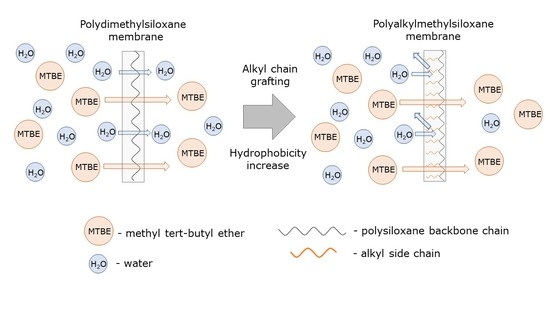
High Selective Composite Polyalkylmethylsiloxane Membranes for Pervaporative Removal of MTBE from Water: Effect of Polymer Side-chain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
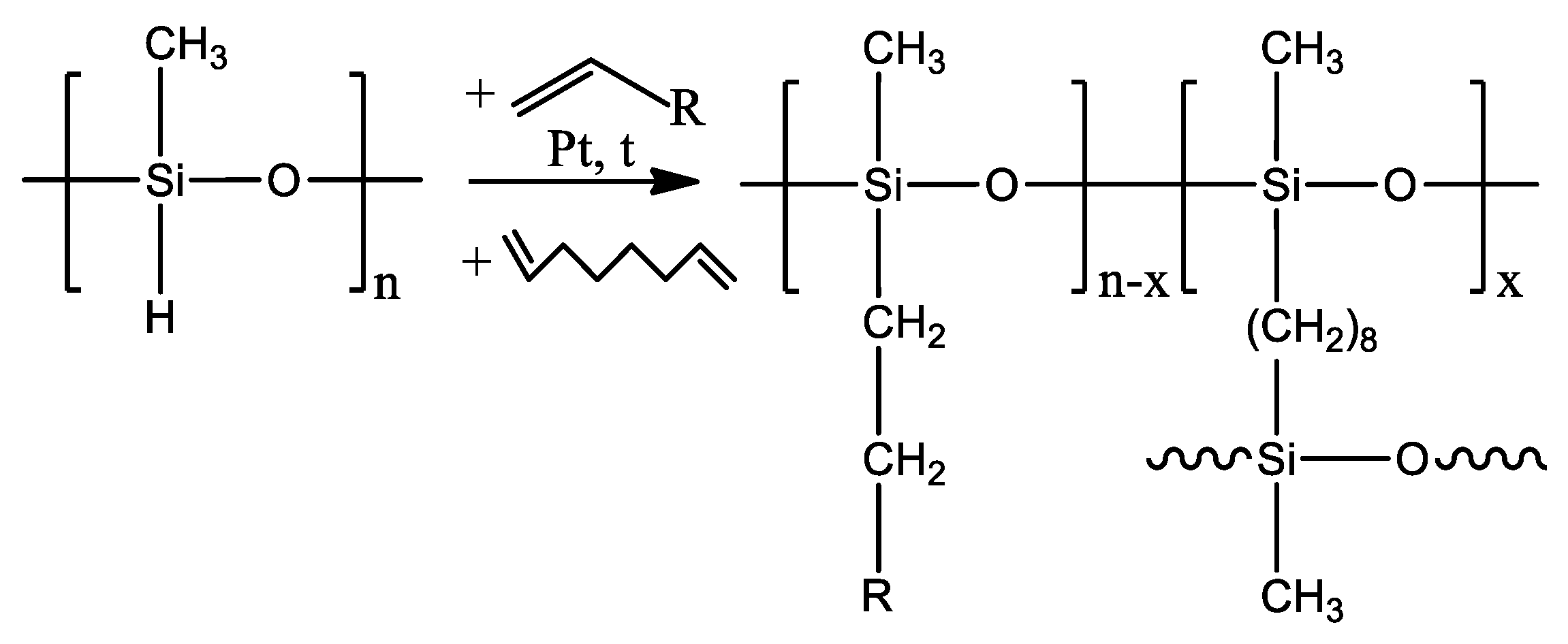
2.2. Synthesis of Polyorganosiloxanes and Preparation of Dense Membranes
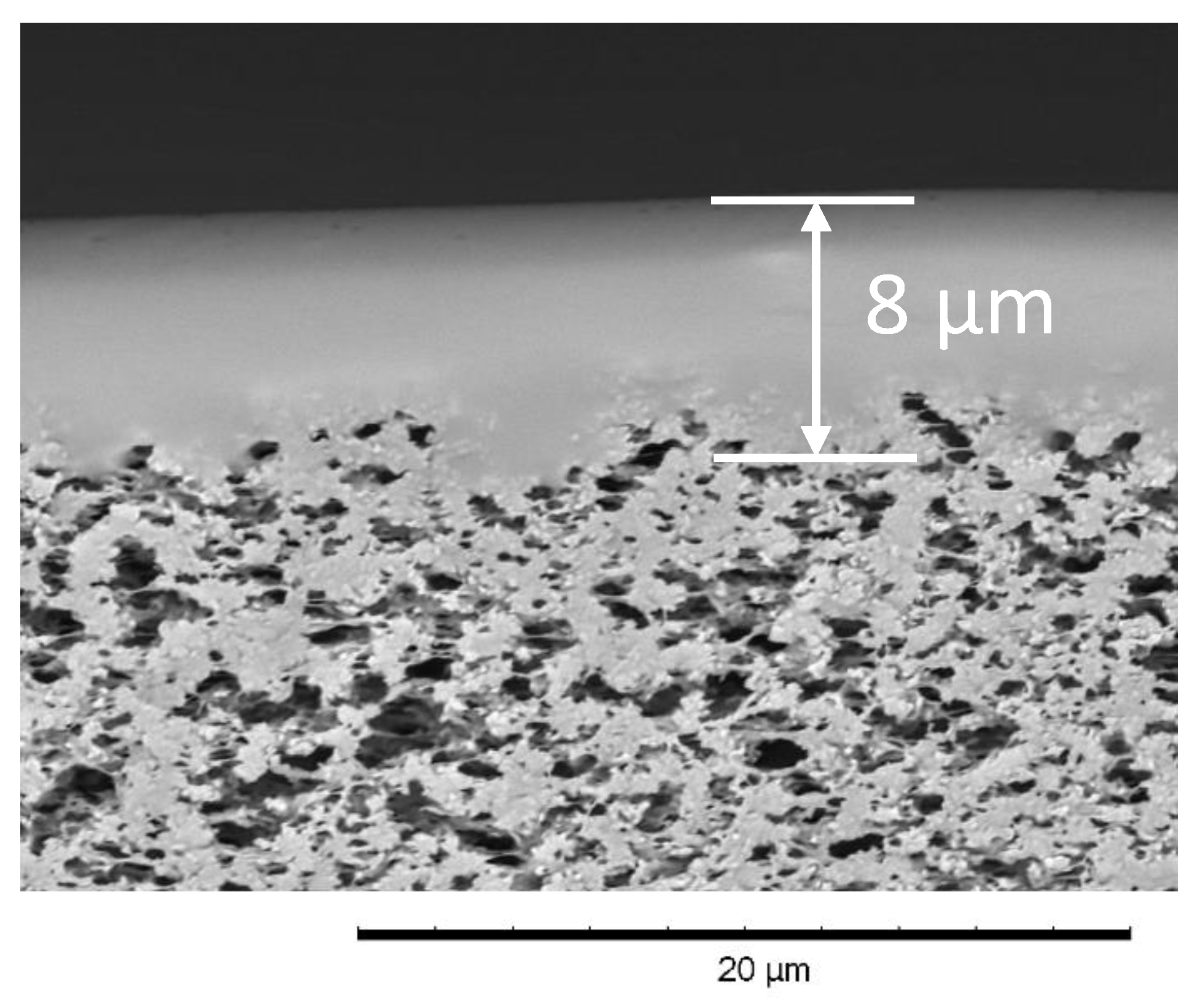
2.3. Composite Membrane Preparation
2.4. Differential Scanning Calorimetry
2.5. Vacuum Pervaporation
2.6. Process Parameters Calculation
3. Results and Discussion
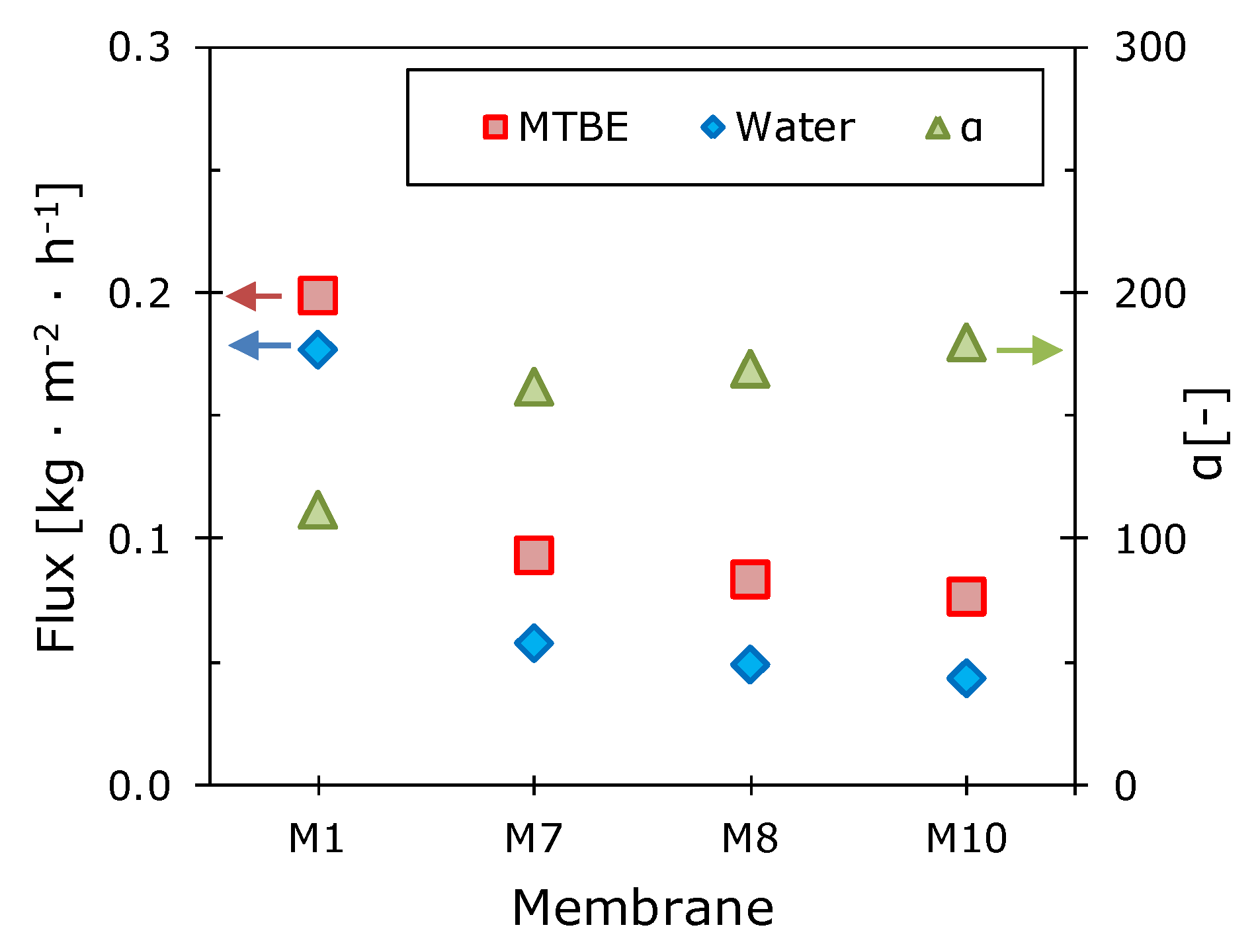
3.1. Effect of Side-Chain Length on the Pervaporation Characteristics of Polyalkylmethylsiloxanes
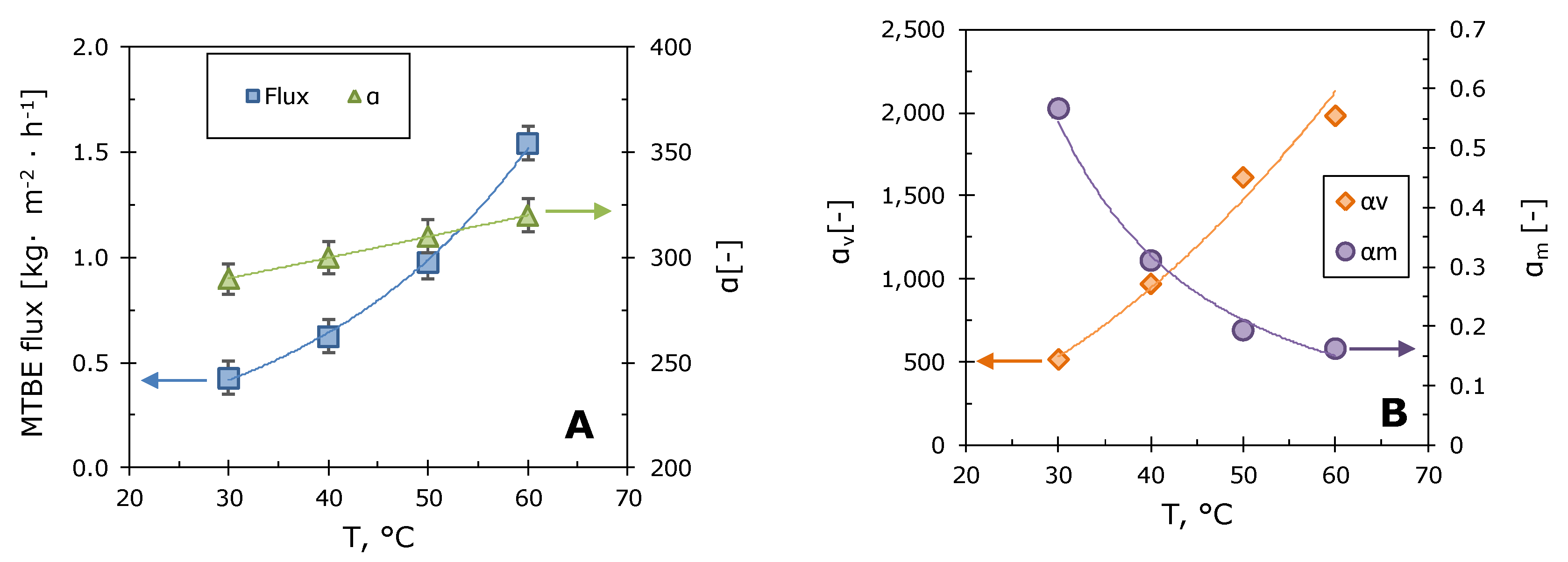
3.2. Pervaporation Characteristics of the Composite Membrane M10/MFFK for MTBE Removal
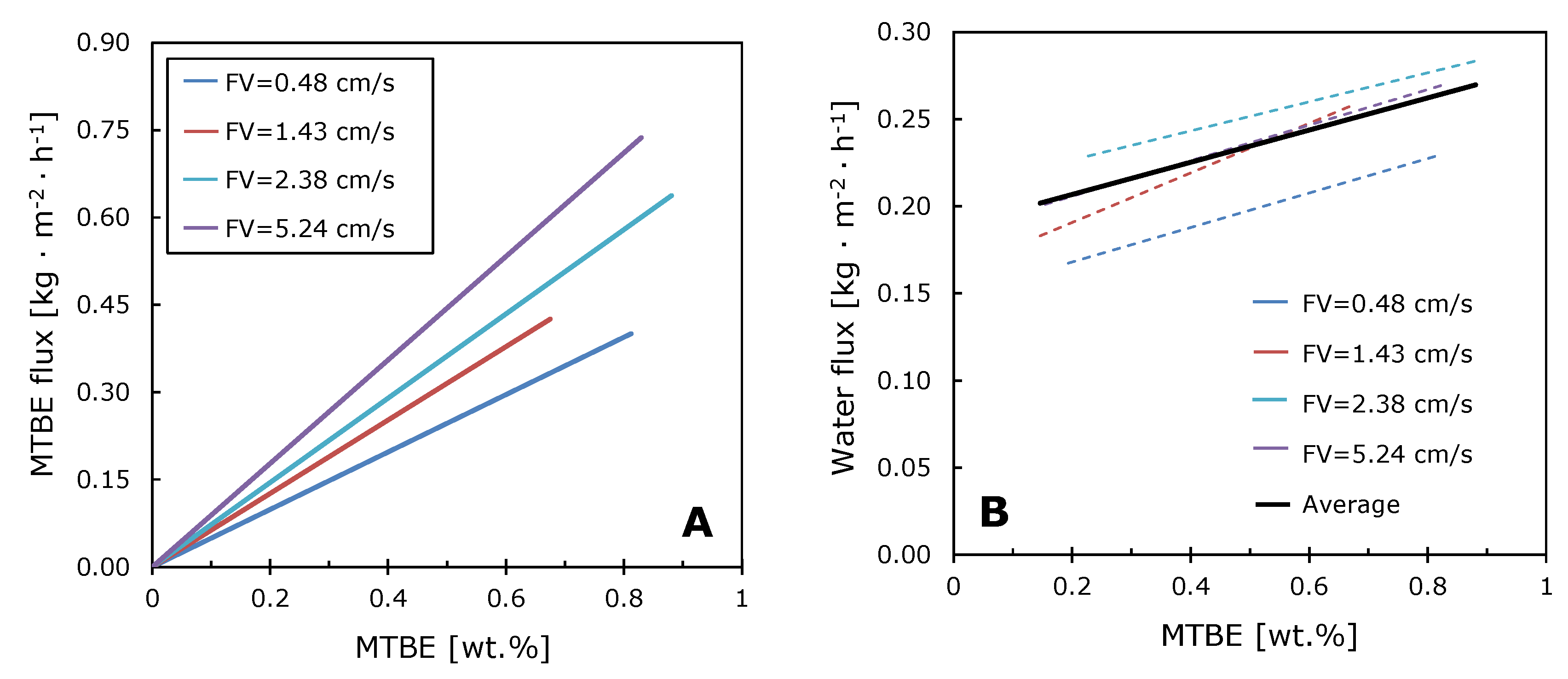
3.3. Concentration Polarization in the Pervaporative Separation of MTBE–Water Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collignon, F.; Loenders, R.; Martens, J.A.; Jacobs, P.A.; Poncelet, G. Liquid phase synthesis of MTBE from methanol andisobutene over acid zeolites and amberlyst-15. J. Catal. 1999, 182, 302–312. [Google Scholar] [CrossRef]
- Salomón, M.A.; Coronas, J.; Menéndez, M.; Santamarı’ıa, J. Synthesis of MTBE in zeolite membrane reactors. Appl. Catal. A Gen. 2000, 200, 201–210. [Google Scholar] [CrossRef]
- Kolb, A.; Püttmann, W. Methyl tert-butyl ether (MTBE) in finished drinking water in Germany. Environ. Pollut. 2006, 140, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Kolb, A.; Püttmann, W. Methyl tert-butyl ether (MTBE) in snow samples in Germany. Atmos. Environ. 2006, 40, 76–86. [Google Scholar] [CrossRef]
- Rosell, M.; Lacorte, S.; Barceló, D. Analysis, occurrence and fate of MTBE in the aquatic environment over the past decade. TrAC Trends Anal. Chem. 2006, 25, 1016–1029. [Google Scholar] [CrossRef]
- Levchuk, I.; Bhatnagar, A.; Sillanpää, M. Overview of technologies for removal of methyl tert-butyl ether (MTBE) from water. Sci. Total Environ. 2014, 476, 415–433. [Google Scholar] [CrossRef]
- Garrett, P.; Moreau, M.; Lowry, J.D. MTBE as a ground water contaminant. In Proceedings of the NWWA-API Conference on Petroleum Hydrocarbons and Organic Chemicals in Ground Water-Prevention, Detection and Restoration, Houston, TX, USA, , 13–15 November 1986; pp. 227–238. [Google Scholar]
- Chen, C.S.; Hseu, Y.C.; Liang, S.H.; Kuo, J.Y.; Chen, S.C. Assessment of genotoxicity of methyl-tert-butyl ether, benzene, toluene, ethylbenzene, and xylene to human lymphocytes using comet assay. J. Hazard Mater. 2008, 153, 351–356. [Google Scholar] [CrossRef]
- Maretto, M.; Blanchi, F.; Vignola, R.; Canepari, S.; Baric, M.; Iazzoni, R.; Tagliabue, M.; Papini, M.P. Microporous and mesoporous materials for the treatment of waste-water produced by petrochemical activities. J. Clean. Prod. 2014, 77, 22–34. [Google Scholar] [CrossRef]
- Sutherland, J.; Adams, C.; Kekobad, J. Treatment of MTBE by air stripping, carbon adsorption, and advanced oxidation: Technical and economic comparison for five groundwaters. Water Res. 2004, 38, 193–205. [Google Scholar] [CrossRef]
- Rossner, A.; Knappe, D.R. MTBE adsorption on alternative adsorbents and packed bed adsorber performance. Water Res. 2008, 42, 2287–2299. [Google Scholar] [CrossRef]
- Hassen, J.A.; Gross, C.P. MTBE: Groundwater remediation technologies. Remediat. J. 2000, 10, 129–139. [Google Scholar] [CrossRef]
- Shih, T.; Wangpaichitr, M.; Suffet, M. Performance and cost evaluations of synthetic resin technology for the removal of methyl tert-butyl ether from drinking water. J. Environ. Eng. 2005, 131, 450–460. [Google Scholar] [CrossRef]
- Pulyalina, A.; Tataurov, M.; Faykov, I.; Rostovtseva, V.; Polotskaya, G. Polyimide Asymmetric Membrane vs. Dense Film for Purification of MTBE Oxygenate by Pervaporation. Symmetry 2020, 12, 436. [Google Scholar] [CrossRef] [Green Version]
- Deeb, R.A.; Scow, K.M.; Alvarez-Cohen, L. Aerobic MTBE biodegradation: An examination of past studies, current challenges and future research directions. Biodegradation 2000, 11, 171–185. [Google Scholar] [CrossRef]
- Stocking, A.J.; Deeb, R.A.; Flores, A.E.; Stringfellow, W.; Talley, J.; Brownell, R.; Kavanaugh, M.C. Bioremediation of MTBE: A review from a practical perspective. Biodegradation 2000, 11, 187–201. [Google Scholar] [CrossRef]
- Suflita, J.M.; Mormile, M.R. Anaerobic biodegradation of known and potential gasoline oxygenates in the terrestrial subsurface. Environ. Sci. Technol. 1993, 27, 976–978. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Butanol fermentation research: Upstream and downstream manipulations. Chem. Rec. 2004, 4, 305–314. [Google Scholar] [CrossRef]
- Liu, G.; Wei, W.; Jin, W. Pervaporation membranes for biobutanol production. ACS Sustain. Chem. Eng. 2014, 2, 546–560. [Google Scholar] [CrossRef]
- Ikegami, T.; Yanagishita, H.; Kitamoto, D.; Haraya, K.; Nakane, T.; Matsuda, H.; Koura, N.; Sano, T. Production of highly concentrated ethanol in a coupled fermentation/pervaporation process using silicalite membranes. Biotechnol. Technol. 1997, 11, 921–924. [Google Scholar] [CrossRef]
- Rozicka, A.; Niemistö, J.; Keiski, R.L.; Kujawski, W. Apparent and intrinsic properties of commercial PDMS based membranes in pervaporative removal of acetone, butanol and ethanol from binary aqueous mixtures. J. Membr. Sci. 2014, 453, 108–118. [Google Scholar] [CrossRef]
- Rom, A.; Friedl, A. Investigation of pervaporation performance of POMS membrane during separation of butanol from water and the effect of added acetone and ethanol. Sep. Purif. Technol. 2016, 170, 40–48. [Google Scholar] [CrossRef]
- Borisov, I.L.; Ushakov, N.V.; Volkov, V.V.; Finkel’shtein, E.S. Polydimethylsilalkylene-dimethylsiloxanes as advanced membrane materials for thermopervaporative recovery of oxygenates from aqueous reaction media. Pet. Chem. 2016, 56, 798–804. [Google Scholar] [CrossRef]
- Žák, M.; Klepic, M.; Štastná, L.C.; Sedláková, Z.; Vychodilová, H.; Hovorka, S.; Friess, K.; Randová, A.; Brozˇová, L.; Jansen, J.C.; et al. Selective removal of butanol from aqueous solution by pervaporation with a PIM-1 membrane and membrane aging. Sep. Purif. Technol. 2015, 151, 108–114. [Google Scholar] [CrossRef]
- Borisov, I.L.; Malakhov, A.O.; Khotimsky, V.S.; Litvinova, E.G.; Finkelshtein, E.S.; Ushakov, N.V.; Volkov, V.V. Novel PTMSP-based membranes containing elastomeric fillers: Enhanced 1-butanol/water pervaporation selectivity and permeability. J. Membr. Sci. 2014, 466, 322–330. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, K.S.; Galebach, P.; Gilbert, C.; Tompsett, G.; Conner, W.C.; Jones, C.W.; Nair, S. Seeded growth, silylation, and organic/water separation properties of MCM-48 membranes. J. Membr. Sci. 2013, 427, 293–302. [Google Scholar] [CrossRef]
- Kanemoto, M.; Negishi, H.; Sakaki, K.; Ikegami, T.; Chohnan, S.; Nitta, Y.; Ohta, H. Efficient butanol recovery from acetone-butanol-ethanol fermentation cultures grown on sweet sorghum juice by pervaporation using silicalite-1 membrane. J. Biosci. Bioeng. 2016, 121, 697–700. [Google Scholar] [CrossRef]
- Pulyalina, A.; Faykov, I.; Nesterova, V.; Goikhman, M.; Podeshvo, I.; Loretsyan, N.; Novikov, A.; Gofman, I.; Toikka, A.; Polotskaya, G. Novel Polyester Amide Membranes Containing Biquinoline Units and Complex with Cu (I): Synthesis, Characterization, and Approbation for n-Heptane Isolation from Organic Mixtures. Polymers 2020, 12, 645. [Google Scholar] [CrossRef] [Green Version]
- Polotskaya, G.A.; Pulyalina, A.Y.; Goikhman, M.Y.; Podeshvo, I.V.; Valieva, I.A.; Toikka, A.M. Aromatic Copolyamides with Anthrazoline Units in the Backbone: Synthesis, Characterization, Pervaporation Application. Polymers 2016, 8, 362. [Google Scholar] [CrossRef] [Green Version]
- Pulyalina, A.Y.; Polotskaya, G.A.; Toikka, A.M. Membrane materials based on polyheteroarylenes and their application for pervaporation. Russ. Chem. Rev. 2016, 85, 81–98. [Google Scholar] [CrossRef]
- Vane, L.M.; Alvarez, F.R.; Mullins, B. Removal of methyl tert-butyl ether from water by pervaporation: Bench-and pilot-scale evaluations. Environ. Sci. Technol. 2001, 35, 391–397. [Google Scholar] [CrossRef]
- Vane, L.M. Pervaporation and vapor permeation tutorial: Membrane processes for the selective separation of liquid and vapor mixtures. Sep. Sci. Technol. 2013, 48, 429–437. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Highly hydrophobic ceramic membranes applied to the removal of volatile organic compounds in pervaporation. Chem. Eng. J. 2015, 260, 43–54. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Removal of hazardous volatile organic compounds from water by vacuum pervaporation with hydrophobic ceramic membranes. J. Membr. Sci. 2015, 474, 11–19. [Google Scholar] [CrossRef]
- Metz, S.J.; Van de Ven, W.J.C.; Potreck, J.; Mulder, M.H.V.; Wessling, M. Transport of water vapor and inert gas mixtures through highly selective and highly permeable polymer membranes. J. Membr. Sci. 2005, 251, 29–41. [Google Scholar] [CrossRef]
- Jullok, N.; Martínez, R.; Wouters, C.; Luis, P.; Sanz, M.T.; Van der Bruggen, B. A biologically inspired hydrophobic membrane for application in pervaporation. Langmuir 2013, 29, 1510–1516. [Google Scholar] [CrossRef]
- Petrusová, Z.; Machanová, K.; Stanovský, P.; Izák, P. Separation of organic compounds from gaseous mixtures by vapor permeation. Sep. Purif. Technol. 2019, 217, 95–107. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Podtynnikov, I.A.; Golubev, G.S.; Volkov, V.V.; Borisov, I.L. Polyheptylmethylsiloxane—A novel material for removal of oxygenates from water by pervaporation. Pet. Chem. 2018, 58, 941–948. [Google Scholar] [CrossRef]
- Börjesson, J.; Karlsson, H.O.; Trägårdh, G. Pervaporation of a model apple juice aroma solution: Comparison of membrane performance. J. Membr. Sci. 1996, 119, 229–239. [Google Scholar] [CrossRef]
- Van Hecke, W.; De Wever, H. High-flux POMS organophilic pervaporation for ABE recovery applied in fed-batch and continuous set-ups. J. Membr. Sci. 2017, 540, 321–332. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Athayde, A.L.; Daniels, R.; Ly, J.H.; Le, M. The effect of concentration polarization on the separation of volatile organic compounds from water by pervaporation. J. Membr. Sci. 1997, 137, 159–172. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Athayde, A.L.; Daniels, R.; Ly, J.H.; Kamaruddin, H.D.; Pinnau, I. The role of boundary layers in the removal of volatile organic compounds from water by pervaporation. J. Membr. Sci. 1996, 109, 135–146. [Google Scholar] [CrossRef]
- Borisov, I.L.; Kujawska, A.; Knozowska, K.; Volkov, V.V.; Kujawski, W. Influence of feed flow rate, temperature and feed concentration on concentration polarization effects during separation of water-methyl acetate solutions with high permeable hydrophobic pervaporation PDMS membrane. J. Membr. Sci. 2018, 564, 1–9. [Google Scholar] [CrossRef]
- Vane, L.M.; Alvarez, F.R. Vibrating pervaporation modules: Effect of module design on performance. J. Membr. Sci. 2005, 255, 213–224. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Bakhtin, D.S.; Legkov, S.A.; Bondarenko, G.N.; Volkov, A.V. Membrane material based on octyl-substituted polymethylsiloxane for separation of C3/C1 hydrocarbons. Pet. Chem. 2017, 57, 334–340. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Bakhtin, D.S.; Bondarenko, G.N.; Levin, I.S.; Volkov, A.V. Silicone rubbers with alkyl side groups for C3+ hydrocarbon separation. React. Funct. Polym. 2019, 134, 156–165. [Google Scholar] [CrossRef]
- Tucker, W.A.; Nelken, L.H. Diffusion Coefficients in Air and Water. In Handbook of Chemical Property Estimation Methods: Environmental Behavior of Organic Compounds; Lyman, W.J., Reehl, W.F., Rosenblatt, D.H., Eds.; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Hansen, C.M. The three-dimensional solubility parameter—Key to paint component affinities: Solvents, plasticizers, polymers, and resins. J. Paint Technol. 1967, 39, 104–117. [Google Scholar]
- Fitch, M.W.; Koros, W.J.; Nolen, R.L.; Carnes, J.R. Permeation of several gases through elastomers, with emphasis on the D2/H2 pair. J. Appl. Polym. Sci. 1993, 47, 1033–1046. [Google Scholar] [CrossRef]
- Charati, S.G.; Stern, S.A. Diffusion of gases in silicone polymers: Molecular dynamics simulations. Macromolecules 1998, 31, 5529–5535. [Google Scholar] [CrossRef]
- Ogden, M.D.; Orme, C.J.; Stewart, F.F. Effects of alkyl substitution on the physical properties and gas transport behavior in selected poly (R-phenoxyphosphazenes). Polymer 2011, 52, 3879–3886. [Google Scholar] [CrossRef]
- Schultz, J.; Peinemann, K.-V. Membranes for separation of higher hydrocarbons from methane. J. Membr. Sci. 1996, 110, 37–45. [Google Scholar] [CrossRef]
- Penkova, A.V.; Polotskaya, G.A.; Toikka, A.M. Separation of acetic acid-methanol-methyl acetate-water reactive mixture. Chem. Eng. Sci. 2013, 101, 586–592. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Removal of methyl tert-butyl ether from water by pervaporation using ceramic-supported polymer membranes. J. Membr. Sci. 2004, 229, 27–32. [Google Scholar] [CrossRef]
- Kujawski, W.; Roszak, R. Pervaporative removal of volatile organic compounds from multicomponent aqueous mixtures. Sep. Sci. Technol. 2002, 37, 3559–3575. [Google Scholar] [CrossRef]
- Raisi, A.; Aroujalian, A.; Kaghazchi, T. Multicomponent pervaporation process for volatile aroma compounds recovery from pomegranate juice. J. Membr. Sci. 2008, 322, 339–348. [Google Scholar] [CrossRef]
- Yakovlev, A.V.; Shalygin, M.G.; Matson, S.M.; Khotimskiy, V.S.; Teplyakov, V.V. Separation of diluted butanol-water solutions via vapor phase by organophilic membranes based on high permeable polyacetylenes. J. Membr. Sci. 2013, 434, 99–105. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. A simple predictive treatment of the permeation process in pervaporation. J. Membr. Sci. 1993, 79, 101–113. [Google Scholar] [CrossRef]
- Borisov, I.L.; Golubev, G.S.; Vasilevsky, V.P.; Volkov, A.V.; Volkov, V.V. Novel hybrid process for bio-butanol recovery: Thermopervaporation with porous condenser assisted by phase separation. J. Membr. Sci. 2017, 523, 291–300. [Google Scholar] [CrossRef]
- Kujawski, W. Pervaporative removal of organics from water using hydrophobic membranes. Binary mixtures. Sep. Sci. Technol. 2000, 35, 89–108. [Google Scholar] [CrossRef]
- Zhmakin, V.V.; Teplyakov, V.V. The evaluation of the C1–C4 hydrocarbon permeability parameters in the thin film composite membranes. Sep. Purif. Technol. 2017, 186, 145–155. [Google Scholar] [CrossRef]
- Pinnau, I.; He, Z. Pure-and mixed-gas permeation properties of polydimethylsiloxane for hydrocarbon/methane and hydrocarbon/hydrogen separation. J. Membr. Sci. 2004, 244, 227–233. [Google Scholar] [CrossRef]
- Park, Y.I.; Yeom, C.K.; Kim, B.S.; Suh, J.K.; Hong, J.S.; Lee, J.M.; Joo, H.J. Quantitative evaluation of concentration polarization in the permeation of VOCs/water mixtures through PDMS membrane using model equation. Desalination 2008, 233, 303–309. [Google Scholar] [CrossRef]
- Feng, X.S.; Huang, R.Y.M. Concentration polarization in pervaporation separation processes. J. Membr. Sci. 1994, 92, 201–208. [Google Scholar] [CrossRef]
- Karlsson, H.O.; Trägårdh, G. Aroma compound recovery with pervaporation-feed flow effects. J. Membr. Sci. 1993, 81, 163–171. [Google Scholar] [CrossRef]
- She, M.; Hwang, S.T. Concentration of dilute flavor compounds by pervaporation: Permeate pressure effect and boundary layer resistance modeling. J. Membr. Sci. 2004, 236, 193–202. [Google Scholar] [CrossRef]






| Membrane Symbol | Polymer | Polymer Abbreviation | Modifier | Tg, °C |
|---|---|---|---|---|
| M1 | polydimethylsiloxane | PDMS | - | −123 |
| M7 | polyheptylmethylsiloxane | PHepMS | 1-hepten | −99 |
| M8 | polyoctylmethylsiloxane | POMS | 1-octen | −93 |
| M10 | polydecylmethylsiloxane | PDecMS | 1-decen | −68 |
| Properties of Solvents | Water | MTBE | ||
|---|---|---|---|---|
| Molecular weight [g mol−1] | 18.01 | 88.15 | ||
| Molecular volume [ml mol−1] | 18 | 119 | ||
| Boiling point, °C | 100.0 | 55.2 | ||
| Kinetic diameter, Å | 2.65 | 6.20 | ||
| δd, MPa1/2 | 15.5 | 14.8 | ||
| δp, MPa1/2 | 16.0 | 4.3 | ||
| δh, MPa1/2 | 42.3 | 5.0 | ||
| Properties of Polymers | M1 | M7 | M8 | M10 |
| δd, MPa1/2 | 16.8 | 16.8 | 16.8 | 16.8 |
| δp, MPa1/2 | 6.0 | 2.5 | 2.3 | 2.0 |
| δh, MPa1/2 | 7.4 | 4.8 | 4.6 | 4.3 |
| Distance Parameters | Water | MTBE | ||
| ∆i-M1, MPa1/2 | 36.6 | 3.6 | ||
| ∆i-M7, MPa1/2 | 39.8 | 2.6 | ||
| ∆i-M8, MPa1/2 | 40.1 | 2.8 | ||
| ∆i-M10, MPa1/2 | 40.6 | 3.1 | ||
| Membrane | Temperature [°C] | CMTBE, Feed Solution [wt.%] | Overall Flux [kg·m−2h−1] | Separation Factor | Ref. |
|---|---|---|---|---|---|
| M10/MFFK | 40 | 1 | 0.82 | 310 | This work |
| PVA/CSP-1 | 20 | 1 | 0.60 | 240 | [54] |
| Pervap 1060 | 40 | 1 | 0.70 | 270 | [55] |
| Pervap 1070 | 40 | 1 | 0.30 | 280 | [55] |
| PEBAX 4033 | 40 | 1 | 0.03 | 33 | [55] |
| Al2O3-5nm-C6 | 35 | 1 | 0.70 | 1.1 | [33] |
| ZrO2-5kD-C6 | 35 | 1 | 1.65 | 56 | [33] |
| TiO2-5kD-C6 | 35 | 1 | 1.95 | 84 | [33] |
| Al2O3-5nm-C6 | 35 | 1 | 2.50 | 3 | [34] |
| TiO2-5kD-C6 | 35 | 1 | 2.16 | 91 | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, I.; Podtynnikov, I.; Grushevenko, E.; Scharova, O.; Anokhina, T.; Makaev, S.; Volkov, A.; Volkov, V. High Selective Composite Polyalkylmethylsiloxane Membranes for Pervaporative Removal of MTBE from Water: Effect of Polymer Side-chain. Polymers 2020, 12, 1213. https://doi.org/10.3390/polym12061213
Borisov I, Podtynnikov I, Grushevenko E, Scharova O, Anokhina T, Makaev S, Volkov A, Volkov V. High Selective Composite Polyalkylmethylsiloxane Membranes for Pervaporative Removal of MTBE from Water: Effect of Polymer Side-chain. Polymers. 2020; 12(6):1213. https://doi.org/10.3390/polym12061213
Chicago/Turabian StyleBorisov, Ilya, Ivan Podtynnikov, Evgenia Grushevenko, Olga Scharova, Tatiana Anokhina, Sergey Makaev, Alexey Volkov, and Vladimir Volkov. 2020. "High Selective Composite Polyalkylmethylsiloxane Membranes for Pervaporative Removal of MTBE from Water: Effect of Polymer Side-chain" Polymers 12, no. 6: 1213. https://doi.org/10.3390/polym12061213