A Smart Strategy to Improve t-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Synthesis of CD Polymers Coated with Magnetic Nanoparticles
2.4. Vitis vinifera cv. Monastrell Cell Culture Treatments
2.5. Extraction and Quantification of t-Resveratrol
2.6. Preliminary Tests for Absorption-Desorption of t-Resveratrol from the Magnetic Polymers
2.7. Statistical Analysis
3. Results and Discussion
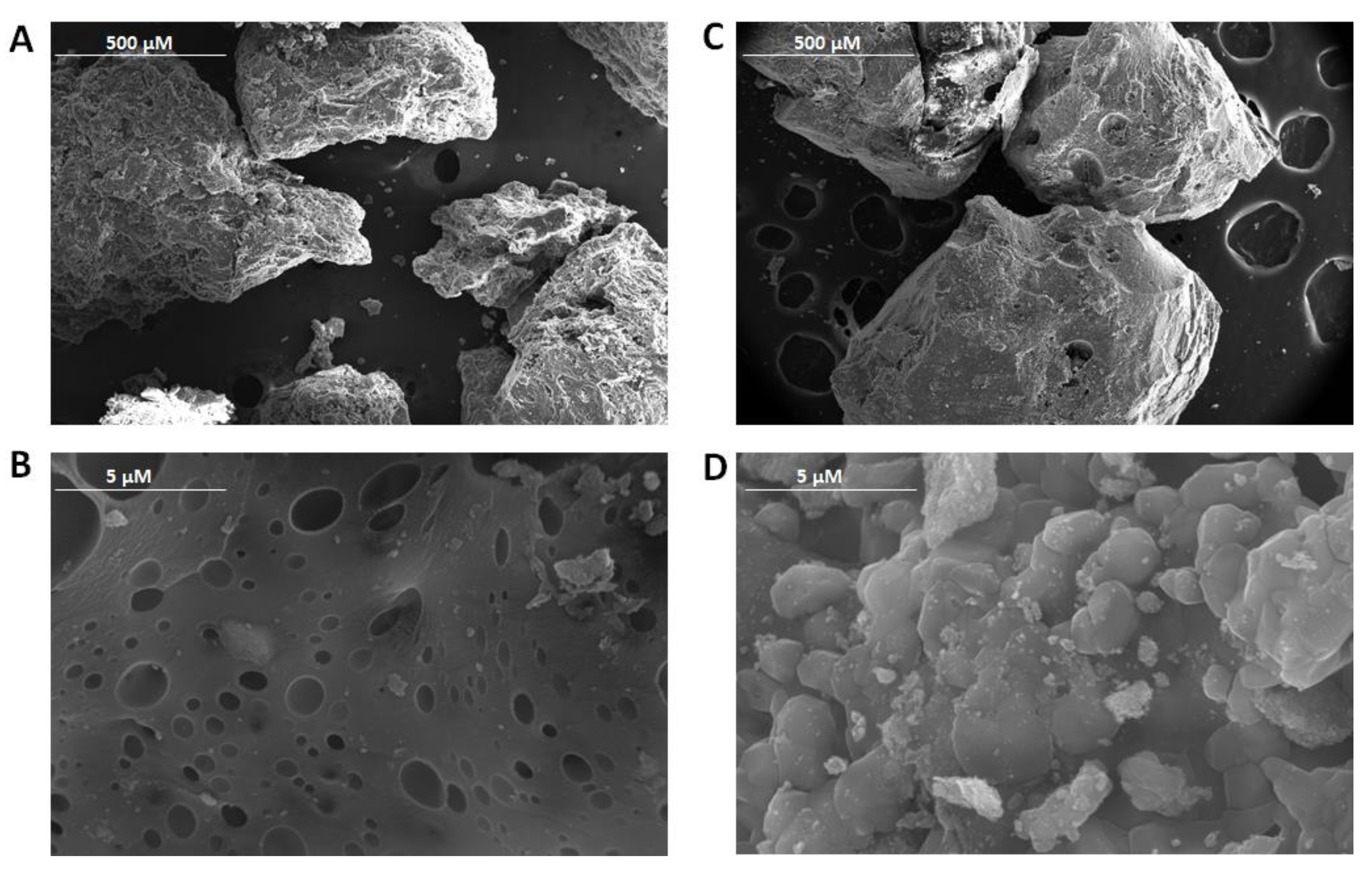
3.1. FE-SEM Characterisation and t-Resveratrol Release Test
3.2. Effect of Different Concentrations of CM-β-CD-EPI-MN or HP-β-CD-EPI-MN Polymers in the Presence of 100 µM MJ on t-Resveratrol Production in V. vinifera Suspension-Cultured Cells
3.3. Effect of Elicitation Time Course on t-Resveratrol Production in V. vinifera Suspension-Cultured Cells Treated with 15 g/L HP-β-CD-EPI-MN Polymer and 100 µM MJ
3.4. Regeneration of HP-β-CD-EPI-MN Polymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusido, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Chu, M.; Almagro, L.; Chen, B.-H.; Burgos, L.; Pedreno, M.A. Recent trends and comprehensive appraisal for the biotechnological production of trans-resveratrol and its derivatives. Phytochem. Rev. 2018, 17, 491–508. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Pedreño, M.A.; Almagro, L. Critical parameters on which the production of trans-resveratrol in Vitis vinifera cv Monastrell cell cultures depends. Plant Cell Tiss. Org. 2019, 138, 395–398. [Google Scholar] [CrossRef]
- Biçer, P.Ö.; Demirci, T.; Aşcı, Ö.A.; Baydar, N.G. Effects of Methyl Jasmonate and Caffeic Acid Applications on Secondary Metabolite Production in Madder (Rubia tinctorum) Root Cultures. Indian J. Pharm. Educ. Res. 2017, 51, 508–512. [Google Scholar] [CrossRef]
- Belch-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru-Martinez, R.; Pedreno, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2011, 31, 81–89. [Google Scholar] [CrossRef]
- Komaikul, J.; Kitisripanya, T.; Likhitwitayawuid, K.; Sritularak, B.; Tanaka, H.; Putalun, W. Improvement of stilbenoid production by 2-hydroxypropyl-β-cyclodextrin in white mulberry (Morus alba L.) callus cultures. Nat. Prod. Res. 2018, 33, 2762–2769. [Google Scholar] [CrossRef]
- Bertini, L.; Palazzi, L.; Proietti, S.; Pollastri, S.; Arrigoni, G.; De Laureto, P.P.; Caruso, C. Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding. Int. J. Mol. Sci. 2019, 20, 2525. [Google Scholar] [CrossRef] [PubMed]
- Giri, C.C.; Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult. 2016, 126, 1–18. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Fortea, M.; Gabaldon, J.; Núñez-Delicado, E. Complexation of resveratrol by native and modified cyclodextrins: Determination of complexation constant by enzymatic, solubility and fluorimetric assays. Food Chem. 2008, 111, 262–267. [Google Scholar] [CrossRef]
- Ros, M.T.M.; Lucas-Abellán, C.; Gabaldón, J.A.; Fortea, M.I.; Martínez-Cachá, A.; Núñez-Delicado, E. Kaempferol Complexation in Cyclodextrins at Basic pH. J. Agric. Food Chem. 2010, 58, 4675–4680. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bru-Martinez, R.; Sellés, S.; Casado-Vela, J.; Belchi-Navarro, S.; Pedreno, M.A. Modified Cyclodextrins Are Chemically Defined Glucan Inducers of Defense Responses in Grapevine Cell Cultures. J. Agric. Food Chem. 2006, 54, 65–71. [Google Scholar] [CrossRef]
- Almagro, L.; Belchí-Navarro, S.; Márquez, A.M.; Bru-Martinez, R.; Pedreno, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef]
- Almagro, L.; Carbonell-Bejerano, P.; Belchí-Navarro, S.; Bru-Martinez, R.; Martínez, M.; Ángeles, L.; Lijavetzky, D.; Pedreño, M.A. Dissecting the Transcriptional Response to Elicitors in Vitis vinifera Cells. PLoS ONE 2014, 9, 109777. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Almagro, L.; Belchí-Navarro, S.; Ferrer, M.A.; Barcelo, A.R.; Pedreno, M.A. Induction of sesquiterpenes, phytoesterols and extracellular pathogenesis-related proteins in elicited cell cultures of Capsicum annuum. J. Plant Physiol. 2010, 167, 1273–1281. [Google Scholar] [CrossRef]
- Easo, S.L.; Mohanan, P. In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids Surfaces B Biointerfaces 2015, 134, 122–130. [Google Scholar] [CrossRef]
- Voliani, V.; Ricci, F.; Signore, G.; Nifosì, R.; Luin, S.; Beltram, F. Multiphoton Molecular Photorelease in Click-Chemistry-Functionalized Gold Nanoparticles. Small 2011, 7, 3271–3275. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2009, 62, 284–304. [Google Scholar] [CrossRef]
- Jain, A.; Escallier, J.; Ganetis, G.; Louie, W.; Marone, A.; Thomas, R.; Wanderer, P. Magnetic Field Measurements for Fast-Changing Magnetic Fields. IEEE Trans. Appl. Supercond. 2005, 15, 1221–1224. [Google Scholar] [CrossRef]
- Lungoci, A.-L.; Turin-Moleavin, I.-A.; Corciova, A.; Mircea, C.; Arvinte, A.; Fifere, A.; Marangoci, N.; Del Prete, S. Multifunctional magnetic cargo-complexes with radical scavenging properties. Mater. Sci. Eng. C 2019, 94, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Ping, W.; Wang, J.; Zhu, X. Cyclodextrin polymer/Fe3O4 nanocomposites as solid phase extraction material coupled with UV–vis spectrometry for the analysis of rutin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Calderón, A.; Zapata, J.M.; Muñoz, R.; Pedreño, M.A.; Barceló, A.R. Resveratrol production as a part of the hypersensitive-like response of grapevine cells to an elicitor from Trichoderma viride. New Phytol. 1993, 124, 455–463. [Google Scholar] [CrossRef]
- Kandpal, N.D.; Sah, N.; Loshali, R.; Joshi, R.; Prasad, J. Co-precipitation method of synthesis and characterization of iron oxide nanoparticles. J. Sci. Ind. Res. 2014, 73, 87–90. Available online: http://hdl.handle.net/123456789/26444 (accessed on 1 February 2014).
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Tay, D.W.J.; Hidajat, K.; Uddin, M.S. Endocrine Disrupters and Toxic Metal Ions Removal by Carboxymethyl-Cyclodextrin Polymer Grafted onto Magnetic Nanoadsorbents. J. Chem. Eng. 2013, 27, 69–73. [Google Scholar] [CrossRef]
- Yu, L.; Xue, W.; Cui, L.; Xing, W.; Cao, X.; Li, H. Use of hydroxypropyl-β-cyclodextrin/polyethylene glycol 400, modified Fe3O4 nanoparticles for congo red removal. Int. J. Boil. Macromol. 2014, 64, 233–239. [Google Scholar] [CrossRef]
- Donnez, D.; Kim, K.-H.; Antoine, S.; Conreux, A.; De Luca, V.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and viniferins by an elicited grapevine cell culture in a 2L stirred bioreactor. Process. Biochem. 2011, 46, 1056–1062. [Google Scholar] [CrossRef]
- Vidal-Limon, H.R.; Almagro, L.; Moyano, E.; Palazon, J.; Pedreño, M.A.; Cusido, R.M. Perfluorodecalins and Hexenol as Inducers of Secondary Metabolism in Taxus media and Vitis vinifera Cell Cultures. Front. Plant Sci. 2018, 9, 335. [Google Scholar] [CrossRef]
- Sivakumar, G.; Paek, K.Y. Methyl Jasmonate Induce Enhanced Production of Soluble Biophenols in PANAX GINSENG Adventitious Roots from Commercial Scale Bioreactors. Chem. Nat. Compd. 2005, 41, 669–673. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of Elicitors on the Production of Resveratrol and Viniferins in Cell Cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef]
- Taurino, M.; Ingrosso, I.; D’Amico, L.; De Domenico, S.; Nicoletti, I.; Corradini, D.; Santino, A.; Giovinazzo, G. Jasmonates elicit different sets of stilbenes in Vitis vinifera cv. Negramaro cell cultures. SpringerPlus 2015, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Zhan, J.-C.; Huang, W.-D. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. 2015, 122, 197–211. [Google Scholar] [CrossRef]
- Cai, Z.; Riedel, H.; Saw, N.M.M.T.; Mewis, I.; Reineke, K.; Knorr, D.; Smetanska, I. Effects of elicitors and high hydrostatic pressure on secondary metabolism of Vitis vinifera suspension culture. Process. Biochem. 2011, 46, 1411–1416. [Google Scholar] [CrossRef]
- Aadinath, W.; Bhushani, A.; Anandharamakrishnan, C. Synergistic radical scavenging potency of curcumin-in-β-cyclodextrin-in-nanomagnetoliposomes. Mater. Sci. Eng. C 2016, 64, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Yassari, M.; Heidari, B. Thermo-responsive molecularly imprinted polymer containing magnetic nanoparticles: Synthesis, characterization and adsorption properties for curcumin. Colloids Surf. B Biointerfaces 2018, 162, 154–162. [Google Scholar] [CrossRef]
- Rastegari, B.; Karbalaei-Heidari, H.R.; Zeinali, S.; Sheardown, H. The enzyme-sensitive release of prodigiosin grafted β-cyclodextrin and chitosan magnetic nanoparticles as an anticancer drug delivery system: Synthesis, characterization and cytotoxicity studies. Colloids Surf. B Biointerfaces 2017, 158, 589–601. [Google Scholar] [CrossRef]
- Li, N.; Chen, J.; Shi, Y.-P. Magnetic reduced graphene oxide functionalized with β-cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phytohormones in tomatoes coupled with high performance liquid chromatography. J. Chromatogr. A 2016, 1441, 24–33. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Gao, Y.; Gao, M.; Huang, X.; Lv, J.; Xu, D. A beta-cyclodextrin-functionalized magnetic metal organic framework for efficient extraction and determination of prochloraz and triazole fungicides in vegetables samples. Ecotoxicol. Environ. Saf. 2019, 183, 109546. [Google Scholar] [CrossRef]
- Ragavan, K.; Rastogi, N.K. β-Cyclodextrin capped graphene-magnetite nanocomposite for selective adsorption of Bisphenol-A. Carbohydr. Polym. 2017, 168, 129–137. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almagro, L.; Gea-Abellán, A.D.; Rodríguez-López, M.I.; Núñez-Delicado, E.; Gabaldón, J.A.; Pedreño, M.A. A Smart Strategy to Improve t-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles. Polymers 2020, 12, 991. https://doi.org/10.3390/polym12040991
Almagro L, Gea-Abellán AD, Rodríguez-López MI, Núñez-Delicado E, Gabaldón JA, Pedreño MA. A Smart Strategy to Improve t-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles. Polymers. 2020; 12(4):991. https://doi.org/10.3390/polym12040991
Chicago/Turabian StyleAlmagro, Lorena, Alicia De Gea-Abellán, María Isabel Rodríguez-López, Estrella Núñez-Delicado, José Antonio Gabaldón, and María Angeles Pedreño. 2020. "A Smart Strategy to Improve t-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles" Polymers 12, no. 4: 991. https://doi.org/10.3390/polym12040991
APA StyleAlmagro, L., Gea-Abellán, A. D., Rodríguez-López, M. I., Núñez-Delicado, E., Gabaldón, J. A., & Pedreño, M. A. (2020). A Smart Strategy to Improve t-Resveratrol Production in Grapevine Cells Treated with Cyclodextrin Polymers Coated with Magnetic Nanoparticles. Polymers, 12(4), 991. https://doi.org/10.3390/polym12040991







