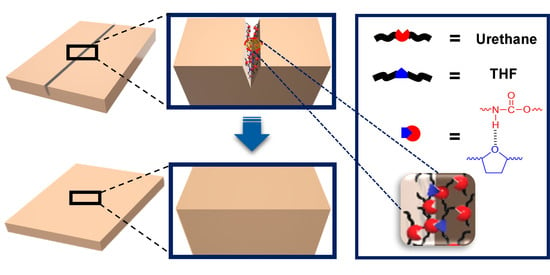
A Heterocyclic Polyurethane with Enhanced Self-Healing Efficiency and Outstanding Recovery of Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyol (Poly(2-hydroxyl methacrylate-r-butyl methacrylate) (poly(HEMA-r-BMA))
2.3. Preparation Conventional Polyurethane (PU), PU with Aromatic Moiety (PUB), PU with Heterocyclic Moiety (PUT), and PU with Aliphatic Moiety (PUD)
2.4. Characterization
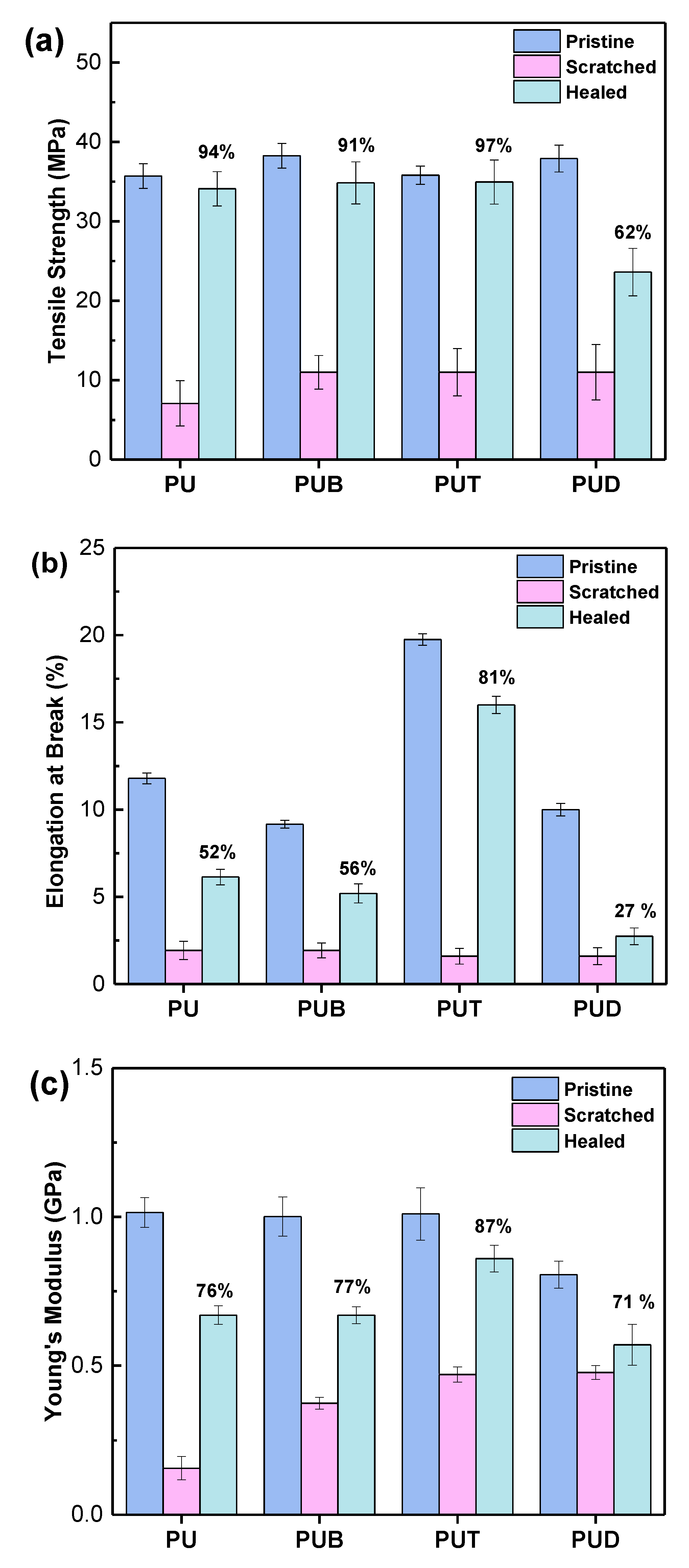
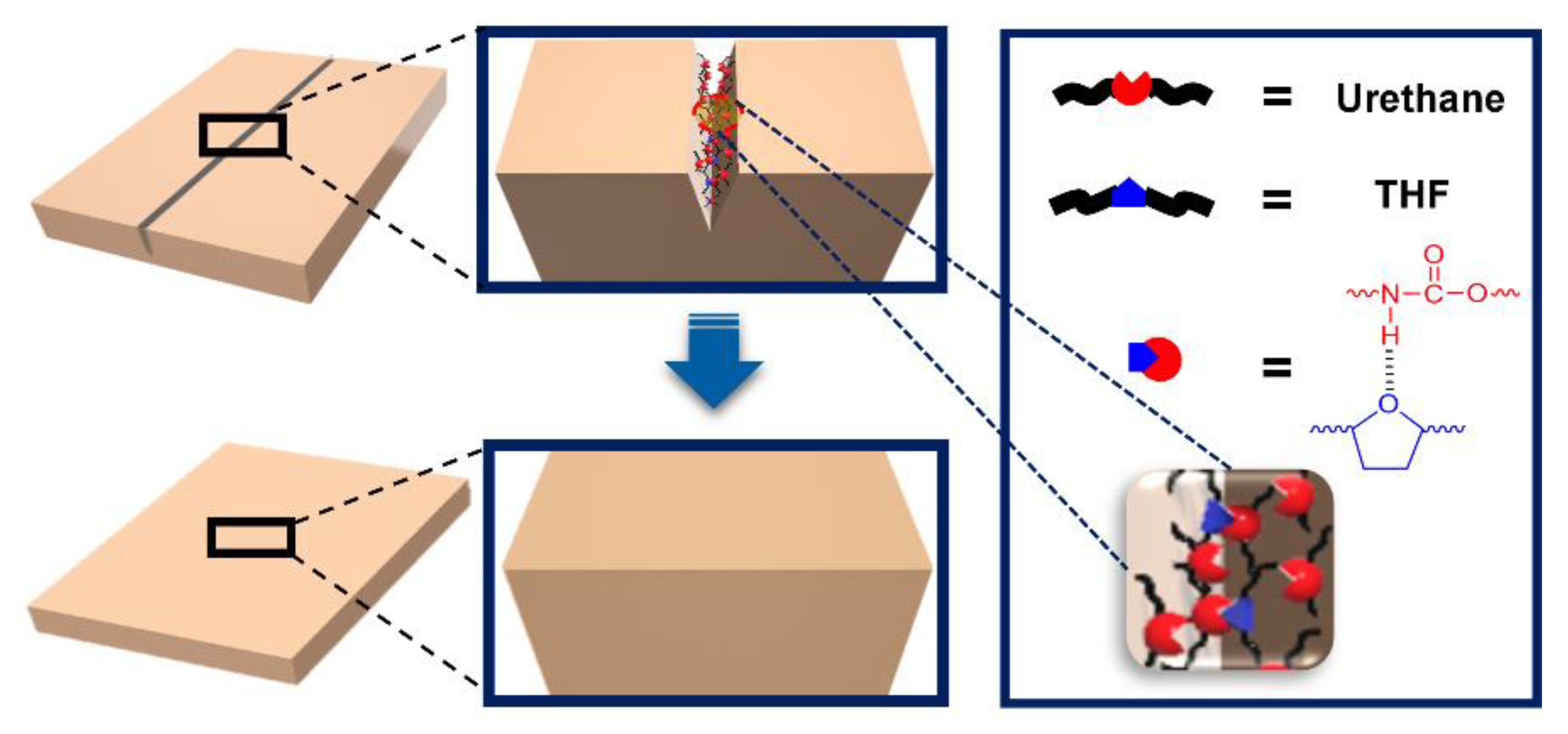
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lai, Y.; Kuang, X.; Zhu, P.; Huang, M.M.; Dong, X.; Wang, D.J. Colorless, Transparent, Robust, and Fast Scratch-Self-Healing Elastomers via a Phase-Locked Dynamic Bonds Design. Adv. Mater. 2018, 30, 1802556. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.B.; Zhou, A.; Pan, T.; Han, J.B.; Wei, M.; Evans, D.G.; Duan, X. Humidity-triggered self-healing films with excellent oxygen barrier performance. Chem. Commun. 2014, 50, 7136–7138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benight, S.J.; Wang, C.; Tok, J.B.H.; Bao, Z.A. Stretchable and self-healing polymers and devices for electronic skin. Prog. Polym. Sci. 2013, 38, 1961–1977. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, K.Q.; Wan, P.B. A Flexible Stretchable Hydrogel Electrolyte for Healable All-in-One Configured Supercapacitors. Small 2018, 14, 1704497. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yi, N.; Wu, Y.; Zhang, Y.; Zhang, Q.; Huang, Y.; Ma, Y.; Chen, Y. Multichannel and repeatable self-healing of mechanical enhanced graphene-thermoplastic polyurethane composites. Adv. Mater. 2013, 25, 2224–2228. [Google Scholar] [CrossRef]
- Huynh, T.P.; Sonar, P.; Haick, H. Advanced Materials for Use in Soft Self-Healing Devices. Adv. Mater. 2017, 29, 1604973. [Google Scholar] [CrossRef] [Green Version]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef]
- Nathan, A.; Ahnood, A.; Cole, M.T.; Lee, S.; Suzuki, Y.; Hiralal, P.; Bonaccorso, F.; Hasan, T.; Garcia-Gancedo, L.; Dyadyusha, A. Flexible Electronic: The Next Ubiquitous Platform. Proc. IEEE 2012, 100, 1486–1517. [Google Scholar] [CrossRef]
- Fischer, H. Self-repairing material system a dream or a reality. Nat. Sci. 2010, 2, 873–901. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.A.; Bao, Z.; Baldwin, K.; Dodabalapur, A.; Crone, B.; Raju, V.; Kuck, V.; Katz, H.; Amundson, K.; Ewing, P. Paper-like electronic displays: Large-area rubber-stamped plastic sheets of electronics and microencapsulated electrophoretic inks. Proc. Natl. Acad. Sci. USA 2001, 98, 4835–4840. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.; White, S.; Sottos, N. Retardation and repair of fatigue cracks in a microcapsule toughened epoxy composite-Part II: In situ self-healing. Compos. Sci. Technol. 2005, 65, 2474–2480. [Google Scholar] [CrossRef] [Green Version]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Blaiszik, B.J.; Kramer, S.L.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-Healing Polymers and Composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Yuan, Y.C.; Yin, T.; Rong, M.Z.; Zhang, M.Q. Self-healing in polymers and polymer composites. Concepts, realization and outlook: A review. Express Polym. Lett. 2008, 2, 238–250. [Google Scholar] [CrossRef]
- Guoqiang, L.; Harper, M. Recent Advances in Smart Self-Healing Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Nathalie, K.G.; Kim, K.; Oehlenschlaeger, J.Z.; Stefan, H.; Friedrich, G.S.; Christopher, B.K. Current Trends in the Field of Self-Healing Materials. Macromol. Chem. Phys. 2012, 213, 131–143. [Google Scholar]
- Herck, V.N.; Du Prez, F.E. Fast Healing of Polyurethane Thermosets Using Reversible Triazolinedione Chemistry and Shape-Memory. Macromolecules 2018, 51, 3405–3414. [Google Scholar] [CrossRef]
- Du, P.; Wu, M.; Liu, X.; Zheng, Z.; Wang, X.; Sun, P.; Joncheray, T.; Zhang, Y. Synthesis of linear polyurethane bearing pendant furan and cross-linked healable polyurethane containing Diels–Alder bonds. New J. Chem. 2014, 38, 770–776. [Google Scholar] [CrossRef]
- Saraf, V.P.; Glasser, W.G. Engineering plastics from lignin. Structure property relationships in solution cast polyurethane films. J. Appl. Polym. Sci. 1984, 29, 1831–1841. [Google Scholar] [CrossRef]
- Mailhot, B.; Komvopoulos, K.; Ward, B.; Tian, Y.; Somarjai, G. Mechanical and friction properties of thermoplastic polyurethanes determined by scanning force microscopy. J. Appl. Phys. 2001, 89, 5712–5719. [Google Scholar] [CrossRef]
- Sonnenschein, M.F. Polyurethanes: Science, Technology, Markets, and Trends; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Lee, S.; Hong, P.H.; Kim, J.; Choi, K.; Moon, G.; Kang, J.; Lee, S.; Ahn, J.B.; Eom, W.; Ko, M.J. Highly Self-Healable Polymeric Blend Synthesized Using Polymeric Glue with Outstanding Mechanical Properties. Macromolecules 2020, 53, 2279–2286. [Google Scholar] [CrossRef]
- Randall, D.; Lee, S. The Polyurethanes Book; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
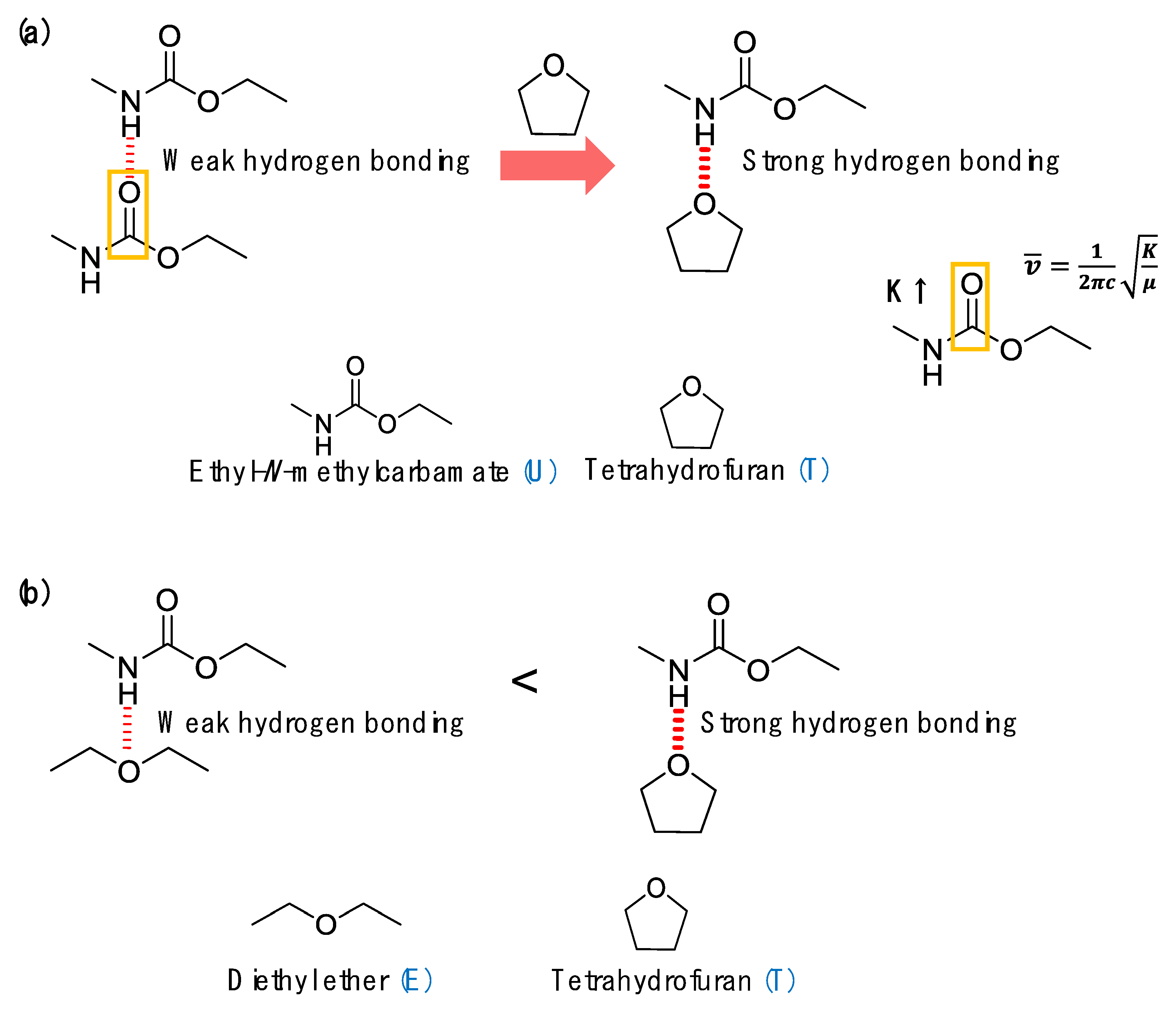
- Mattia, J.; Painter, P. A comparison of hydrogen bonding and order in a polyurethane and poly(urethane-urea) and their blends with poly(ethylene glycol). Macromolecules 2007, 40, 1546–1554. [Google Scholar] [CrossRef]
- Huacuja-Sań chez, J.; Muller, K.; Possart, W. Water diffusion in a crosslinked polyether-based polyurethane adhesive. Int. J. Adhes. Adhes. 2016, 66, 167–175. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, K.Y.; Hsu, L.S. Spectroscopic analysis of phase separation behavior of model polyurethanes. Macromolecules 1987, 20, 2089–2095. [Google Scholar] [CrossRef]
- Coleman, M.M.; Skrovanek, J.D.; Hu, J.; Painter, C.P. Hydrogen bonding in polymer blends. 1. FTIR studies of urethane-ether blends. Macromolecules 1988, 21, 59–65. [Google Scholar] [CrossRef]
- Skrovanek, D.J.; Howe, S.E.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymer: Infrared temperature studies of an amorphous polyamide. Macromolecules 1985, 18, 1676–1683. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frenquencies; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Fessenden, R.J.; Fessenden, J.S. Organic Chemistry, 5th ed.; Brooks Cole: Pacific Grove, CA, USA, 1993. [Google Scholar]


| Sample Designations | Compositions | ||
|---|---|---|---|
| Polyol | Crosslinker (HDIt) | Functional Diols | |
| PU | 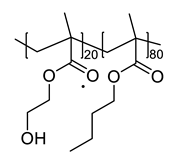 | 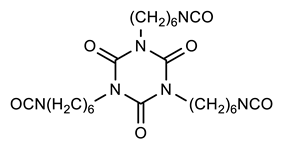 | None |
| PUB | 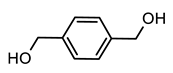 BHMB | ||
| PUT | 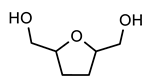 BHMTHF | ||
| PUD |  DHE | ||
| Sample Designations | Optical Properties | Thermal Property | Thickness (µm) | ||
|---|---|---|---|---|---|
| Tr (%) | YI | Haze | Tg (°C) | ||
| PU | 91.7 ± 0.12 | 0.05 ± 0.02 | 0.2 ± 0.03 | 58.0 | 12.5 ± 0.23 |
| PUB | 91.6 ± 0.15 | 0.02 ± 0.01 | 0.3 ± 0.02 | 60.0 | 11.2 ± 0.33 |
| PUT | 91.7 ± 0.14 | 0.05 ± 0.01 | 0.1 ± 0.04 | 57.2 | 10.9 ± 0.21 |
| PUD | 91.7 ± 0.21 | 0.06 ± 0.03 | 0.2 ± 0.02 | 59.1 | 10.0 ± 0.13 |
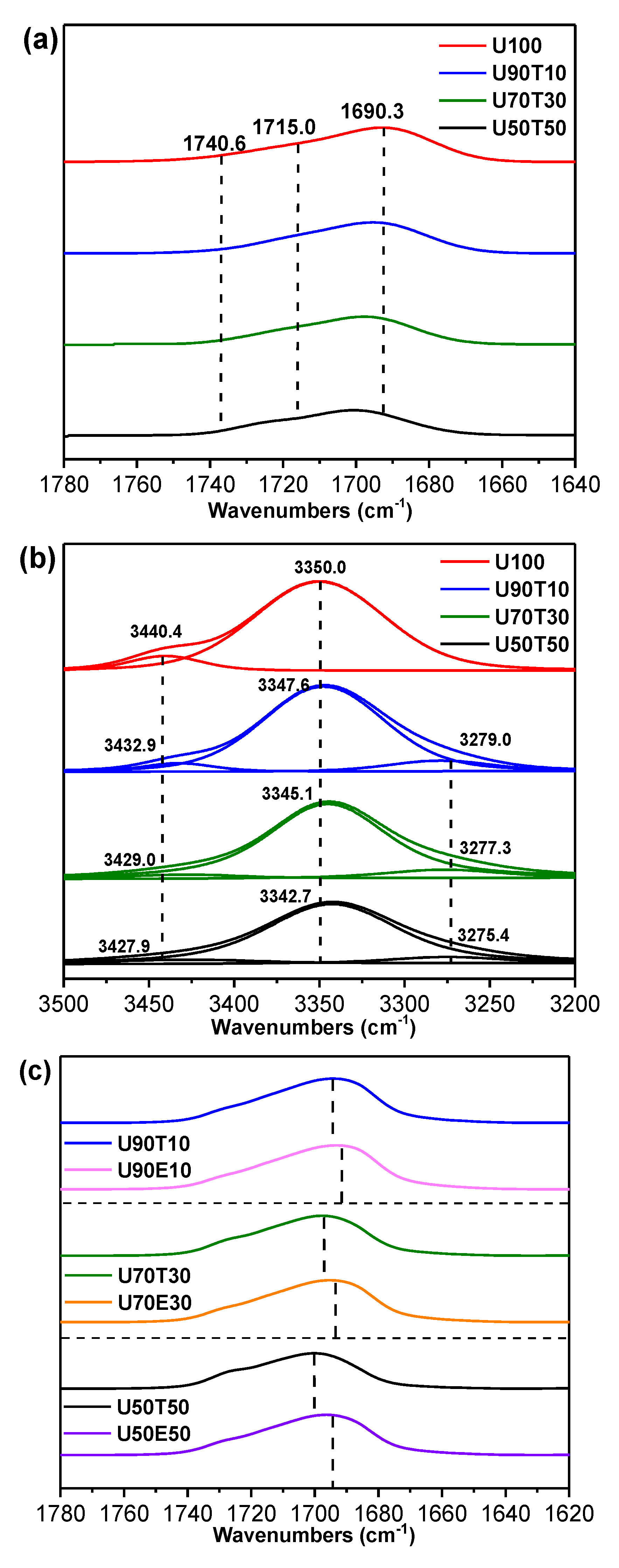
| Sample Designations | Compositions (wt.%) | Free N–H | Hydrogen Bonded N–H (to U) | Hydrogen Bonded N–H (to T) | Hydrogen Bonded C=O | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| U | CHCl3 | T | Wave Number (cm−1) | Area (%) | Wave Number (cm−1) | Area (%) | Wave Number (cm−1) | Area (%) | Wave Number (cm−1) | |
| Solvent for U | ||||||||||
| U100 | 70 | 30 | 0 | 3440.4 | 8.5 | 3350.0 | 91.5 | - | - | 1690.3 |
| U90T10 | 63 | 31 | 6 | 3432.9 | 5.4 | 3347.8 | 85.4 | 3279.0 | 9.2 | 1694.3 |
| U70T30 | 49 | 36 | 15 | 3429.0 | 6.0 | 3345.1 | 85.0 | 3277.3 | 9.7 | 1697.7 |
| U50T50 | 35 | 47 | 18 | 3427.9 | 6.6 | 3342.7 | 82.5 | 3275.4 | 10.9 | 1700.2 |
| U90E10 | 63 | 31 | 6 | - | - | - | - | - | - | 1693.2 |
| U70E30 | 49 | 36 | 15 | - | - | - | - | - | - | 1695.3 |
| U50E50 | 35 | 47 | 18 | - | - | - | - | - | - | 1696.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Hong, P.H.; Choi, K.; Moon, G.; Kang, J.; Lee, S.; Lee, S.; Jung, H.W.; Ko, M.J.; Hong, S.W. A Heterocyclic Polyurethane with Enhanced Self-Healing Efficiency and Outstanding Recovery of Mechanical Properties. Polymers 2020, 12, 968. https://doi.org/10.3390/polym12040968
Kim J, Hong PH, Choi K, Moon G, Kang J, Lee S, Lee S, Jung HW, Ko MJ, Hong SW. A Heterocyclic Polyurethane with Enhanced Self-Healing Efficiency and Outstanding Recovery of Mechanical Properties. Polymers. 2020; 12(4):968. https://doi.org/10.3390/polym12040968
Chicago/Turabian StyleKim, Jinsil, Pyong Hwa Hong, Kiwon Choi, Gyeongmin Moon, Jungsoon Kang, Seoyun Lee, Sungkoo Lee, Hyun Wook Jung, Min Jae Ko, and Sung Woo Hong. 2020. "A Heterocyclic Polyurethane with Enhanced Self-Healing Efficiency and Outstanding Recovery of Mechanical Properties" Polymers 12, no. 4: 968. https://doi.org/10.3390/polym12040968
APA StyleKim, J., Hong, P. H., Choi, K., Moon, G., Kang, J., Lee, S., Lee, S., Jung, H. W., Ko, M. J., & Hong, S. W. (2020). A Heterocyclic Polyurethane with Enhanced Self-Healing Efficiency and Outstanding Recovery of Mechanical Properties. Polymers, 12(4), 968. https://doi.org/10.3390/polym12040968