The Effect of Germinated Sorghum Extract on the Pasting Properties and Swelling Power of Different Annealed Starches
Abstract
1. Introduction
2. Materials and Methods
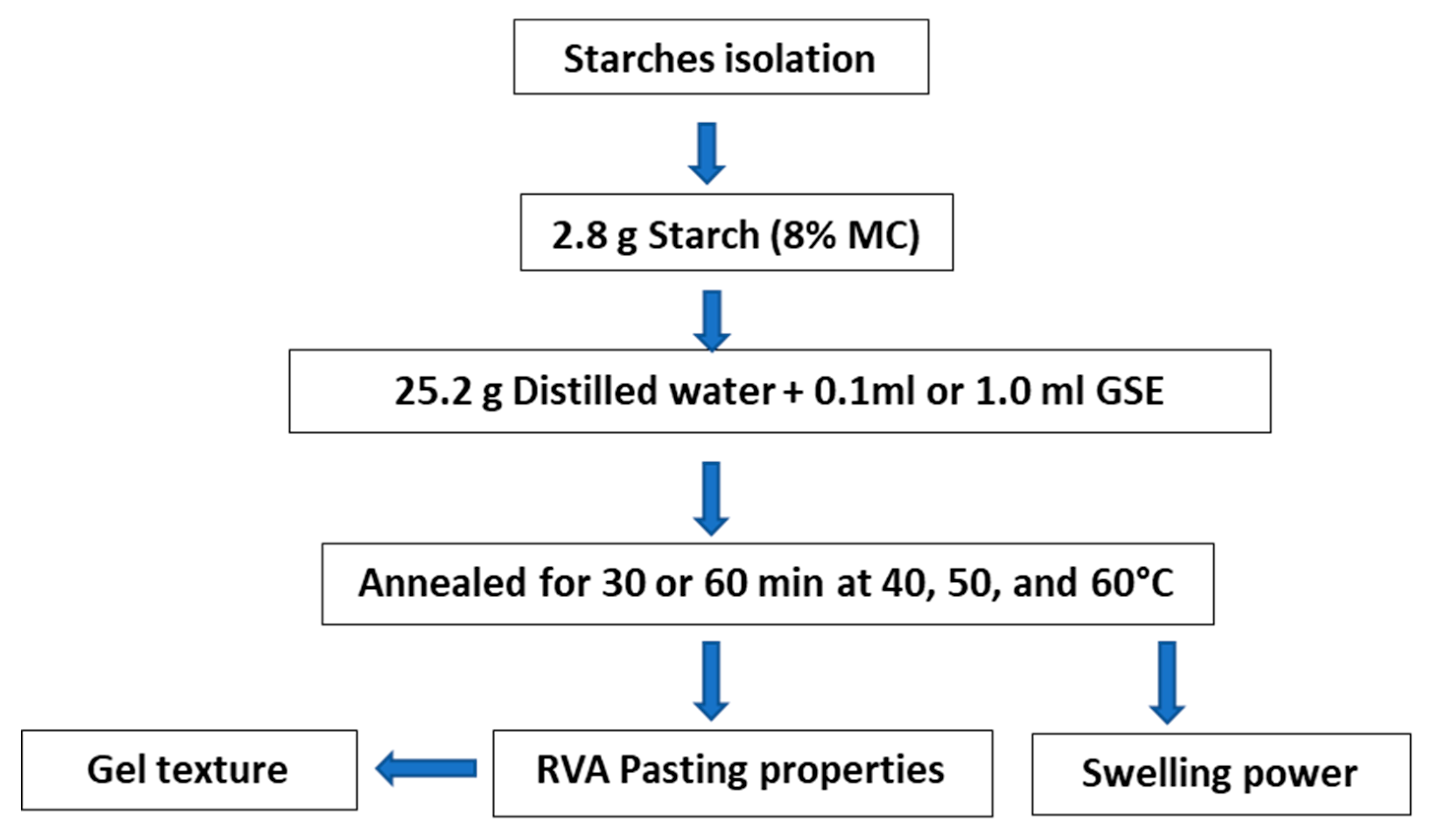
2.1. Starch Isolation
2.1.1. Chickpea and Turkish Bean Starch Isolation
2.1.2. Wheat Starches Isolation
2.1.3. Sweet Potato Starches Isolation
2.2. Amylose Content
2.3. Sorghum Germination
2.4. Annealing of Starch in Germinated Sorghum Extract (GSE)
2.5. Pasting Properties
2.6. Gel Texture
2.7. Swelling Power
2.8. Estimation of the Amount of α-Amylase in Extract
2.9. Statistical Analysis
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohyama, K.; Sasaki, T. Differential scanning calorimetry and a model calculation of starches annealed at 20 and 50 °C. Carbohydr. Polym. 2006, 63, 82–88. [Google Scholar] [CrossRef]
- Biliaderis, C.G. The structure and interactions of starch with food constituents. Can. J. Physiol. Pharmacol. 1991, 69, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Tester, R.F.; DeBon, S.J. Annealing of starch—A review. Int. J. Boil. Macromol. 2000, 27, 1–12. [Google Scholar] [CrossRef]
- Kiseleva, V.I.; Krivandin, A.V.; Fornal, J.; Błaszczak, W.; Jeliński, T.; Yuryev, V.P. Annealing of normal and mutant wheat starches. LM, SEM, DSC, and SAXS studies. Carbohydr. Res. 2005, 340, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.H.; Wong, D.W.S.; Lee, C.C.; Wagschal, K.; Smith, M.R.; Orts, W.J. Native or Raw Starch Digestion: A Key Step in Energy Efficient Biorefining of Grain. J. Agric. Food Chem. 2006, 54, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.; Pagès, G.; Gilbert, R.G.; Kuchel, P. Digestion of starch: In vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydr. Polym. 2010, 80, 599–617. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-amylase and starch digestion: An interesting marriage. Starch-Stärke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Gallant, D.J.; Bouchet, B.; Buléon, A.; Pérez, S. Physical characteristics of starch granules and susceptibility to enzymatic degradation. Eur. J. Clin. Nutr. 1992, 46, S3–S16. [Google Scholar]
- Baldwin, P.; Adler, J.; Davies, M.; Melia, C. High Resolution Imaging of Starch Granule Surfaces by Atomic Force Microscopy. J. Cereal Sci. 1998, 27, 255–265. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Tester, R.; Qi, X.; Karkalas, J. Hydrolysis of native starches with amylases. Anim. Feed. Sci. Technol. 2006, 130, 39–54. [Google Scholar] [CrossRef]
- Warren, F.J.; Butterworth, P.J.; Ellis, P.R. The surface structure of a complex substrate revealed by enzyme kinetics and Freundlich constants for α-amylase interaction with the surface of starch. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3095–3101. [Google Scholar] [CrossRef]
- Singh, N.; Sandhu, K.S.; Kaur, M. Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J. Food Eng. 2004, 63, 441–449. [Google Scholar] [CrossRef]
- Kasemsuwan, T.; Jane, J.; Schnable, P.; Stinard, P.; Robertson, D. Characterization of the dominant mutant amylose-extender (Ae1-5180) maize starch. Cereal Chem. 1995, 72. [Google Scholar]
- Sit, N.; Misra, S.; Deka, S.C. Physicochemical, functional, textural and colour characteristics of starches isolated from four taro cultivars of North-East India. Starch-Stärke 2013, 65, 1011–1021. [Google Scholar] [CrossRef]
- Williams, V.R.; Wu, W.-T.; Tsai, H.Y.; Bates, H.G. Rice Starch, Varietal Differences in Amylose Content of Rice Starch. J. Agric. Food Chem. 1958, 6, 47–48. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; El-Salam, S.M.A.; Omran, A.A. Protein Solubility, Digestibility and Fractionation after Germination of Sorghum Varieties. PLoS ONE 2012, 7, e31154. [Google Scholar] [CrossRef]
- Alamri, M.S.; Mohamed, A.A.; Hussain, S.; Almania, H.A. Legume Starches and Okra (Abelmoschus esculentus) Gum Blends: Pasting, Thermal, and Viscous Properties. Food Sci. Technol. Res. 2013, 19, 381–392. [Google Scholar] [CrossRef]
- Sandhu, K.; Singh, N. Some properties of corn starches II: Physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007, 101, 1499–1507. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; A Aparicio, M.; A Bello, L.; A Monroy, J. Changes of banana starch by chemical and physical modification. Carbohydr. Polym. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Waduge, R.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of annealing on the structure and physicochemical properties of barley starches of varying amylose content. Food Res. Int. 2006, 39, 59–77. [Google Scholar] [CrossRef]
- O’Brien, S.; Wang, Y.-J. Susceptibility of annealed starches to hydrolysis by α-amylase and glucoamylase. Carbohydr. Polym. 2008, 72, 597–607. [Google Scholar] [CrossRef]
- Zhou, Y.; Hoover, R.; Liu, Q. Relationship between α-amylase degradation and the structure and physicochemical properties of legume starches. Carbohydr. Polym. 2004, 57, 299–317. [Google Scholar] [CrossRef]
- Blazek, J.; Salman, H.; Rubio, A.L.; Gilbert, E.; Hanley, T.; Copeland, L. Structural characterization of wheat starch granules differing in amylose content and functional characteristics. Carbohydr. Polym. 2009, 75, 705–711. [Google Scholar] [CrossRef]
- Walker, G.; Hope, P.; Mould, D.L.; Thomas, G.J. The action of some α-amylases on starch granules. Biochem. J. 1963, 86, 452–462. [Google Scholar] [CrossRef]
- Leach, H.; Schoch, T. Structure of starch granule. II. Action of various amylases on starches. Cereal Chem. 1963, 38, 318–327. [Google Scholar]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch-Stärke 2013, 66, 41–57. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Effect of Enzymatic Hydrolysis on Native Starch Granule Structure. Biomacromolecules 2010, 11, 3275–3289. [Google Scholar] [CrossRef]
- Crosbie, G. The relationship between starch swelling properties, paste viscosity and boiled noodle quality in wheat flours. J. Cereal Sci. 1991, 13, 145–150. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsuki, J. Effect of Wheat Starch Structure on Swelling Power. Cereal Chem. J. 1998, 75, 525–529. [Google Scholar] [CrossRef]
- López-Rubio, A.; Flanagan, B.M.; Shrestha, A.K.; Gidley, M.J.; Gilbert, E.P. Molecular Rearrangement Of Starch During In Vitro Digestion: Toward A Better Understanding Of Enzyme Resistant Starch Formation In Processed Starches. Biomacromolecules 2008, 9, 1951–1958. [Google Scholar] [CrossRef] [PubMed]

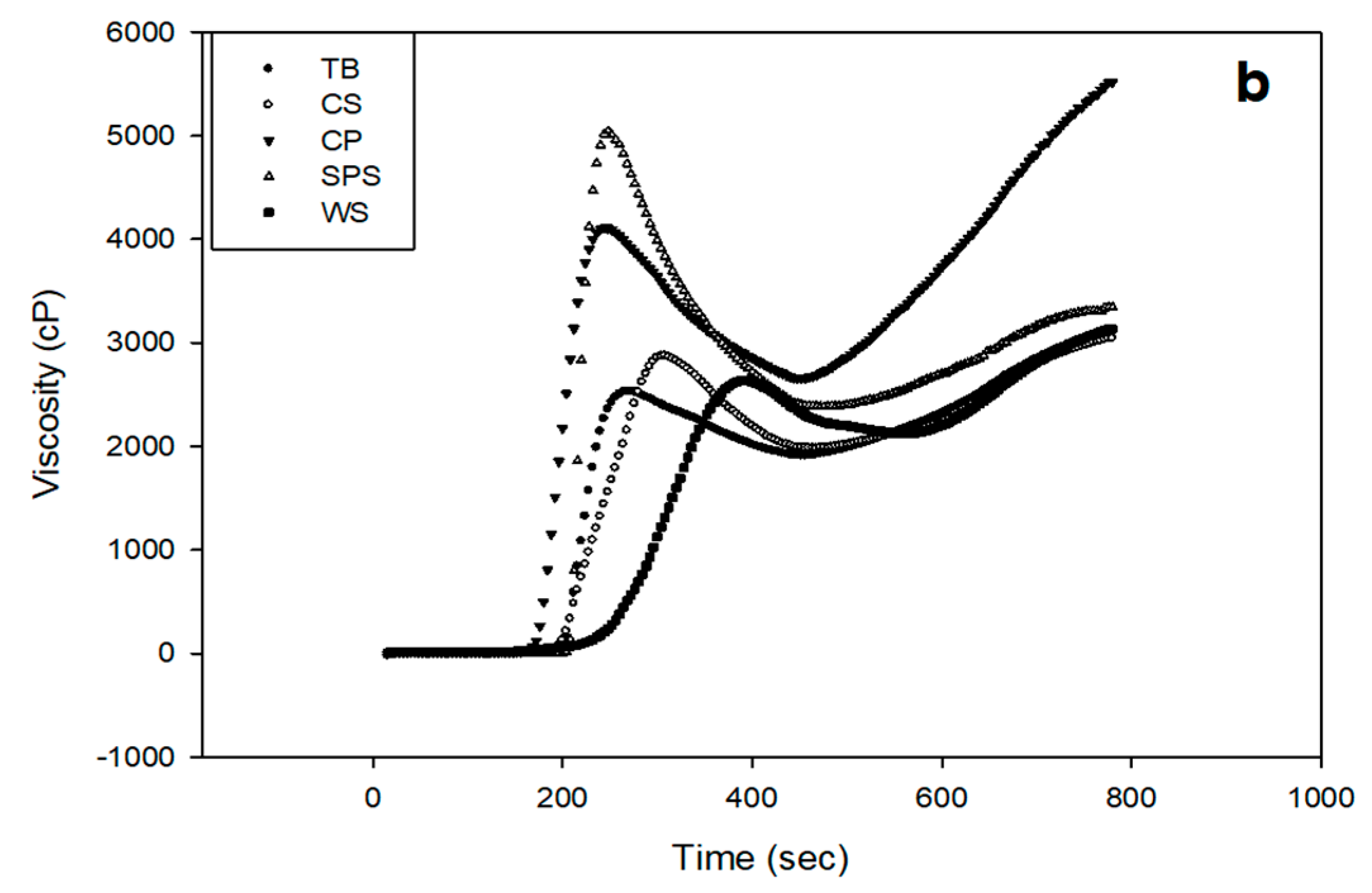
| 40 °C | ||||||
|---|---|---|---|---|---|---|
| C. P | C.S | T.B | W.S | S.P.S | ||
| 30 min | No GSE | 1 3580 b ± 78 | 2482 d ± 6 | 2504 c ± 29 | 2514 c ± 14 | 4787 a ± 72 |
| 0.1 mL | 2453 b ± 6.0 2 31.6% | 2263 c ± 20 8.8% | 2264 c ± 11 9.5% | 1307 d ± 8.0 48% | 3375 a ± 83 29.8% | |
| 1.0 mL | 851 ± 13 c 73.4% | 1348 b ± 17 49.8% | 1523 a ± 6.0 39.5% | 363 d ± 8.0 85.5% | 1549 a ± 31 67.6% | |
| 60 min | No GSE | 3858 b ± 12.3 | 2589 d ± 34.7 | 2642 c ± 15.1 | 2633 c ± 12.3 | 4989 a ± 4.9 |
| 0.1 mL | 2639 b ± 35 31.5% | 2464 c ± 31 4.8% | 2430 c ± 18 8% | 1479 d ± 8.0 43.8% | 3496 a ± 81 29.9% | |
| 1.0 mL | 1070 b ± 17 72.5% | 1510 c ± 88 41.7% | 1553 c ± 12 41.2% | 364 d ± 18 86.5% | 1562 a ± 17 68.9% | |
| 50 °C | ||||||
| 30 min | No GSE | 1 4174 b ± 10.4 | 2685 c ± 34.2 | 2539 d ± 61.2 | 2620 cd ± 21 | 5057 a ± 15.2 |
| 0.1 mL | 2944 b ± 13.5 2 29.46% | 2559 c ± 41.2 4.7% | 2319 d ± 15.6 8.66% | 1606 e ± 14 38.70% | 3739 a ± 8.6 26.06% | |
| 1.0 mL | 1698 b ± 333 59.31% | 1836 a ± 18.5 31.62% | 1686 b ± 23.1 33.59% | 368 e ± 11.1 85.95% | 1478 c ± 14.2 70.77% | |
| 60 min | No GSE | 4672 b ± 65.2 | 2707 c ± 56.2 | 2738 c ± 15.3 | 2712 c ± 10 | 5348 a ± 21.1 |
| 0.1 mL | 3309 b ± 19.3 29.17% | 2661 c ± 71.1 1.69% | 2564 d ± 8.2 6.35% | 1531 e± 23.5 43.54% | 4200 a ± 9.6 21.46% | |
| 1.0 mL | 1703 a ± 18.1 63.54% | 1658 b ± 12.6 38.75% | 1697 ab ± 4.6 38.02% | 354 d ± 7.3 86.94% | 1481 c ± 12.3 72.30% | |
| 60 °C | ||||||
| 30 min | No GSE | 1 3250 b ± 18.5 | 2824 c ± 12.1 | 2519 d ± 7.5 | Gelatinized | 5675 a ± 51 |
| 0.1 mL | 2302 c ± 69 2 29.16% | 2668 b ± 18 5.52% | 2286 d ± 40 9.24% | Gelatinized | 4269 a ± 14 24.77% | |
| 1.0 mL | 1209 d ± 8.3 3 62.8% | 2038 b ± 35 27.83% | 1711 c ± 10.6 32.07% | Gelatinized | 2457 a ± 47 56.70% | |
| 60 min | No GSE | 4689 b ± 19 | 3179 c ± 22 | 2856 d ± 14 | Gelatinized | 6122 a ± 78 |
| 0.1 mL | 3428 b ± 25 26.89% | 3010 c ± 20 5.31% | 2575 d ± 36 9.83% | Gelatinized | 4659 a ± 93 23.89% | |
| 1.0 mL | 1309 d ± 32 72.08% | 2345 b ± 24 26.23% | 1920 c ± 49 32.77% | Gelatinized | 2880 a ± 39 52.95% | |
| 40 °C | ||||||
|---|---|---|---|---|---|---|
| C.P. | C.S. | T.B. | W.S. | S.P.S. | ||
| 30 min | No GSE | 1 2553 a ± 61 | 799 d ± 4.4 | 1172 b ± 58.3 | 968 c ± 28.8 | 958 c ± 85.1 |
| 0.1 mL | 1517 a ±61.5 2 40.58% | 724 d ± 90.8 9.38% | 1105 b ±44.7 5.7 1% | 568 e ± 91.8 41.32% | 858 c ± 19.3 10.43% | |
| 1.0 mL | 280 c ± 58.5 3 89.03% | 279 c ± 41.7 65.08% | 626 a ± 2.0 46.6% | 81.3 d± 9.6 91.60% | 521.3 b ±30.4 45.58% | |
| 60 min | No GSE | 2717 a ± 49 | 922 d ± 24.5 | 1273 b ± 66.4 | 1091 c ± 69.4 | 942 d ±5 8.31 |
| 0.1 mL | 1540 a ± 1 1.4 43.32% | 771 c ± 11.1 16.37% | 1254 b ± 82.8 1.49% | 663 e ± 40.6 39.23% | 707 cd ± 87.5 24.94% | |
| 1.0 mL | 404 b ± 63.8 85.13% | 327 c ±41.8 64.53% | 686 a ± 64.6 46.11% | 46 d ± 3.46 95.78% | 388 bc ± 1.5 58.81% | |
| 50 °C | ||||||
| 30 min | No GSE | 1 3481 a ± 89 | 920 d ± 13.1 | 1254 b ± 62 | 1018 c ± 20 | 924 d ± 30.7 |
| 0.1 mL | 1539 a ± 36.5 2 55.80% | 881 d ± 49 4.23% | 1134 b ± 39.9 9.57% | 688 c ± 88.1 32.41% | 671 c ± 17.7 27.38% | |
| 1.0 mL | 711 ab ± 61.1 3 79.58% | 507 c ± 20.6 44.89% | 746 a ± 46.7 40.51% | 102 e ± 8.54 89.98% | 222 d ± 10.5 75.97% | |
| 60 min | No GSE | 3251 a ± 14 | 940 d ± 81.4 | 1319 b ± 12 | 1074 c ± 40 | 1034 c± 92 |
| 0.1 mL | 2188 a ± 15.6 32.69% | 872 c ± 53.6 7.23% | 1210 b ±11.7 8.26% | 666 d ± 70.3 37.98% | 671 d ± 25.11 35.10% | |
| 1.0 mL | 793 a ± 95.1 75.60% | 537 b ± 27.6 42.87% | 792 a ± 75.8 39.95% | 107 d ± 33.2 90.03% | 162 c ± 25.4 84.33% | |
| 60 °C | ||||||
| 30 min | No GSE | 1 1839 a ± 12 | 895 d ± 26.5 | 1222 b ± 1.7 | Gelatinized | 1011 c ± 9.7 |
| 0.1 mL | 1053 b ± 47 2 42.74% | 883 c ± 22.9 1.34% | 1152 a ± 39 5.72% | Gelatinized | 749 d ± 36.3 25.91% | |
| 1.0 mL | 413 c ±55.5 3 77.54% | 655 b ± 21.8 26.81% | 757 a ± 8.3 38.05% | Gelatinized | 376 d ± 11.6 62.80% | |
| 60 min | No GSE | 4623 a ± 46 | 922 d ± 12.4 | 1222 b ± 20 | Gelatinized | 1075 c ± 23 |
| 0.1 mL | 3561 a ± 25.9 22.97% | 884 c ± 8 4.12% | 1100 b ± 19.4 9.98% | Gelatinized | 781 d ± 6.1 27.34% | |
| 1.0 mL | 1309 a ± 32.5 71.68% | 736 c ± 13.8 20.17% | 855 b ± 82.8 30.03% | Gelatinized | 384 d ± 12.7 64.27% | |
| 40 °C | ||||||
|---|---|---|---|---|---|---|
| C.P. | C.S. | T.B. | W.S. | S.P.S. | ||
| Native | 6.04 ± 0.06 a | 4.26 ± 0.08 c | 1.83 ± 0.05 d | 1.86 ± 0.05 d | 5.49 ± 0.05 b | |
| 30 min | No GSE | 5.30 c ± 0.35 | 5.61 b ± 0.01 | 3.61 d ± 0.23 | 6.86 a ± 0.07 | 3.01 de ± 0.22 |
| 0.1 mL | 5.00 c ± 0.04 | 5.32 b ± 0.15 | 3.15 d ± 0.08 | 6.61 a ± 0.19 | 2.84 e ± 0.05 | |
| 1.0 mL | 4.82 b ± 0.47 | 4.65 c ± 0.21 | 2.53 d ± 0.03 | 5.32 a ± 2.54 | 2.40 d ± 0.06 | |
| 60 min | No GSE | 5.58 b ± 0.10 | 5.08 c ± 0.19 | 3.82 d ± 0.10 | 6.64 a ± 0.22 | 3.31 e ± 0.37 |
| 0.1 mL | 5.33 b ± 0.08 | 4.23 c ± 0.28 | 3.20 d ± 0.03 | 6.60 a ± 0.51 | 2.23 e ± 0.14 | |
| 1.0 mL | 4.82 b ± 0.13 | 4.23 cd ± 0.04 | 2.26 e ± 0.07 | 6.00 a ± 0.12 | 2.15 ef ± 0.23 | |
| 50 °C | ||||||
| 30 min | No GSE | 5.25 bc ± 0.08 | 5.54 b ± 0.11 | 2.59 d ± 0.08 | 6.60 a ± 0.29 | 2.56 d ± 0.1 |
| 0.1 mL | 5.11 b ± 0.51 | 5.09 b ± 0.44 | 1.89 d ± 0.53 | 5.24 a ± 0.09 | 2.07 c ± 0.13 | |
| 1.0 mL | 5.01 ab ± 0.55 | 4.37 c ± 0.08 | 1.79 d ± 0.10 | 5.35 a ± 0.14 | 1.96 d ± 0.14 | |
| 60 min | No GSE | 5.66 a ± 0.16 | 5.32 b ± 0.04 | 2.56 c ± 0.10 | 5.24 b ± 0.18 | 2.58 c ± 0.02 |
| 0.1 mL | 5.16 a ± 0.05 | 5.08 ab ± 0.13 | 2.79 d ± 0.10 | 4.80 c ± 0.1 | 2.09 e ± 0.08 | |
| 1.0 mL | 5.01 a ± 0.36 | 4.76 b ± 0.02 | 2.99 d ± 0.06 | 4.60 bc ± 0.35 | 1.98 e ± 0.03 | |
| 60 °C | ||||||
| 30 min | No GSE | 5.39 ab ± 0.36 | 5.54 a ± 0.07 | 2.35 c ± 0.15 | Gelatinized | 1.88 d ± 0.42 |
| 0.1 mL | 5.24 ab ± 0.07 | 5.47 a ± 0.23 | 2.38 c ± 0.02 | Gelatinized | 1.94 d ± 0.27 | |
| 1.0 mL | 5.20 ab ± 0.18 | 5.49 a ± 0.22 | 2.90 c ± 0.02 | Gelatinized | 1.99 d ± 0.27 | |
| 60 min | No GSE | 6.12 a ± 0.22 | 5.64 b ± 0.13 | 2.25 c ± 0.12 | Gelatinized | 2.25 c ± 0.24 |
| 0.1 mL | 5.91 a ± 0.76 | 5.33 b ± 0.08 | 2.37 c ± 0.19 | Gelatinized | 2.15 cd ± 0.21 | |
| 1.0 mL | 5.50 a ± 0.09 | 4.82 b ± 0.05 | 2.25 c ± 0.19 | Gelatinized | 2.34 c ± 0.15 | |
| 40 °C | ||||||
|---|---|---|---|---|---|---|
| C.P. | C.S. | T.B. | W.S. | S.P.S. | ||
| 30 min | No GSE | 677 a ± 0.4 | 136 c ± 0.1 | 481.7 b ± 23 | 141 c ± 1.3 | 80 d ± 0.1 |
| 0.1 mL | 670 a ± 0.2 | 161 c ± 0.2 | 479 b ± 8.1 | 98 d ± 2.4 | 119 c ± 0.1 | |
| 1.0 mL | 544 a ± 0.1 | 68.7 d ± 0.7 | 307 b ± 14.2 | 18.7 e ± 0.4 | 194 c ± 0.2 | |
| 60 min | No GSE | 657 a ± 0.8 | 140.7 c ± 8.2 | 568 b ± 9.1 | 128.7 c ± 0.8 | 87 d ± 0.9 |
| 0.1 mL | 646 a ± 0.1 | 106 c ± 1.3 | 468 a ± 1.1 | 102 c ± 0.6 | 127 b ± 0.4 | |
| 1.0 mL | 242 b ± 4.2 | 102 d ± 2.6 | 320 a ± 0.8 | 12.7 e ± 0.1 | 195 c ± 0.3 | |
| 50 °C | ||||||
| 30 min | No GSE | 721 a ± 0.45 | 162 c ± 1.1 | 588 b ± 4.1 | 157 c ± 11.2 | 156 c ± 2.3 |
| 0.1 mL | 698 a ± 1.2 | 145 d ± 12.1 | 503 b ± 12.3 | 112 d ± 13.2 | 198 c ± 1.6 | |
| 1.0 mL | 274 b ± 1.2 | 144 c ± 3.1 | 363 a ± 16.1 | 19 d ± 4.5 | 222 b ± 2.5 | |
| 60 min | No GSE | 730 a ± 0.9 | 144 c ± 1.2 | 467 b ± 3.8 | 160 c ± 61 | 127 c ± 3.1 |
| 0.1 mL | 750 a ±2.1 | 149 c ± 2.3 | 562 b ± 21.3 | 117 c ± 1.2 | 144 c ± 2.6 | |
| 1.0 mL | 334 b ± 23.1 | 172 c ± 1.1 | 402 a ± 9.1 | 19 d ± 3.8 | 161 c ± 1.2 | |
| 60 °C | ||||||
| 30 min | No GSE | 580 a ± 12.3 | 178 b ± 2.5 | 579 a ± 2.3 | Gelatinized | 115 bc ± 2.1 |
| 0.1 mL | 361 b ± 8.2 | 171 c ± 1.5 | 432 a ± 1.2 | Gelatinized | 137 c ± 1.1 | |
| 1.0 mL | 224 a ± 4.5 | 170 c ± 4.6 | 254 a ± 2.1 | Gelatinized | 210 bc ± 1.3 | |
| 60 min | No GSE | 554 a ± 6.1 | 185 b ± 1.2 | 590 a ± 3.2 | Gelatinized | 109 c ± 3.2 |
| 0.1 mL | 367 b ± 1.4 | 183 c ± 1.4 | 471 a ± 1.6 | Gelatinized | 130 c ± 2.5 | |
| 1.0 mL | 260.7 b ± 3.4 | 146.7 cd ± 2.6 | 296 a ± 4.2 | Gelatinized | 181 c ± 1.3 | |
| 40 °C | ||||||
|---|---|---|---|---|---|---|
| C.P. | C.S. | T.B. | W.S. | S.P.S. | ||
| 30 min | No GSE | 0.54 b ± 0.06 | 0.64 a ± 0.08 | 0.44 c ± 0.06 | 0.38 d ± 0.09 | 0.47 c ± 0.07 |
| 0.1 mL | 0.50 ab ± 0.03 | 0.48 b ± 0.01 | 0.40 bc ± 0.06 | 0.59 a ± 0.06 | 0.39 c ± 0.06 | |
| 1.0 mL | 0.48 b ± 0.01 | 0.47 b ± 0.03 | 0.40 bc ± 0.04 | 0.57 a ± 0.03 | 0.33 d ± 0.03 | |
| 60 min | No GSE | 0.45 c ± 0.1 | 0.48 c ± 0.05 | 0.41 d ± 0.1 | 0.55 a ± 0.07 | 0.50 b ± 0.1 |
| 0.1 mL | 0.42 c ± 0.05 | 0.48 b ± 0.02 | 0.41 c ± 0.08 | 0.55 a ± 0.01 | 0.48 b ± 0.08 | |
| 1.0 mL | 0.37 c ± 0.03 | 0.50 b ± 0.01 | 0.35 c ± 0.04 | 0.58 a ± 0.01 | 0.33 d ± 0.06 | |
| 50 °C | ||||||
| 30 min | No GSE | 0.48 a ± 0.01 | 0.48 a ± 0.03 | 0.39 b ± 0.06 | 0.46 a ± 0.03 | 0.46 a ± 0.01 |
| 0.1 mL | 0.35 d ± 0.06 | 0.49 b ± 0.01 | 0.39 c ± 0.09 | 0.60 a ± 0.02 | 0.45 b ± 0.04 | |
| 1.0 mL | 0.39 b ± 0.01 | 0.40 b ± 0.05 | 0.37 c ± 0.02 | 0.50 a ± 0.03 | 0.42 b ± 0.14 | |
| 60 min | No GSE | 0.41 cd ± 0.04 | 0.61 a ± 0.2 | 0.49 c ± 0.17 | 0.48 c ± 0.03 | 0.55 b ± 0.02 |
| 0.1 mL | 0.42 b ± 0.07 | 0.43 b ± 0.01 | 0.39 c ± 0.06 | 0.53 a ± 0.02 | 0.52 a ± 0.08 | |
| 1.0 mL | 0.40 c ± 0.06 | 0.51 b ± 0.01 | 0.40 c ± 0.13 | 0.79 a ± 0.14 | 0.49 b ± 0.13 | |
| 60 °C | ||||||
| 30 min | No GSE | 0.37 c ± 0.1 | 0.52 a ± 0.2 | 0.40 b ± 0.07 | Gelatinized | 0.43 b ± 0.02 |
| 0.1 mL | 0.26 c ± 0.2 | 0.50 a ± 0.1 | 0.51 a ± 0.08 | Gelatinized | 0.42 b ± 0.07 | |
| 1.0 mL | 0.42 c ± 0.2 | 0.56 b ± 0.6 | 0.68 a ± 0.15 | Gelatinized | 0.34 d ± 0.05 | |
| 60 min | No GSE | 0.45 b ± 0.02 | 0.43 c ± 0.3 | 0.41 c ± 0.12 | Gelatinized | 0.50 a ± 0.02 |
| 0.1 mL | 0.45 a ± 0.09 | 0.47 a ± 0.04 | 0.42 a ± 0.07 | Gelatinized | 0.41 ab ± 0.05 | |
| 1.0 mL | 0.49 a ± 0.02 | 0.40 b ± 0.08 | 0.45 a ± 0.08 | Gelatinized | 0.37 c ± 0.03 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqah, H.; Alamri, M.S.; Mohamed, A.A.; Hussain, S.; Qasem, A.A.; Ibraheem, M.A.; Ababtain, I.A. The Effect of Germinated Sorghum Extract on the Pasting Properties and Swelling Power of Different Annealed Starches. Polymers 2020, 12, 1602. https://doi.org/10.3390/polym12071602
Alqah H, Alamri MS, Mohamed AA, Hussain S, Qasem AA, Ibraheem MA, Ababtain IA. The Effect of Germinated Sorghum Extract on the Pasting Properties and Swelling Power of Different Annealed Starches. Polymers. 2020; 12(7):1602. https://doi.org/10.3390/polym12071602
Chicago/Turabian StyleAlqah, Hesham, M. S. Alamri, A. A. Mohamed, S. Hussain, A. A. Qasem, M. A. Ibraheem, and I. A. Ababtain. 2020. "The Effect of Germinated Sorghum Extract on the Pasting Properties and Swelling Power of Different Annealed Starches" Polymers 12, no. 7: 1602. https://doi.org/10.3390/polym12071602
APA StyleAlqah, H., Alamri, M. S., Mohamed, A. A., Hussain, S., Qasem, A. A., Ibraheem, M. A., & Ababtain, I. A. (2020). The Effect of Germinated Sorghum Extract on the Pasting Properties and Swelling Power of Different Annealed Starches. Polymers, 12(7), 1602. https://doi.org/10.3390/polym12071602






