Abstract
This work aims to enhance the photocatalytic antibacterial performance of plastics according to the JIS Z 2801:2010 standard, and to determine their mechanical properties by studying: (i) the influence of calcination on titanium dioxide (TiO2); (ii) modification with different TiO2 concentrations, and; (iii) the effect of silane as a coupling agent. Acrylonitrile-butadiene-styrene plastics (ABS) and Escherichia coli (E. coli) were chosen as the model plastic and bacteria, respectively. The 500 °C calcined TiO2 successfully provided the best photoantibacterial activity, with an approximately 62% decrease of E. coli colony counts following 30 min of exposure. Heat treatment improved the crystallinity of anatase TiO2, resulting in low electron-hole recombination, while effectively adsorbing reactants on the surface. ABS with 500 °C-calcined TiO2 at the concentration of 1 wt % gave rise to the highest performance due to the improved distribution of TiO2. At this point, blending silane coupling agent could further improve the efficacy of photoantibacterial activity up to 75% due to greater interactions with the polymer matrix. Moreover, it could promote a 1.6-fold increase of yield strength via increased adherent bonding between TiO2 and the ABS matrix. Excellent photocatalytic and material stability can be achieved, with constant photocatalytic efficiency remaining for up to five reuse cycles without loss in the yield strength.
1. Introduction
Thermoplastic polymers are widely used for appliances such as sanitary ware, medical appliances, furniture and children’s toys due to their favorable properties, such as excellent impact resistance, good machinability and excellent aesthetic qualities [1]. Durable plastics with a useful life of three years or more, such as Acrylonitrile-Butadiene-Styrene (ABS) plastics, could be potentially colonized by myriad microorganisms. Therefore, one of the biggest problems these plastics pose is microorganism contamination, including viruses, fungi and bacteria, which are harmful to the environment, hazardous to humans, and are difficult to disinfect. The generated reactive oxygen species (ROS) play a crucial role in bacterial disinfection. ROS can be generated by light irradiation or, more recently, by harnessing the power of microwaves using a conventional microwave oven [2,3].
Photocatalysis have attracted considerable attention in solving bacterial contamination as a clean, energy-efficient, low-cost and environmentally friendly technology. Photocatalytic bacterial inactivation relies upon the generation of highly reactive short-lived hydroxyl radicals and reactive oxygen that efficiently damage the cell membranes of microorganisms. TiO2 is widely used for photocatalytic applications due to its high stability, low cost and non-toxicity [4,5]. For example, Ti-O nanostructures synthesized from TiO2 in alkaline hydrothermal conditions have enhance photocatalytic reaction rates and sedimentability [6]. Lee and Chang reviewed composite photocatalysts widely used for degradation of hazardous chemicals, antibacterial, and photocatalytic hydrogen production [7]. Polymeric membranes with TiO2 showed excellent degradation of pollutants in water due to active sites of TiO2 available for interacting with photons [8]. Moreover, in-situ-formed granular TiO2 on polyvinylidene fluoride (PVDF) fiber with graphitic carbon nitride exhibited excellent self-cleaning properties [9].
Coating TiO2 on polymers such as polypropylene [10,11], polyethylene terephthalate [12], polycarbonate [13], and polystyrene [14] has been proposed to reduce the abundance of Escherichia coli (E. coli) and other harmful microrganisms. The properties of TiO2 such as crystallinity, surface defects, and particle size may affect its photocatalytic activities. However, potential flaking-off of TiO2 is an issue when the polymer is used many times. This may increase the risk to human health and result in environmental hazards. Apart from the effect on microorganisms, awareness of the mechanical properties of thermoplastic polymers is another concern. Many studies have reported that the filler adhesion and dispersion on the matrix of polymers were the main factors [15].
There is a body of literature on the effects of TiO2 on physical and chemical characteristics of ABS composites. Adding commercially surface-treated TiO2 pigment to ABS shows some optical benefits; i.e., doing so can impart opacity and whiten the ABS polymer as indicated by an increasing whiteness index and decreasing yellowness index. Impact strength of TiO2-pigmented ABS improved with a small loading of TiO2 pigment [16]. The friction and wear properties of TiO2-ABS were investigated by varying the TiO2 content, normal load, and sliding speed. With optimization of these parameters, the friction and wear properties of TiO2-ABS could be improved [17]. Recently, TiO2-ABS nanocomposite filaments have been produced and applied in 3D printing. TiO2-ABS with 5 and 10 wt % loading possess higher breaking point stress thresholds than those printed from the pure ABS polymer. A 10% TiO2-ABS preparation exhibited a marked photocatalytic activity leading to dye degradation, as observed by decreasing fluorescence emission spectra of rhodamine 6G by approx. 22% over 4 h of light exposure [18].
Although many studies have demonstrated the development of thermoplastic polymers in terms of their mechanical properties or in relation to microorganism suppression, there has been limited assessment of the antibacterial role of TiO2, and its effects on the strength of plastics. In particular, there is no previous study available on ABS plastic—a material that provides effective and quality products for various applications. The most relevant literature is our preliminary study of TiO2/ABS on photoantibacterial activity, without functionality or interfacial affinity modification, which resulted in unimpressive photoactivity [19]. Moreover, the impact of calcination and fillers on TiO2 properties, and its potential photoantibacterial effect, have not been investigated of late. This study focuses on improving photoantibacterial performance following the standard JIS Z 2801, which covers the ability of plastic surfaces to inhibit the growth of microorganisms. We also investigate strength properties (per standard ASTM D639 Type I) of ABS plastic with modified TiO2. The role of calcination temperature, concentration and silane-coupling agent is also assessed.
2. Materials and Methods
2.1. Reagents
TiO2 (anatase 100%), acrylonitrile-butadiene-styrene (ABS Resin, PA—717C Grade), 3-aminopropyltriethoxysilane (APTES) (Sigma–Aldrich, St. Louis, MI, USA), stock phosphate buffer solution (PBS) (Sigma–Aldrich), plate count agar (PCA), tryptic soy broth (TSB), peptone (J.T.Baker) were used.
2.2. Calcination of TiO2 Powder
Anatase TiO2 particles were preheated in an oven at 90 °C for 2 h, and then calcined at temperatures of 300, 500 and 800 °C for 2 h with a ramping rate of 5 °C min−1.
2.3. Surface Modification of TiO2 with Silane
A 0.5 g mass of calcined TiO2 was dispersed in 50 cm3 of 2.5% v/v ethanol. Then, 0.15 g of the silane-coupling agents (APTES) at standard conditions were added in the dispersion, and stirred for 45 min. The resulting slurry was centrifuged and dried in an oven for 24 h at 80 °C
2.4. Preparation of TiO2/ABS Compositions
Prior to blending polymers, ABS plastic was dried in an oven at 90 °C for 2 h to remove moisture. The TiO2 or calcined TiO2 particles (concentration of 0.5, 1 and 2 wt %) or calcined TiO2 treated with silane, and ABS plastic were loaded into a twin-screw extruder, and then mixed well in an internal mixer at 250 °C with speed round mixing at 60 rpm for 6 min. The melting material was put in the mold (5 × 5 cm2) by using a compression molding at a temperature of 250 °C and pressure of 125 kg cm−2 for 5 min, and rapidly cooled for 5 min. The resulting TiO2/ABS compositions were air-cooled at room temperature before being tested.
2.5. E. coli Bacteria Preparation and Photoantibacterial Activity
Photoantibacterial activity and efficacy assessments followed the standard JIS Z 2801 (The test for antibacterial activity and efficacy on surfaces of antibacterial products). A colony of E. coli bacteria was transferred into TSB solution, and then incubated at 32 ± 0.5 °C for 24 h. The bacterial suspensions were diluted into 2.5 × 108 cfu cm−3, dropped on TiO2/ABS workpieces, and covered with a polyethylene (PE) film size 4 × 4 cm2. The sample was illuminated by UVC light (15 W) for 30 min. The bacterial population was determined by plated serial dilution, and plates were incubated at 32 ± 0.5 °C for 24 h. All photoantibacterial activity experiments were performed with three replicates (independent experiments), and the data were represented as the average mean ± SD (error bar).
2.6. Sample Characterization
Crystal and structural characteristics of the products and crystallinity were investigated using powder X-ray diffraction (XRD) system with monochromatized Cukα radiation (λ = 1.5406 Å). Full width at half maximum (FWHM) derived from XRD patterns at 2θ = 25° indicated the degree of crystallinity. Sample morphology was investigated by a scanning electron microscope, and the surface area and pore size distribution were determined by N2 adsorption.
2.7. Mechanical Tensile Strength
The workpieces were tested using a universal testing machine according to the guidelines set in ASTM D639 Type I (Standard Test Method for Tensile Properties of Plastics). All experiments were performed with three replicates, and the data were represented as the average mean ± SD (error bar).
3. Results and Discussion
3.1. Effect of Calcination Temperatures on TiO2
The crystal structure of TiO2 was investigated by XRD analysis as shown in Figure 1 and corresponding crystallite size was calculated by using the Debye–Scherrer formula. The XRD pattern demonstrated that TiO2 structures were influenced by calcination temperatures. The initial TiO2 results included peaks at 2θ = 25.4°, 37.8°, 48°, 54.5° and 62.8°, corresponding to the anatase phase of TiO2 with the FWHM value of 0.5 and particle size of 6.12 nm. No peaks caused by impurities were observed. TiO2 calcined at 300 °C showed that the intensity of crystallinity of anatase increased, with a lower FWHM value of 0.41 and particle size of 7.08 nm. Further increases in temperature to 500 and 800 °C increased crystallinity of anatase, as shown by recorded FWHM values of 0.27 and 0.08, respectively. Therefore, crystalline structure was slightly developed by increasing the calcination temperatures. The particle sizes of 500 and 800 °C calcined TiO2 increased slightly to 7.13 and 7.15 nm, respectively.
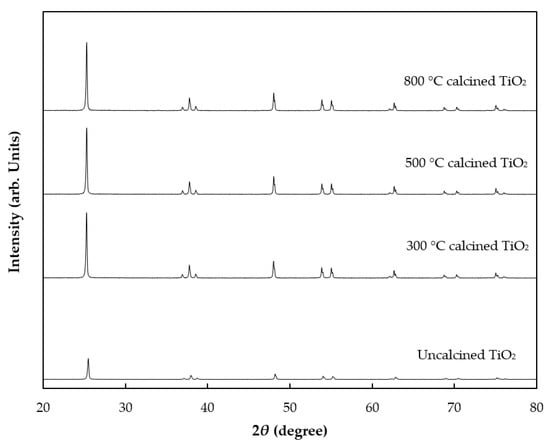
Figure 1.
XRD patterns of uncalcined TiO2 (bottom) and TiO2 calcined at different temperatures.
The initial surface area of TiO2 was 10 m2 g−1. At 300 °C, the specific surface area was reduced to 9 m2 g −1. A continuous decrease in surface area with the rise of calcination temperature was observed in TiO2 calcined at 500 and 800 °C, with respective surface areas of 7 and 4 m2 g−1. The influence of calcination temperature on morphology was investigated by SEM imaging as presented in Figure 2. Initially, the TiO2 particles had a diameter of approximately 200 nm as shown in Figure 2a. When mixing TiO2 particles with ABS plastic, the particles appeared to be embedded in the ABS plastic, resulting in the rough surface, as observed in Figure 2b. Calcining at 300 °C led to structural aggregation of TiO2 particles (Figure 2c), whereby the surface of ABS plastic contained assemblies of particles in some areas (Figure 2d). At 500 °C, it was also observed that a continuous aggregation in TiO2 particles occurred (Figure 2e) and these were visibly assembled on the ABS surface (Figure 2f). When the temperature was increased to 800 °C, the TiO2 particles became obviously aggregating as seen in Figure 2g. Calcination of TiO2 results in partial or total collapse of the structure, decreasing of surface area, and the appearance of particle agglomerations. This resulted in poorly dispersed distribution of TiO2 particles on ABS plastic as shown in Figure 2h. The higher degree of agglomeration was ordered as follows: 800 °C-TiO2/ABS > 500 °C-TiO2/ABS > 300 °C-TiO2/ABS > uncalcined-TiO2/ABS, and could be affirmed by orderly lowering yield strength, as shown in the next section.
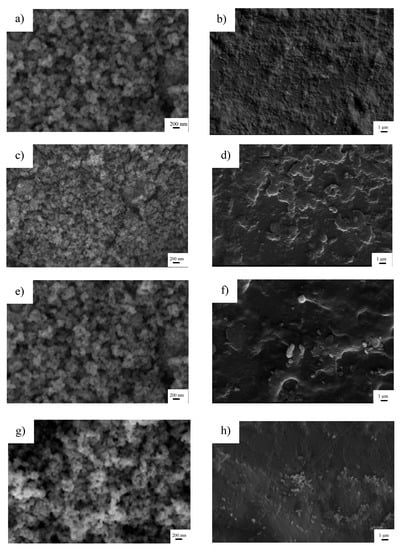
Figure 2.
SEM images of (a) TiO2, (b) TiO2/ABS, (c) 300 °C calcined TiO2, (d) 300 °C calcined TiO2/ABS, (e) 500 °C calcined TiO2, (f) 500 °C calcined TiO2/ABS, (g) 800 °C calcined TiO2, (h) 800 °C calcined TiO2/ABS.
3.2. Effect of TiO2 with and without Calcination on Photoantibacterial Activity and Yield Strength of ABS
The influence of TiO2 and calcined TiO2 mixed with ABS on photoantibacterial Escherichia coli (E. coli) as assessed by the JIS Z 2801: 2010 standard test are illustrated in Figure 3. Photoantibacterial effectiveness for E. coli was presented in the form of bacterial survival. The result obtained for pure ABS under UV illumination for 30 min showed little antibacterial activity, while the E. coli survival of 60 ± 2.8% was observed in ABS with TiO2, highlighting the dominant impact of TiO2 in influencing photocatalytic activity. Figure 4 illustrates the mechanism of photocatalytic antibacterial action proposed, in which the OH radicals and reactive oxygen species (ROS) generated by TiO2 would damage the cell membrane, resulting in the leakage of bacterial cytoplasm, leading to cell death [20,21,22]. Moreover, the calcined TiO2 mixed with ABS provided a greater reduction of E. coli compared to uncalcined TiO2/ABS. Calcining TiO2 at 300 °C could decrease E. coli on ABS by 45 ± 2.1% (remaining bacterial survival 55%), further decreasing to 60 ± 2.6% at 500°C (remaining bacterial survival 40%), despite a decrease in surface area. The photocatalytic improvement could be plausibly explained by a higher degree of crystallinity as mentioned earlier. That is, the more-active crystal phase was improved and surface defects were reduced as documented by several studies [23,24,25,26]. However, when increasing calcination temperature up to 800°C, the photoantibacterial performance decreased. This may derive from sintering and agglomeration effects during calcination at high temperature, as shown in SEM imagery (Figure 2).
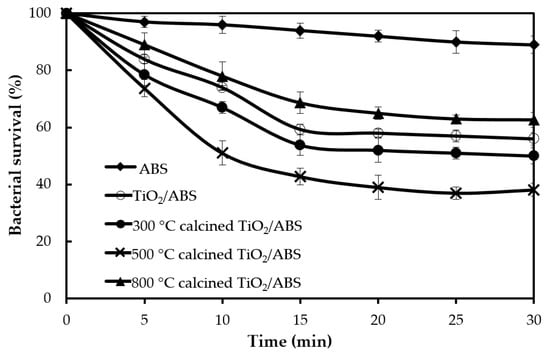
Figure 3.
Bacterial survival following photocatalytic reaction of ABS, TiO2/ABS and 300–800 °C calcined TiO2/ABS (TiO2 concentration of 1 wt %).
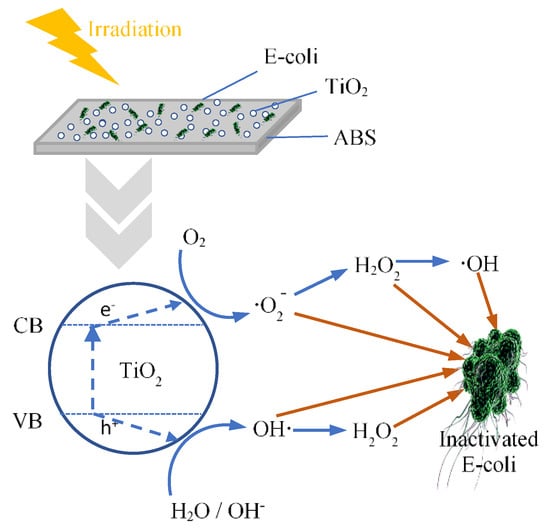
Figure 4.
The mechanism underlying the photocatalytic antibacterial effect.
Figure 5 shows that the yield strength of plain ABS was 16.7 ± 0.1 MPa, which was in the standard range of mechanical strength for this material. ABS containing TiO2 could further improve the yield strength of the workpiece. The enhancement of strength can be explained by TiO2 creating temporary crosslinks among the polymer chains during the deformation process. In particular, uncalcined TiO2/ABS showed the highest values of yield strength with a yield point of 17.2 ± 0.2 MPa. A slight decrease of yield strength was observed when increasing calcination temperature of TiO2. The yield strengths of 300 °C TiO2/ABS, 500 °C TiO2/ABS and 800 °C TiO2/ABS were 17.1 ± 0.3, 17.0 ± 0.3 and 16.9 ± 0.2 MPa, respectively. The decrease of yield strength may be attributed to increasing agglomeration and reduced surface area of TiO2 (e.g., a lower interfacial area for bonding to the ABS polymer) with increasing calcination temperature, as seen from SEM imagery (Figure 2), which contributed to formation of fewer crosslinks among the polymer chains.
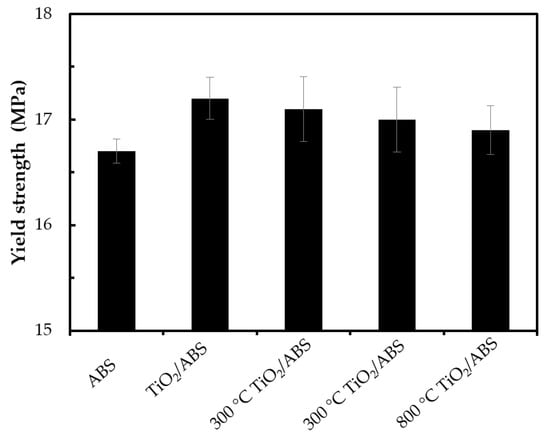
Figure 5.
Yield strength of ABS, TiO2/ABS and 300 °C–800 °C-calcined TiO2/ABS (TiO2 concentration of 1 wt %).
3.3. Effect of Concentration of Calcined TiO2 on Photoantibacterial Activity and Yield Strength of ABS
The previous section demonstrates that optimum performance on photocatalytic performance occurred at 500 °C for calcined TiO2/ABS. In this section, the influence of 500 °C-calcined TiO2 concentration in ABS on photocatalytic performance was considered. From the 40% bacterial survival by 1 wt % calcined TiO2/ABS, changing of TiO2 concentration had been considered, as shown in Figure 6. The reduced concentration of TiO2 resulted in the increase of bacterial survival by 50 ± 2.2%. This indicated that the smaller amount of TiO2 was not enough to produce OH radicals and reactive oxygen species (ROS) to inactivate the bacteria. However, by increasing the concentration to 2 wt %, the bacterial survival increased to 70 ± 2.5%. This showed that presence of large amounts of TiO2 did not always lead to the high photocatalytic activity, but may in fact suppress the activity due to their aggregation.
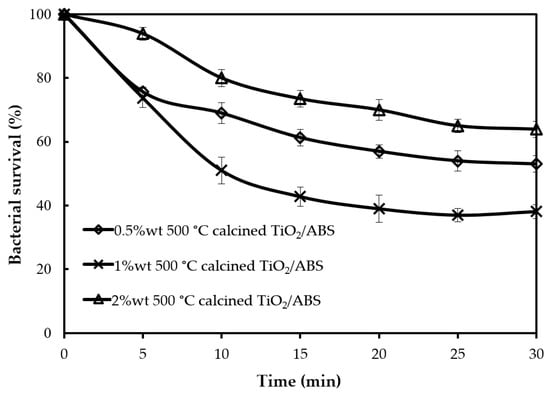
Figure 6.
Bacterial survival by photocatalytic reaction of ABS with 500 °C calcined TiO2 at different concentrations.
Figure 7 shows that yield strength decreased with increasing of TiO2 loading. A 0.5 w t% TiO2/ABS had the highest yield strength of 18.0 ± 0.3 MPa. This was to be expected given the higher TiO2 inducing the effective matrix reduction. In other words, higher TiO2 loading (1 and 2 wt %) increase “particle-to-particle” interactions rather than “particle-to polymer” interactions, thus lowering yield strength [27].
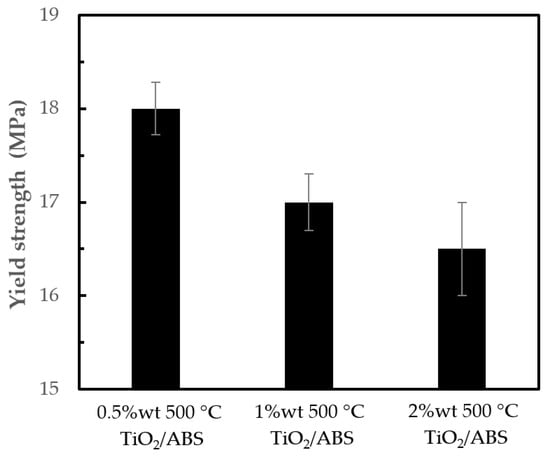
Figure 7.
Yield strength of ABS with 500 °C calcined TiO2 at different concentrations.
3.4. Effect of Silane on Photoantibacterial Activity and Yield Strength of ABS
The influence of silane on TiO2/ABS was observed in the SEM images shown in Figure 8, in which the 500 °C-calcined TiO2/ABS without silane possessed rough surfaces and smaller particles on the ABS surface, as illustrated in Figure 8a. After mixing silane, the morphology of blended polymer in Figure 8b shows the presence of a smooth surface and better dispersion, rather than TiO2/ABS. The difference was attributed to a greater interactions with the polymer matrix, which silane coupling agent showed the compatible behaviour of ABS/TiO2 by creating more adherent bonding between TiO2 and ABS matrix, as similarly explained for the modified SiO2 with silane [28,29,30].
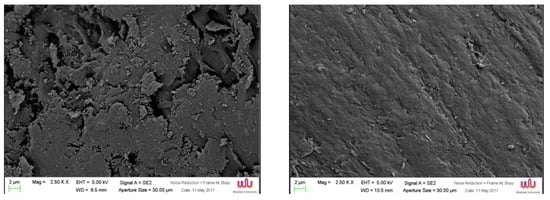
Figure 8.
SEM of (a) 500 °C calcined TiO2/ABS and (b) 500 °C calcined TiO2/ABS with silane.
To investigate the effect of silane on photocatalytic activity, the ratio of TiO2 to silane during catalyst preparation was varied over the weight ratio of 1:0.2-1:0.5. The optimum photocatalytic activity occurred for samples at a ratio of TiO2 to silane of 1:0.3 as presented in Figure 9. Figure 9 reveals that 500 °C-calcined TiO2/ABS with silane (called “silane-TiO2/ABS” for brevity) displays a better photobacterial activity compared with 500 °C-calcined TiO2/ABS without silane. The silane-TiO2/ABS could reduce 75% of E. coli (remaining bacteria survival 25 ± 2.6%). The higher silane-TiO2/ABS photobacterial activity was attributed to the comparatively greater distribution of TiO2 on ABS which promoted good UV absorption; however this result did not agree with the findings of Pazokifard et al. [31], who reported that in a case of degradation of rhodamine, TiO2 P25 nanoparticles showed a better activity than silane-treated particles due to the reduced surface area of TiO2 affecting poor photon absorption. The differences between the photocatalytic activity findings for bacteria and rhodamine likely originate from the operating conditions when blending the composite and the particle itself. The inset of Figure 9 shows that the yield strength of silane-TiO2/ABS was 1.6 times higher than TiO2/ABS without silane. The yield strength improvement was ascribed to better dispersion and adhesion of TiO2 in the ABS matrix, which arose from the silane coupling agent, enhancing interfacial bonding between the TiO2 and the matrix. We could highlight one key finding here, that the incorporation of silane results in improvement of TiO2 particls dispersion within the ABS polymeric matrix (shown as higher photocatalytic activity) and increasing possible interactions between TiO2 particles and the matrix (shown as higher yield strength).
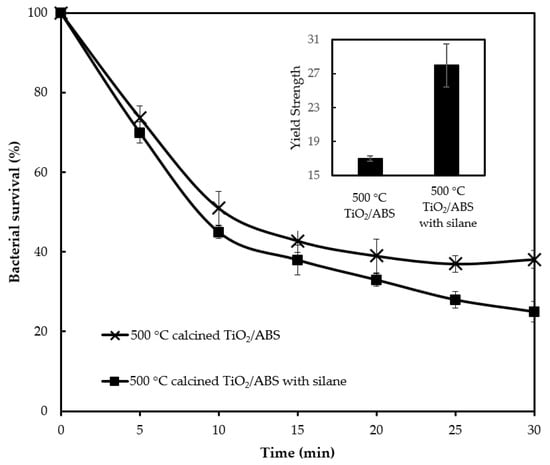
Figure 9.
Bacterial survival by photocatalytic reaction of ABS with 500 °C calcined TiO2 (1 wt % loading) with and without silane and corresponding their yield strength (inset).
3.5. Reusability and Robustness
The stability and reusability in terms of photocatalytic activity and the robustness of the composite material are crucial for practical applications. Therefore, the antibacterial experiments were repeated without any treatment on the specimen between the cycle runs. Constant photocatalytic efficiency was observed after five reuse cycles without loss in the yield strength of material, as shown in Figure 10. This suggests that blending calcined TiO2 into ABS prevented the loss of TiO2 photocatalyst particles from the surface, which is usually observed when coating TiO2 on the polymer surface. As expected, Ti content in the ABS plastic after the fifth run was identical to the as-prepared material (as determined by inductively coupled plasma atomic emission spectroscopy (ICP-OES). This robust ABS material could minimize detachment and release of TiO2 leading to lessening of the possibly negative environmental fates, transport, transformation and toxicity.
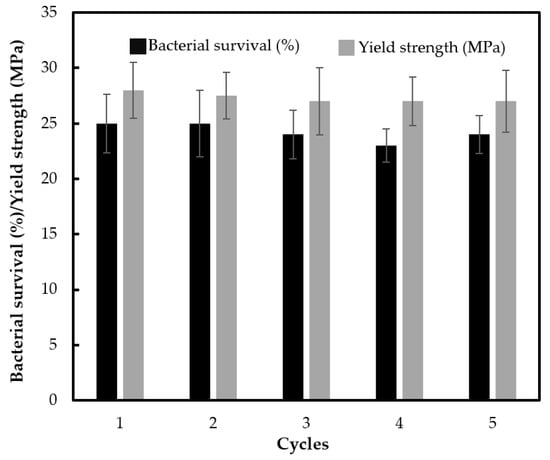
Figure 10.
Reusability of ABS with 500 °C calcined TiO2 (1 wt % loading) with silane grafting up to the 5th cycle in terms of bacterial survival by photocatalytic reaction, and the yield strength of the material (photo irradiation time of 30 min for each cycle).
4. Conclusions
Titanium dioxide (TiO2) was found to influence antibacterial performance and yield strength enhancement when blended with acrylonitrile-butadiene-styrene plastics (ABS). The optimum photoantibacterial activity occurred for ABS in the 500 °C-calcined TiO2 at a concentration of 1 wt %. At this temperature and concentration, the high degree of crystallinity and optimal amount of TiO2, were sufficient to produce OH radicals and reactive oxygen species (ROS), resulting in damage to bacterial cell membranes. The photoantibacterial performance for 500 °C calcined TiO2 at 1 wt % in ABS was more efficient than plain ABS over 62%. With optimal conditions, silane addition could further improve TiO2 dispersion on ABS. This resulted in a decrease of bacterial survival by 75%. Moreover, the benefit of TiO2-embedded ABS plastic could improve yield strength than pure ABS. The yield strength of TiO2/ABS with silane was 67.7% higher than of pure ABS. The efficiency of TiO2/ABS with silane photocatalyst showed an excellent photocatalytic antibacterial stability after five reuses, without loss in the yield strength.
Author Contributions
Conceptualization, K.K.; investigation, K.K.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, K.K., J.W.L., C.K.C, W.K. and S.A.; funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the by the Thailand Research Fund (TRF) and Office of the Higher Education Commission (Grant No. MRG6180262), and King Mongkut’s Institute of Technology Ladkrabang, KMITL with the Grant No. CRT28/2561.
Acknowledgments
Financial support by the Thailand Research Fund (TRF) and Office of the Higher Education Commission (Grant No. MRG6180262) and King Mongkut’s Institute of Technology Ladkrabang (Grant No. CRT28/2561) is gratefully acknowledged. The authors thank Miss Kullaporn Chotigkrai, Miss Nannapat Limnoi and Miss Thanathorn Jantharothai for their assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.B.; Milliron, D.J.; Aich, N.; Katz, L.E.; Liljestrand, H.M.; Kirisits, M.J. Importance of doping, dopant distribution, and defects on electronic band structure alteration of metal oxide nanoparticles: Implications for reactive oxygen species. Sci. Total Environ. 2016, 568, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Plazas-Tuttle, J.; Das, D.; Sabaraya, I.V.; Saleh, N.B. Harnessing the power of microwaves for inactivating Pseudomonas aeruginosa with nanohybrids. Environ. Sci. Nano 2018, 5, 72–82. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. JJAP 2005, 44, 8269. [Google Scholar]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Kiatkittipong, K.; Assabumrungrat, S. A comparative study of sodium/hydrogen titanate nanotubes/nanoribbons on destruction of recalcitrant compounds and sedimentation. J. Clean. Prod. 2017, 148, 905–914. [Google Scholar] [CrossRef]
- Lee, S.L.; Chang, C.-J. Recent developments about conductive polymer based composite photocatalysts. Polymers 2019, 11, 206. [Google Scholar] [CrossRef]
- Mukherjee, D.; Barghi, S.; Ray, A.K. Preparation and characterization of the TiO2 immobilized polymeric photocatalyst for degradation of aspirin under UV and solar light. Processes 2014, 2, 12–23. [Google Scholar] [CrossRef]
- Zhou, T.-T.; Zhao, F.-H.; Cui, Y.-Q.; Chen, L.-X.; Yan, J.-S.; Wang, X.-X.; Long, Y.-Z. Flexible TiO2/PVDF/g-C3N4 Nanocomposite with Excellent Light Photocatalytic Performance. Polymers 2020, 12, 55. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiol. 2008, 123, 288–292. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Ohmori, A. Behavior of TiO2 coating formation on PET plate by plasma spraying and evaluation of coating’s photocatalytic activity. Surf. Coat. Tech. 2005, 197, 45–50. [Google Scholar] [CrossRef]
- Ratova, M.; West, G.; Kelly, P. Optimisation of HiPIMS photocatalytic titania coatings for low temperature deposition. Surf. Coat. Tech. 2014, 250, 7–13. [Google Scholar] [CrossRef]
- Loddo, V.; Marcì, G.; Palmisano, G.; Yurdakal, S.; Brazzoli, M.; Garavaglia, L.; Palmisano, L. Extruded expanded polystyrene sheets coated by TiO2 as new photocatalytic materials for foodstuffs packaging. Appl. Surf. Sci. 2012, 261, 783–788. [Google Scholar] [CrossRef]
- Selvin, T.P.; Kuruvilla, J.; Sabu, T. Mechanical properties of titanium dioxide-filled polystyrene microcomposites. Mater. Lett. 2004, 58, 281–289. [Google Scholar] [CrossRef]
- Asiaban, S.; Taghinejad, S.F. Investigation of the effect of Titanium Dioxide on optical aspects and physical and mechanical characteristics of ABS Polymer. J. Elastomers Plast. 2010, 42, 267–274. [Google Scholar] [CrossRef]
- Sudeepan, J.; Kumar, K.; Barman, T.; Sahoo, P. Tribological behavior of ABS/TiO2 polymer composite using Taguchi statistical analysis. Mater. Sci. 2014, 5, 41–49. [Google Scholar] [CrossRef]
- Skorski, M.R.; Esenther, J.M.; Ahmed, Z.; Miller, A.E.; Hartings, M.R. The chemical, mechanical, and physical properties of 3D printed materials composed of TiO2-ABS nanocomposites. Sci. Technol. Adv. Mater. 2016, 17, 89–97. [Google Scholar] [CrossRef]
- Sangkatip, R.; Sriseubsai, W.; Kiatkittipong, K. Antibacterial and Mechanical Properties of the TiO2/ABS Composites. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2017; pp. 209–213. [Google Scholar]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mat. Sci. Semicon. Proc. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Ratova, M.; Mills, A. Antibacterial titania-based photocatalytic extruded plastic films. J. Photoch. Photobio. A 2015, 299, 159–165. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B 2015, 176, 70–75. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Trapalis, C. Effects of calcination temperature on the microstructures and photocatalytic activity of titanate nanotubes. J. Mol. Catal. A Chem. 2006, 249, 135–142. [Google Scholar] [CrossRef]
- An, H.; Zhu, B.; Li, J.; Zhou, J.; Wang, S.; Zhang, S.; Wu, S.; Huang, W. Synthesis and characterization of thermally stable nanotubular TiO2 and its photocatalytic activity. J. Phys. Chem. 2008, 112, 18772–18775. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Rangari, V. Polymer nanocomposite materials for structural applications. In Advances in Nanocomposites-Synthesis, Characterization Industrial Applications; Reddy, D.B., Ed.; IntechOpen: London, UK, 2011; pp. 61–84. [Google Scholar]
- Li, H.; Zhang, Z.; Ma, X.; Hu, M.; Wang, X.; Fan, P. Synthesis and characterization of epoxy resin modified with nano-SiO2 and γ-glycidoxypropyltrimethoxy silane. Surf. Coat. Technol. 2007, 201, 5269–5272. [Google Scholar] [CrossRef]
- Xu, X.; Li, B.; Lu, H.; Zhang, Z.; Wang, H. The interface structure of nano-SiO2/PA66 composites and its influence on material’s mechanical and thermal properties. Appl. Surf. Sci. 2007, 254, 1456–1462. [Google Scholar] [CrossRef]
- Li, X.; Cao, Z.; Zhang, Z.; Dang, H. Surface-modification in situ of nano-SiO2 and its structure and tribological properties. Appl. Surf. Sci. 2006, 252, 7856–7861. [Google Scholar] [CrossRef]
- Pazokifard, S.; Mirabedini, S.; Esfandeh, M.; Mohseni, M.; Ranjbar, Z. Silane grafting of TiO2 nanoparticles: Dispersibility and photoactivity in aqueous solutions. Surf. Interface Anal. 2012, 44, 41–47. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).