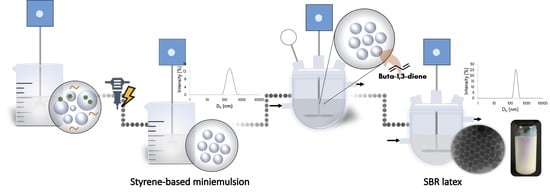
Styrene-Butadiene Rubber by Miniemulsion Polymerization Using In Situ Generated Surfactant
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
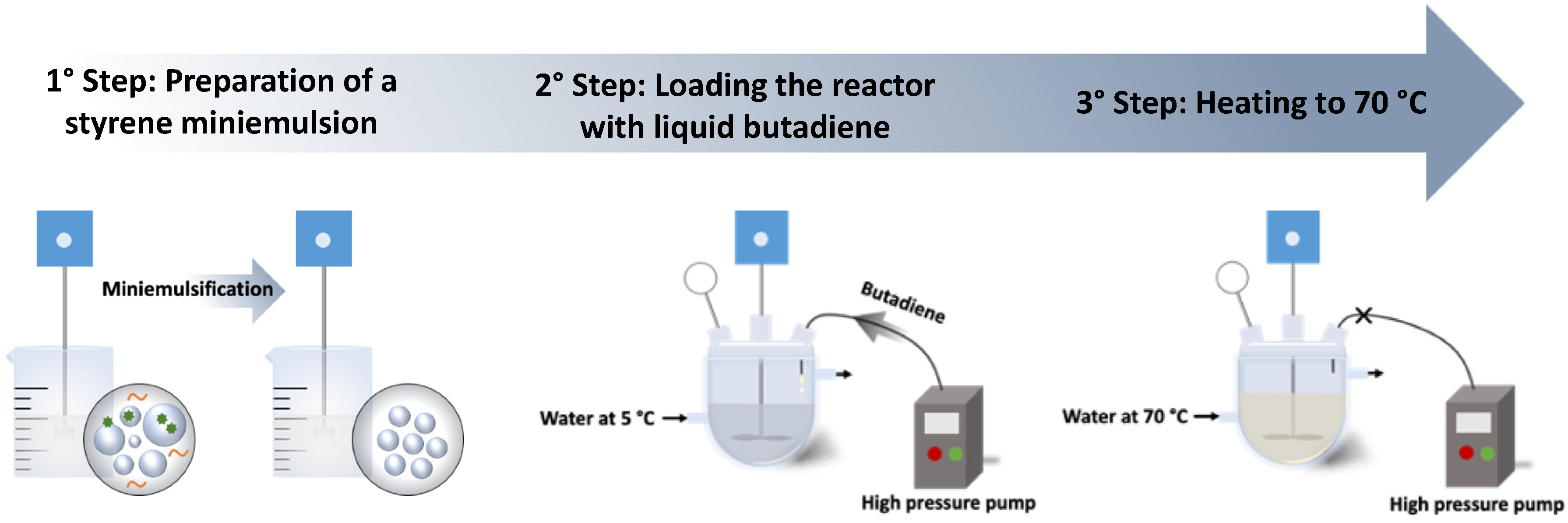
2.2. Preparation of SBR Latex by Miniemulsion Polymerization
2.3. Characterizations
2.3.1. Dynamic Light Scattering (DLS)
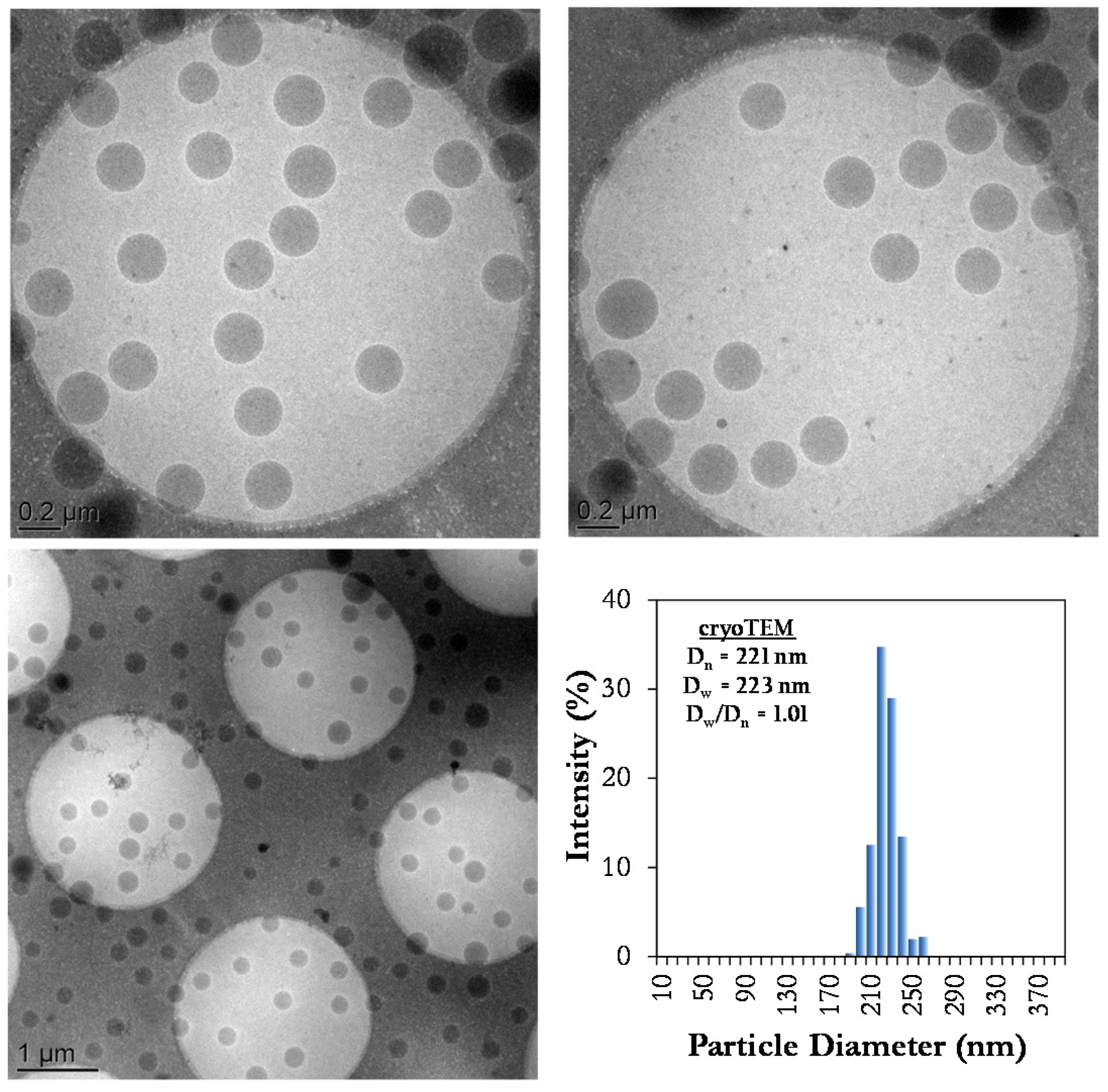
2.3.2. Cryo-Transmission Electronic Microscopic (Cryo-TEM)
2.3.3. Nuclear Magnetic Resonance (1H NMR and 13C NMR)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Size Exclusion Chromatography (SEC)
2.3.6. Swelling Experiments
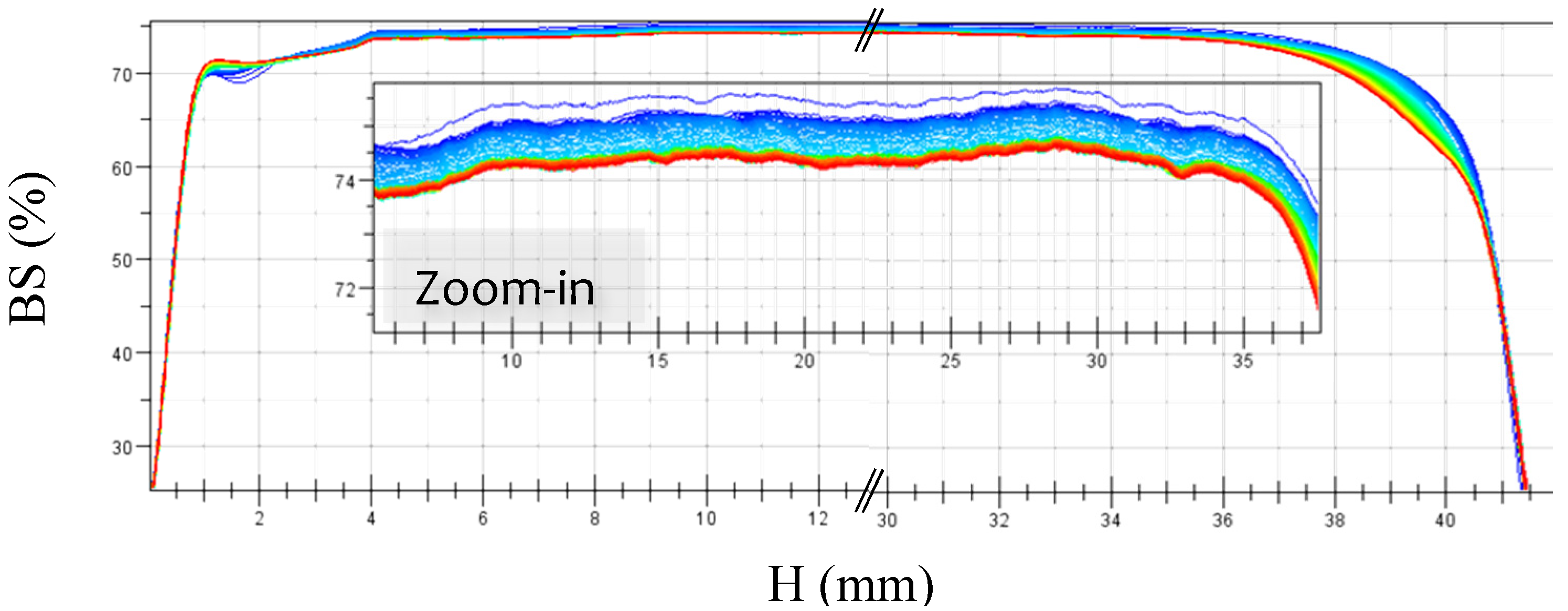
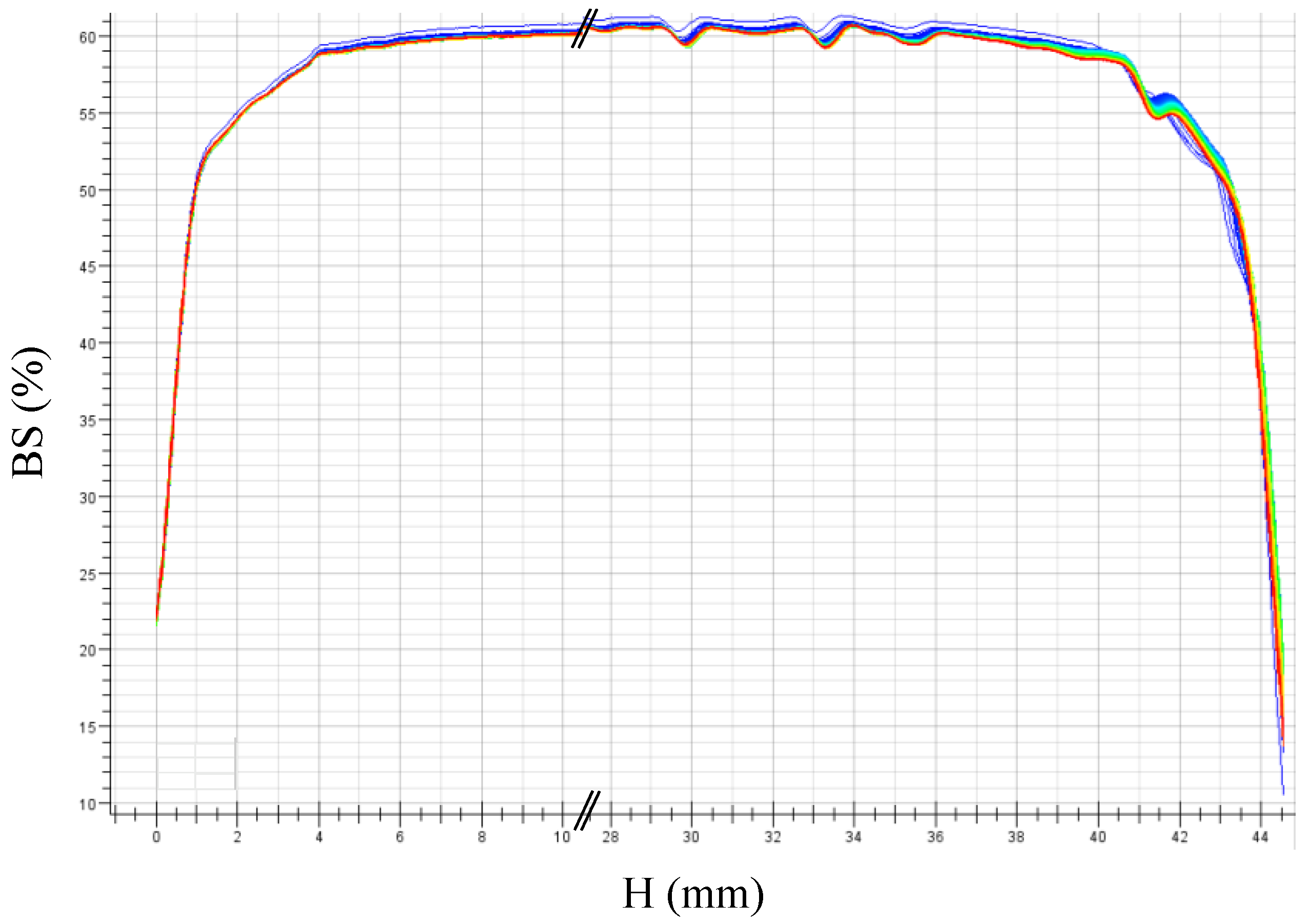
2.3.7. Turbiscan® Measurements
3. Results and Discussion
3.1. Stability of Styrene Miniemulsions
3.2. Synthesis of High Solids Content SBR Latexes
3.3. Effect of the Nature of the Hydrophobe
3.4. Scaling Up of SBR Miniemulsion Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinez Delfa, G.; Olivieri, A.; Boschetti, C.E. Multiple response optimization of styrene–butadiene rubber emulsion polymerization. Comput. Chem. Eng. 2009, 33, 850–856. [Google Scholar] [CrossRef]
- Zubov, A.; Pokorny, J.; Kosek, J. Styrene–butadiene rubber (SBR) production by emulsion polymerization: Dynamic modeling and intensification of the process. Chem. Eng. J. 2012, 207–208, 414–420. [Google Scholar] [CrossRef]
- Filho, A.P.; Araujo, O.; Giudici, R.; Sayer, C. Batch and Semicontinuous Styrene–Butadiene Emulsion Copolymerization Reactions. Macromol. Symp. 2006, 243, 114–122. [Google Scholar] [CrossRef]
- Li, D.; David Sudol, E.; El-Aasser, M.S. Miniemulsion and conventional emulsion copolymerization of styrene and butadiene: A comparative kinetic study. J. Appl. Polym. Sci. 2006, 101, 2304–2312. [Google Scholar] [CrossRef]
- Kohnle, M.-V.; Ziener, U.; Landfester, K. Synthesis of styrene–butadiene rubber latex via miniemulsion copolymerization. Colloid Polym. Sci. 2009, 287, 259–268. [Google Scholar] [CrossRef]
- Charmot, D.; Guillot, J. Kinetic modelling of network formation in styrene-butadiene emulsion copolymers: A comparative study with the generalized form of Flory’s theory of gelation. Polymer 1992, 33, 352–360. [Google Scholar] [CrossRef]
- Dubé, M.A.; Li, L. In-line monitoring of SBR emulsion polymerization using ATR-FTIR spectroscopy. Polym. Plast. Technol. Eng. 2010, 49, 648–656. [Google Scholar] [CrossRef]
- Landfester, K. Miniemulsions for Nanoparticle Synthesis. In Colloid Chemistry II; Antonietti, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 75–123. ISBN 978-3-540-36412-2. [Google Scholar]
- Li, M.K.; Fogler, H.S. Acoustic emulsification. Part 1. The instability of the oil-water interface to form the initial droplets. J. Fluid Mech. 1978, 88, 499–511. [Google Scholar] [CrossRef]
- Udagama, R. Synthesis of Polymer-Polymer Hybrids by Miniemulsion Polymerisation and Characterisation of Hybrid Latex. PhD Thesis, Université Claude Bernard-Lyon I, Villeurbanne, France, 2009. [Google Scholar]
- Schork, F.J.; Luo, Y.; Smulders, W.; Russum, J.P.; Butté, A.; Fontenot, K. Miniemulsion Polymerization. In Polymer Particles; Okubo, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 129–255. ISBN 978-3-540-31565-0. [Google Scholar]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Hecht, L.L.; Schoth, A.; Muñoz-Espí, R.; Javadi, A.; Köhler, K.; Miller, R.; Landfester, K.; Schuchmann, H.P. Determination of the Ideal Surfactant Concentration in Miniemulsion Polymerization. Macromol. Chem. Phys. 2013, 214, 812–823. [Google Scholar] [CrossRef]
- Chern, C.S.; Liou, Y.C.; Chen, T.J. Particle nucleation loci in styrene miniemulsion polymerization using alkyl methacrylates as the reactive cosurfactant. Macromol. Chem. Phys. 1998, 199, 1315–1322. [Google Scholar] [CrossRef]
- Jia, G.; Cai, N.; Xu, Y.; Liu, C.; Tan, X. Miniemulsion polymerization of styrene with liquid polybutadiene as the sole co-stabilizer. Eur. Polym. J. 2007, 43, 4453–4459. [Google Scholar] [CrossRef]
- Lin, C.T.; Shiau, F.T.; Chern, C.S. Synthesis and characterization of miniemulsion copolymers of methyl methacrylate (or styrene) and stearyl methacrylate. Colloid Polym. Sci. 2009, 287, 1139–1144. [Google Scholar] [CrossRef]
- Moustafa, A.F. Miniemulsion Polymerization of Butadiene: Kinetics Study. J. Colloid Sci. Biotechnol. 2015, 4, 14–19. [Google Scholar] [CrossRef]
- Chern, C.S.; Chen, T.J. Miniemulsion polymerization of styrene using alkyl methacrylates as the reactive cosurfactant. Colloid Polym. Sci. 1997, 275, 546–554. [Google Scholar] [CrossRef]
- El-Jaby, U.; Cunningham, M.; Enright, T.; McKenna, T.F.L. Polymerisable Miniemulsions Using Rotor-Stator Homogenisers. Macromol. React. Eng. 2008, 2, 350–360. [Google Scholar] [CrossRef]
- El-Jaby, U.; Cunningham, M.; McKenna, T.F.L. The Advantages of In Situ Surfactant Generation for Miniemulsions. Macromol. Rapid Commun. 2010, 31, 558–562. [Google Scholar] [CrossRef]
- Guo, Y.; Teo, V.L.; Ting, S.R.S.; Zetterlund, P.B. Miniemulsion polymerization based on in situ surfactant formation without high-energy homogenization: Effects of organic acid and counter ion. Polym. J. 2012, 44, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.; Goikoetxea, M.; Barandiaran, M.J. Surfactant-Free Miniemulsion Polymerization of a Bio-Based Oleic Acid Derivative Monomer. Macromol. React. Eng. 2014, 8, 434–441. [Google Scholar] [CrossRef]
- Saygı-Arslan, Ö.; Sudol, E.D.; Daniels, E.S.; El-Aasser, M.S.; Klein, A. In situ surfactant generation as a means of miniemulsification? J. Appl. Polym. Sci. 2009, 111, 735–745. [Google Scholar]
- Senn, W.L. Analysis of styrene-butadiene copolymers by NMR spectroscopy. Anal. Chim. Acta 1963, 29, 505–509. [Google Scholar] [CrossRef]
- Sato, H.; Takebayashi, K.; Tanaka, Y. Analysis of carbon-13 NMR of polybutadiene by means of low molecular weight model compounds. Macromolecules 1987, 20, 2418–2423. [Google Scholar] [CrossRef]
- Sato, H.; Ishikawa, T.; Takebayashi, K.; Tanaka, Y. Carbon-13 NMR signal assignment of styrene-butadiene copolymer. Macromolecules 1989, 22, 1748–1753. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Poly-mer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Henríquez, C.; Bueno, C.; Lissi, E.A.; Encinas, M.V. Thiols as chain transfer agents in free radical polymerization in aqueous solution. Polymer 2003, 44, 5559–5561. [Google Scholar] [CrossRef]





| Chemicals | m (g) | wt% Based on Styrene |
|---|---|---|
| Styrene | 30 | - |
| Deionized water | 100 | - |
| Hexadecane or Octadecylacrylate | 1.2 | 4 |
| Oleic Acid | 0.9 | 3 |
| Potassium hydroxide a | 0.3 | - |
| tert-Dodecylmercaptan | 1.2 | 4 |
| Chemicals | Weight (g) | wt% Based on Organic Phase |
|---|---|---|
| Styrene | 30 | - |
| Butadiene a | 65 | - |
| Deionized water | 100 | - |
| Hexadecane or Octadecylacrylate b | 1.2 | 1.2 |
| tert-Dodecylmercaptan c | 3.8 | 4 |
| VAZO 67 | 1.9 | 2 |
| Oleic Acid b | 0.9 | 1 |
| Potassium hydroxide d | 0.3 | - |
| Time (Days) | Dh (nm) a | PdI a |
|---|---|---|
| 0 | 177 | 0.08 |
| 1 | 174 | 0.09 |
| 2 | 177 | 0.08 |
| 3 | 171 | 0.09 |
| 4 | 174 | 0.07 |
| 7 | 184 | 0.08 |
| Poly(butadiene-co-styrene) | |||
|---|---|---|---|
| Butadiene (%) | Styrene (%) | ||
| 87.2 | 12.8 | ||
| 1,4-cis/1,4-trans (%) | 1,2-vinyl (%) | ||
| 71.4 | 15.8 | ||
| 1,4-cis (%) | 1,4-trans (%) | ||
| 15 | 56.4 | ||
| Entry | Insoluble Fraction (%) | Soluble Fraction (%) |
|---|---|---|
| HD-SBR#01 | 5.3 | 94.7 |
| HD-SBR#02 | 5.8 | 94.2 |
| HD-SBR#03 | 5.3 | 94.7 |
| Average | 5.5 | 94.5 |
| Sample | Tg (°C) | Molar Mass | Gel Content (%) | Composition (mol %) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mw (g mol−1) | Mn (g mol−1) | Ð | Styrene | 1.2-Vinyl | 1.4-cis | 1.4-trans | |||
| HD-SBR#01 | −52 | 30,382 | 8040 | 3.8 | 5.3 | 11.9 | 15.7 | 14.5 | 57.9 |
| HD-SBR#02 | −56 | 31,664 | 6980 | 4.5 | 5.8 | 12.8 | 15.8 | 15 | 56.4 |
| HD-SBR#03 | −56 | 27,458 | 6177 | 4.4 | 5.3 | 13.0 | 16.2 | 15.2 | 55.6 |
| ODA-SBR#01 | −50 | 45,877 | 10,164 | 4.5 | 4.6 | 13.5 | 15.0 | 15.3 | 56.2 |
| ODA-SBR#02 | −50 | 47,992 | 10,369 | 4.6 | 3.2 | 13.2 | 15.5 | 15.5 | 55.8 |
| ODA-SBR#03 | −53 | 46,514 | 8288 | 5.2 | 3.8 | 12.9 | 14.9 | 15.3 | 56.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medeiros, A.M.S.; Bourgeat-Lami, E.; McKenna, T.F.L. Styrene-Butadiene Rubber by Miniemulsion Polymerization Using In Situ Generated Surfactant. Polymers 2020, 12, 1476. https://doi.org/10.3390/polym12071476
Medeiros AMS, Bourgeat-Lami E, McKenna TFL. Styrene-Butadiene Rubber by Miniemulsion Polymerization Using In Situ Generated Surfactant. Polymers. 2020; 12(7):1476. https://doi.org/10.3390/polym12071476
Chicago/Turabian StyleMedeiros, Anderson M. S., Elodie Bourgeat-Lami, and Timothy F. L. McKenna. 2020. "Styrene-Butadiene Rubber by Miniemulsion Polymerization Using In Situ Generated Surfactant" Polymers 12, no. 7: 1476. https://doi.org/10.3390/polym12071476
APA StyleMedeiros, A. M. S., Bourgeat-Lami, E., & McKenna, T. F. L. (2020). Styrene-Butadiene Rubber by Miniemulsion Polymerization Using In Situ Generated Surfactant. Polymers, 12(7), 1476. https://doi.org/10.3390/polym12071476