Macroscopic Poly Schiff Base-Coated Bacteria Cellulose with High Adsorption Performance
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
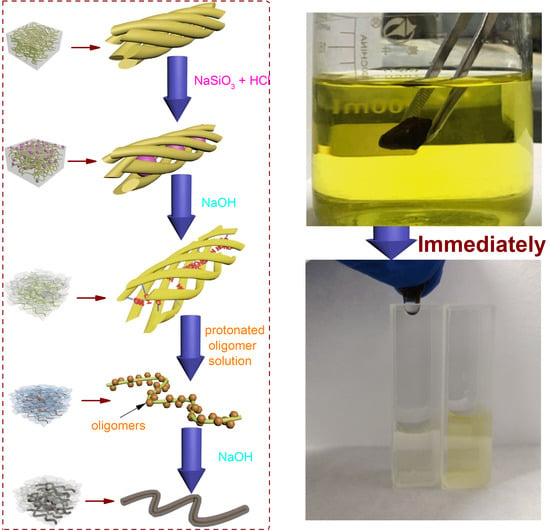
2.2. Preparation of Porous Bacteria Cellulose/Poly Schiff Base
- Pretreatment
- Polymer Coating
2.3. Characterization
2.4. Batch Adsorption
2.5. Dynamic Adsorption
2.6. Desorption and Reusability
3. Result and Discussion
3.1. Pretreatment of Bacteria Cellulose
3.2. Schiff Base Loading in Bacteria Cellulose
3.3. Characterization
3.4. Adsorption Performance
3.4.1. Adsorption Isotherm
3.4.2. Adsorption Kinetics
3.4.3. Dynamic Adsorption
3.4.4. Recyclability
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hausladen, D.M.; Alexander-Ozinskas, A.; McClain, C.; Fendorf, S. Hexavalent Chromium Sources and Distribution in California Groundwater. Environ. Sci. Technol. 2018, 52, 8242–8251. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Z.; Zhao, L.; Ma, J.; Li, X.; He, F.; Hou, H. Toxicity of Exogenous Hexavalent Chromium to Soil-Dwelling Springtail Folsomia Candida in Relation to Soil Properties and Aging Time. Chemosphere 2019, 224, 734–742. [Google Scholar] [CrossRef]
- Yan, X.; Song, M.; Zhou, M.; Ding, C.; Wang, Z.; Wang, Y.; Yang, W.; Yang, Z.; Liao, Q.; Shi, Y. Response of Cupriavidus Basilensis B-8 to Cuo Nanoparticles Enhances Cr(Vi) Reduction. Sci. Total Environ. 2019, 688, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption Mechanism of Hexavalent Chromium onto Layered Double Hydroxides-Based Adsorbents: A Systematic in-Depth Review. J. Hazard Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Kalidhasan, S.; Kumar, A.S.K.; Rajesh, V.; Rajesh, N. The Journey Traversed in the Remediation of Hexavalent Chromium and the Road Ahead toward Greener Alternatives—a Perspective. Coord. Chem. Rev. 2016, 317, 157–166. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Li, C.; Yang, W.; Song, T.; Tang, C.; Meng, Y.; Dai, S.; Wang, H.; Chai, L.; et al. Synthesis of Core-Shell Magnetic Fe3o4@Poly(M-Phenylenediamine) Particles for Chromium Reduction and Adsorption. Environ. Sci. Technol. 2015, 49, 5654–5662. [Google Scholar] [CrossRef]
- Liang, H.; Song, B.; Peng, P.; Jiao, G.; Yan, X.; She, D. Preparation of Three-Dimensional Honeycomb Carbon Materials and Their Adsorption of Cr(Vi). Chem. Eng. J. 2019, 367, 9–16. [Google Scholar] [CrossRef]
- Huang, M.-R.; Lu, H.-J.; Li, X.-G. Synthesis and Strong Heavy-Metal Ion Sorption of Copolymer Microparticles from Phenylenediamine and Its Sulfonate. J. Mater. Chem. 2012, 22, 34. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Shen, Y.; Huang, L.; He, Y.; Yang, W.; Yuan, T.; Jin, L.; Tang, C.J.; Zhang, L. Synthesis of Core-Shell Uio-66-Poly(M-Phenylenediamine) Composites for Removal of Hexavalent Chromium. Environ Sci. Pollut. Res. Int. 2020, 27, 4115–4126. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Chai, L.; Lei, H.; Yang, W.; Hou, L.; Yuan, T.; Jin, L.; Tang, C.; Luo, J. Highly-Dispersed Fe2o3@C Electrode Materials for Pb2+ Removal by Capacitive Deionization. Carbon 2019, 153, 12–20. [Google Scholar] [CrossRef]
- Hemavathy, R.V.; Kumar, P.S.; Kanmani, K.; Jahnavi, N. Adsorptive Separation of Cu (Ii) Ions from Aqueous Medium Using Thermally/Chemically Treated Cassia Fistula Based Biochar. J. Clean. Prod. 2020, 249, 1–12. [Google Scholar] [CrossRef]
- Luo, J.; Crittenden, J.C. Nanomaterial Adsorbent Design: From Bench Scale Tests to Engineering Design. Environ. Sci. Technol. 2019, 53, 10537–10538. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, I.; Molina, L.; González, P.; Gaete, J.; Valenzuela, F.; Marco, J.F.; Sáez, C.; Basualto, C. Silica-Coated Magnetite Nanoparticles Functionalized with Betaine and Their Use as an Adsorbent for Mo (Vi) and Re(Vii) Species from Acidic Aqueous Solutions. J. Ind. Eng. Chem. 2019, 78, 271–283. [Google Scholar] [CrossRef]
- Du, Z.; Zheng, T.; Wang, P. Experimental and Modelling Studies on Fixed Bed Adsorption for Cu(Ii) Removal from Aqueous Solution by Carboxyl Modified Jute Fiber. Powder Technol. 2018, 338, 952–959. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Chai, L.; Zhang, L. Nano-Functionalized Filamentous Fungus Hyphae with Fast Reversible Macroscopic Assembly & Disassembly Features. Chem. Commun. 2015, 51, 8524–8527. [Google Scholar]
- Wang, D. A Critical Review of Cellulose-Based Nanomaterials for Water Purification in Industrial Processes. Cellulose 2018, 26, 687–701. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Wang, J.; Yao, F.; Yang, Z.; Wan, Y. Step-by-Step Self-Assembly of 2d Few-Layer Reduced Graphene Oxide into 3d Architecture of Bacterial Cellulose for a Robust, Ultralight, and Recyclable All-Carbon Absorbent. Carbon 2018, 139, 824–832. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, F.; Sun, Y.; Xu, X.; Chen, X.; Pan, B.; Sun, D.; Qian, J. Bacterial Cellulose Derived Paper-Like Purifier with Multifunctionality for Water Decontamination. Chem. Eng. J. 2019, 371, 730–737. [Google Scholar] [CrossRef]
- Carpenter, A.W.; de Lannoy, C.F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr (Ii) from Water by Mercerized Bacterial Cellulose Membrane Modified with Edta. J. Hazard Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Yang, L.; Cui, J.; Hao, Q.; Sun, D. Handy Purifier Based on Bacterial Cellulose and Ca-Montmorillonite Composites for Efficient Removal of Dyes and Antibiotics. Carbohydr. Polym. 2019, 222, 115017. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 17. [Google Scholar] [CrossRef]
- Mensah, A.; Lv, P.; Narh, C.; Huang, J.; Wang, D.; Wei, Q. Sequestration of Pb (Ii) Ions from Aqueous Systems with Novel Green Bacterial Cellulose Graphene Oxide Composite. Materials 2019, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-Bacterial Cellulose Bioadsorbent for Effective Removal of Copper and Lead Ions from Aqueous Solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Ng, P.F.; Lee, K.I.; Fei, B.; Xin, J.H.; Wu, J.Y. Polyethylenimine Coated Bacterial Cellulose Nanofiber Membrane and Application as Adsorbent and Catalyst. J. Colloid Interface Sci. 2015, 440, 32–38. [Google Scholar] [CrossRef]
- Jahan, K.; Kumar, N.; Verma, V. Removal of Hexavalent Chromium from Potable Drinking Using a Polyaniline-Coated Bacterial Cellulose Mat. Environ. Sci. Water Res. Technol. 2018, 4, 1589–1603. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, L.; Jin, L.; Huang, L.; He, Y.; Tang, J.; Yang, W.; Wang, H. In-Situ Functionalization of Poly(M-Phenylenediamine) Nanoparticles on Bacterial Cellulose for Chromium Removal. Chem. Eng. J. 2018, 344, 441–452. [Google Scholar] [CrossRef]
- He, C.; Huang, J.; Li, S.; Meng, K.; Zhang, L.; Chen, Z.; Lai, Y. Mechanically Resistant and Sustainable Cellulose-Based Composite Aerogels with Excellent Flame Retardant, Sound-Absorption, and Superantiwetting Ability for Advanced Engineering Materials. ACS Sustain. Chem. Eng. 2017, 6, 927–936. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Chen, C.; Chen, X.; Huang, Y.; Sun, D. In Situ Controllable Fabrication of Porous Bacterial Cellulose. Mater. Lett. 2019, 249, 104–107. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of Bacterial Cellulose and Bacterial Cellulose Nanocrystals for Their Applications in the Stabilization of Olive Oil Pickering Emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken Experimental Design for Chromium(Vi) Ions Removal by Bacterial Cellulose-Magnetite Composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of Reusable Novel Membranes Based on Bacterial Cellulose and Chitosan for the Filtration of Copper in Wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. Ftir Analysis of Cellulose Treated with Sodium Hydroxide and Carbon Dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, Y. Bacterial Cellulose Nanofibers Decorated with Phthalocyanine: Preparation, Characterization and Dye Removal Performance. Mater. Lett. 2015, 142, 235–237. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Zhang, Q.; Zhan, X.; Chen, F. A Shape Recovery Zwitterionic Bacterial Cellulose Aerogel with Superior Performances for Water Remediation. Langmuir 2019, 35, 11959–11967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chai, L.; Liu, J.; Wang, H.; Yu, W.; Sang, P. Ph Manipulation: A Facile Method for Lowering Oxidation State and Keeping Good Yield of Poly (M-Phenylenediamine) and Its Powerful Ag+ Adsorption Ability. Langmuir 2011, 27, 13729–13738. [Google Scholar] [CrossRef]
- Ren, L.; Yang, Z.; Jin, L.; Yang, W.; Shi, Y.; Wang, S.; Yi, H.; Wei, D.; Wang, H.; Zhang, L. Hydrothermal Synthesis of Chemically Stable Cross-Linked Poly-Schiff Base for Efficient Cr (Vi) Removal. J. Mater. Sci. 2019, 55, 3259–3278. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Li, Z.; Ma, M.; Wang, B. A Microwave Synthesized Mesoporous Carbon Sponge as an Efficient Adsorbent for Cr (Vi) Removal. RSC Adv. 2018, 8, 7892–7898. [Google Scholar] [CrossRef]
- Li, L.; Wei, Z.; Liu, X.; Yang, Y.; Deng, C.; Yu, Z.; Guo, Z.; Shi, J.; Zhu, C.; Guo, W.; et al. Biomaterials Cross-Linked Graphene Oxide Composite Aerogel with a Macro–Nanoporous Network Structure for Efficient Cr (Vi) Removal. Int. J. Biol. Macromol. 2019, (in press). [CrossRef]
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Three-Dimensional Magnetic Graphene Oxide Foam/Fe3o4 Nanocomposite as an Efficient Absorbent for Cr (Vi) Removal. J. Mater. Sci. 2014, 49, 4236–4245. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Teng, J.; Zhou, N.; Zhou, Z.; Jiang, X.; Jiao, F.; Yu, J. Three-Dimensional Porous Graphene Oxide-Maize Amylopectin Composites with Controllable Pore-Sizes and Good Adsorption-Desorption Properties: Facile Fabrication and Reutilization, and the Adsorption Mechanism. Ecotoxicol. Environ. Saf. 2019, 176, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liao, Q.; Xie, J.; Qian, Z.; Zhu, W.; Chen, X.; Su, X.; Meng, R.; Yao, J. Synthetic Control of Three-Dimensional Porous Cellulose-Based Bioadsorbents: Correlation between Structural Feature and Metal Ion Removal Capability. Cellulose 2016, 23, 3819–3835. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D Hierarchical Graphene Oxide-Nife Ldh Composite with Enhanced Adsorption Affinity to Congo Red, Methyl Orange and Cr (Vi) Ions. J. Hazard Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, F.; Huang, L.; Chang, Z.; Yang, B.; Liu, S.; Zheng, M.; Lu, Y.; Chen, J. Cyclodextrin Functionalized 3d-Graphene for the Removal of Cr (Vi) with the Easy and Rapid Separation Strategy. Environ. Pollut. 2019, 254, 112854. [Google Scholar] [CrossRef]
- Kim, M.K.; Sundaram, K.S.; Iyengar, G.A.; Lee, K.-P. A Novel Chitosan Functional Gel Included with Multiwall Carbon Nanotube and Substituted Polyaniline as Adsorbent for Efficient Removal of Chromium Ion. Chem. Eng. J. 2015, 267, 51–64. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, Z.; Li, J.; Wang, H.; Huang, M.-R.; Li, X.-G.; Liao, Y. Template-Free Synthesis of Tunable Hollow Microspheres of Aniline and Aminocarbazole Copolymers Emitting Colorful Fluorescence for Ultrasensitive Sensors. Chem. Eng. J. 2019, 357, 776–786. [Google Scholar] [CrossRef]







| Samples | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qmax(mg g−1) | b(L mg−1) | RL2 | Kf (mg g−1 (L mg−1)1/n) | 1/n | Rf2 | |
| pBC-Polym-0.08 | 312.5 | 0.0467 | 0.995 | 152.848 | 0.1093 | 0.7374 |
| pBC-Polym-0.04 | 321.5 | 0.0227 | 0.986 | 104.993 | 0.1674 | 0.7321 |
| pBC-Polym-0.02 | 210.1 | 0.0094 | 0.991 | 21.901 | 0.3388 | 0.8990 |
| BC-Polym-0.08 | 197.2 | 0.0218 | 0.991 | 67.590 | 0.1589 | 0.9083 |
| BC-Polym-0.04 | 161.8 | 0.0303 | 0.999 | 61.018 | 0.1503 | 0.9830 |
| BC-Polym-0.02 | 104.6 | 0.0151 | 0.995 | 22.716 | 0.2288 | 0.9795 |
| Materials | Optimum pH | Kinetics | Adsorption Capacity (mg g−1) | Ref |
|---|---|---|---|---|
| Mesoporous carbon sponge | 2~4 | Pseudo-second-model | 93.9 | [38] |
| Polydopamine and Chitosan cross-linked graphene oxide | 3 | Pseudo-second-model | 312.0 | [39] |
| Magnetic graphene oxide foam | 2 | Pseudo-second-model | 258.6 | [40] |
| 3D porous graphene oxide-maize amylopectin composites | 5 | Pseudo-second-model | 13.6 | [41] |
| 3D porous cellulose | 3 | Pseudo second model | 220.6 | [42] |
| 3D graphene oxide-NiFe LDH composite | 3 | Pseudo second model | 53.6 | [43] |
| Cyclodextrin functionalized 3D-graphene | 2 | Pseudo second model | 107 | [44] |
| Tetraethylenepentamine crosslinked chitosan oligosaccharide hydrogel | 3 | Pseudo second model | 148.1 | [45] |
| Polyaniline-coated bacterial cellulose mat | 2 | Pseudo second model | 128 | [26] |
| pBC-Polym-0.04 | 2 | Pseudo second model | 321.5 | This study |
| Samples | qexp | Pseudo-First-Order Model | Pseudo-Second-Order Model | Intra Particles Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe | k1 | R2 | qe | k2 | R2 | kid1 | C1 | kid2 | C2 | kid3 | C3 | ||
| pBC-Polym-0.04 | 285.2 | 132.3 | 0.010 | 0.9903 | 289.8 | 0.11 | 0.9971 | 141.2 | 0 | 15.8 | 121.8 | 7.2 | 177.2 |
| BC-Polym-0.08 | 170.9 | 115.1 | 0.012 | 0.8993 | 183.8 | 0.056 | 0.9915 | 58.3 | 0 | 10.8 | 39.7 | 0.052 | 170.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Yang, Z.; Huang, L.; He, Y.; Wang, H.; Zhang, L. Macroscopic Poly Schiff Base-Coated Bacteria Cellulose with High Adsorption Performance. Polymers 2020, 12, 714. https://doi.org/10.3390/polym12030714
Ren L, Yang Z, Huang L, He Y, Wang H, Zhang L. Macroscopic Poly Schiff Base-Coated Bacteria Cellulose with High Adsorption Performance. Polymers. 2020; 12(3):714. https://doi.org/10.3390/polym12030714
Chicago/Turabian StyleRen, Lili, Zhihui Yang, Lei Huang, Yingjie He, Haiying Wang, and Liyuan Zhang. 2020. "Macroscopic Poly Schiff Base-Coated Bacteria Cellulose with High Adsorption Performance" Polymers 12, no. 3: 714. https://doi.org/10.3390/polym12030714
APA StyleRen, L., Yang, Z., Huang, L., He, Y., Wang, H., & Zhang, L. (2020). Macroscopic Poly Schiff Base-Coated Bacteria Cellulose with High Adsorption Performance. Polymers, 12(3), 714. https://doi.org/10.3390/polym12030714