | 364 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Huang, C.; Chen, Q.; Ingram, I.D.V.; Zeng, X.; Ren, T.; Xie, H. Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis. Polymers 2020, 12, 586. https://doi.org/10.3390/polym12030586
Zhao C, Huang C, Chen Q, Ingram IDV, Zeng X, Ren T, Xie H. Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis. Polymers. 2020; 12(3):586. https://doi.org/10.3390/polym12030586
Chicago/Turabian StyleZhao, Changbo, Caijuan Huang, Qin Chen, Ian D. V. Ingram, Xiankui Zeng, Tianhua Ren, and Haibo Xie. 2020. "Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis" Polymers 12, no. 3: 586. https://doi.org/10.3390/polym12030586
APA StyleZhao, C., Huang, C., Chen, Q., Ingram, I. D. V., Zeng, X., Ren, T., & Xie, H. (2020). Sustainable Aromatic Aliphatic Polyesters and Polyurethanes Prepared from Vanillin-Derived Diols via Green Catalysis. Polymers, 12(3), 586. https://doi.org/10.3390/polym12030586





