Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Hyacinth Biomass and Chemicals
2.2. Pretreatment Studies and Enzymatic Hydrolysis
2.3. PHB Production Using WH Hydrolysates by R. eutropha
Effects of Supplementation of Cheap N Sources on PHB Production
2.4. Analytical Methods
2.5. PHB Extraction and Analytical Characterization
2.6. Thermal Analysis and Molecular Mass Determination of Produced PHB
2.7. Statistical Analysis
3. Results and Discussion
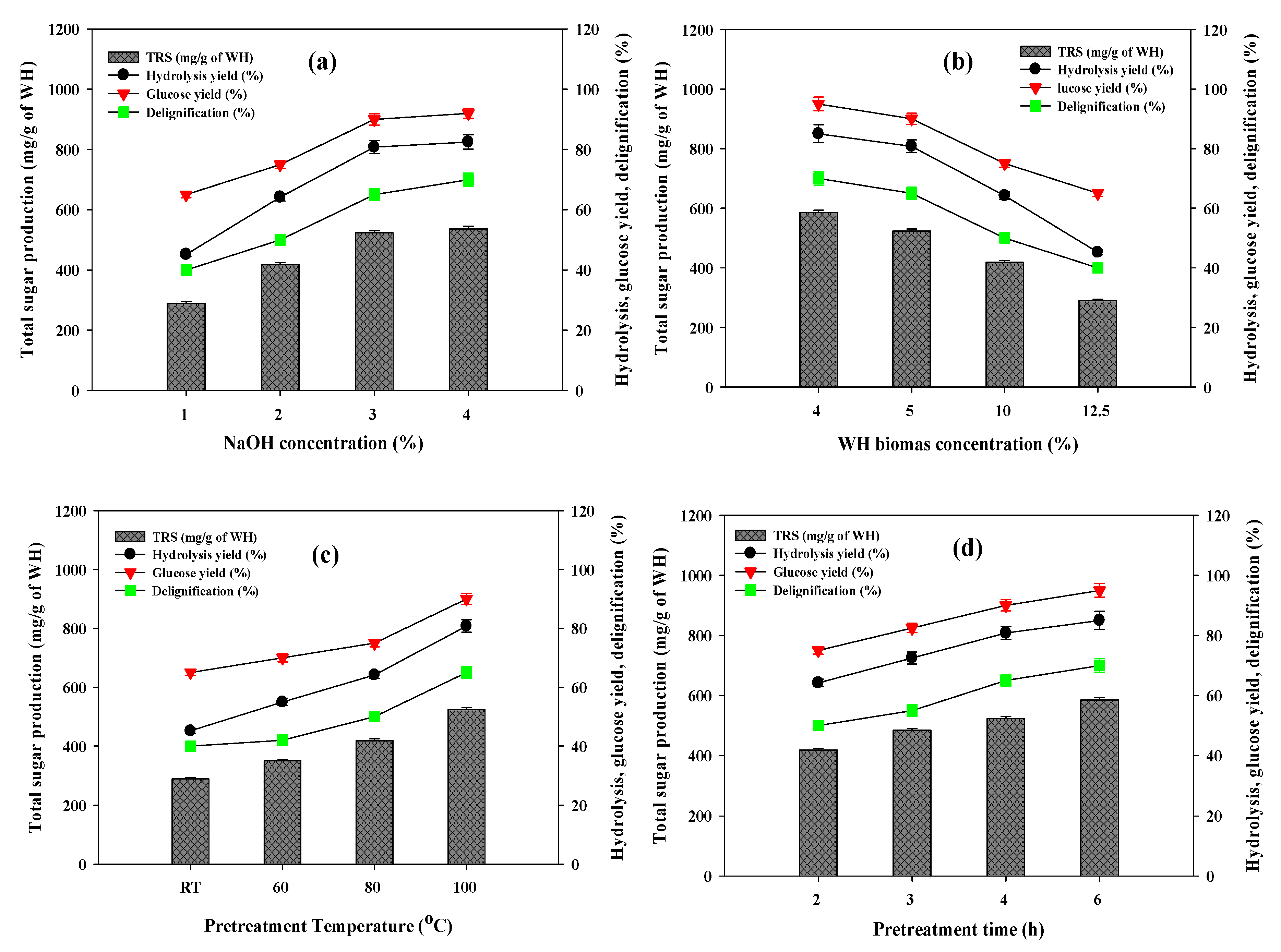
3.1. Effects of Chemical Pretreatment on Enzymatic Hydrolysis of WH
3.2. Determination of the Best Pretreatment Conditions
3.3. Ambient Conditions for Enzymatic Hydrolysis of NaOH Pretreated WH Biomass
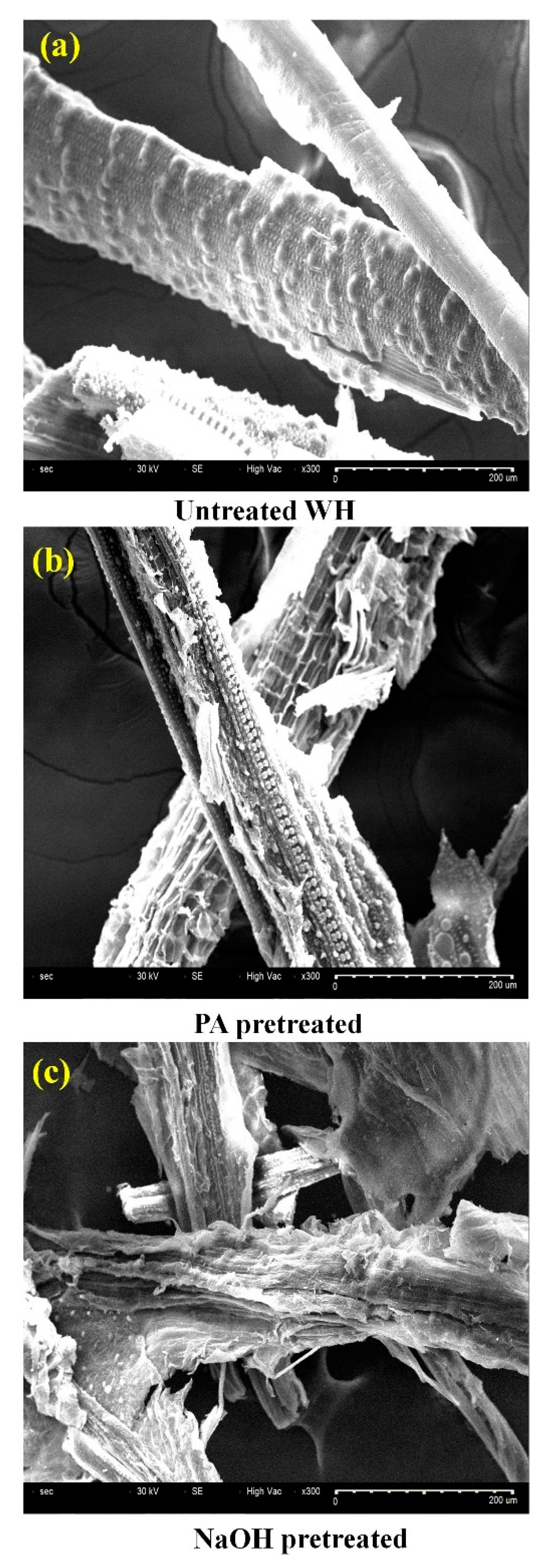
3.4. Physicochemical Changes of Water Hyacinth after Pretreatment
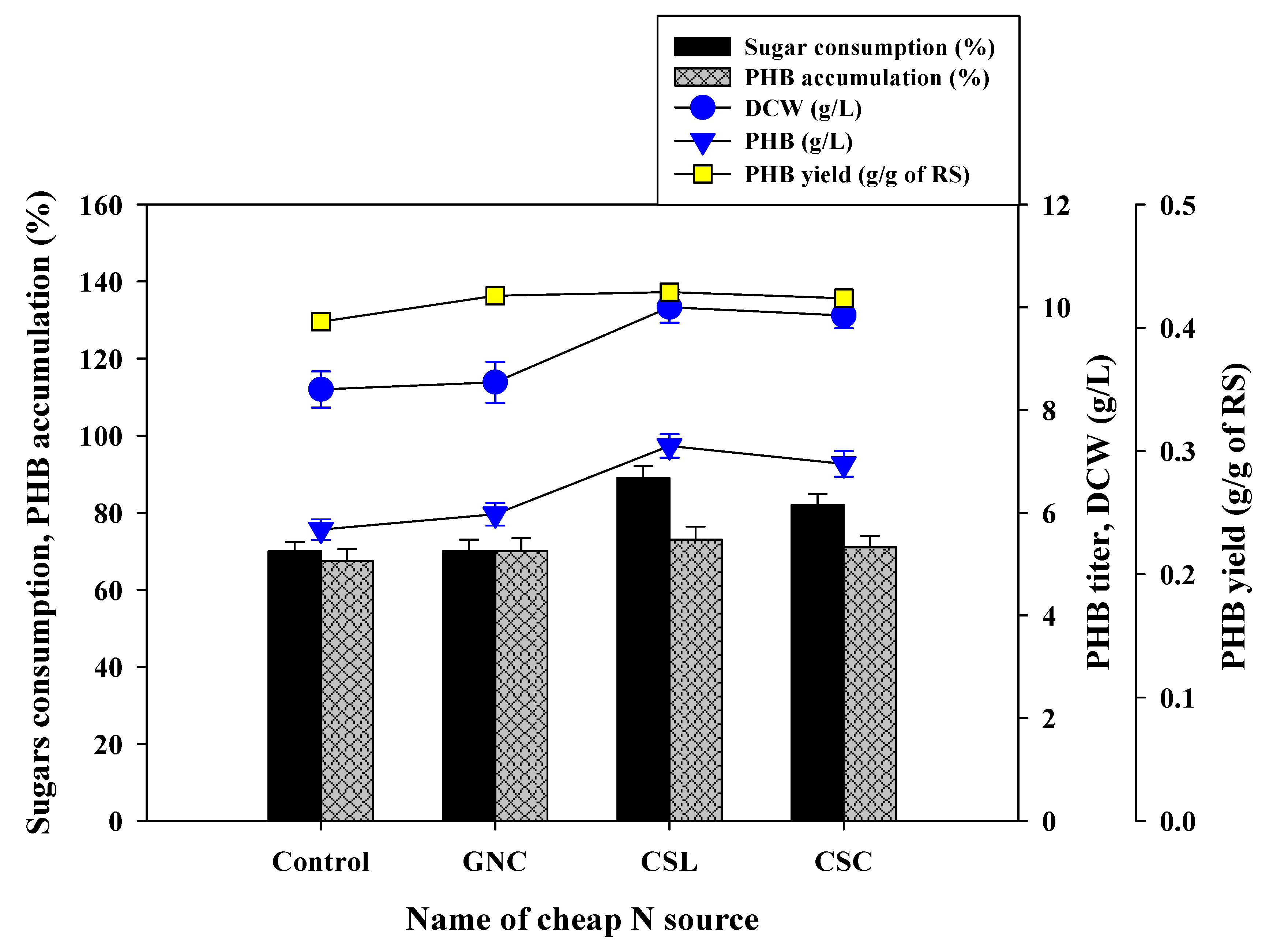
3.5. PHB Production Using WH Hydrolysates
Effects of Supplementation of Cheap Nitrogen Source on PHB Production
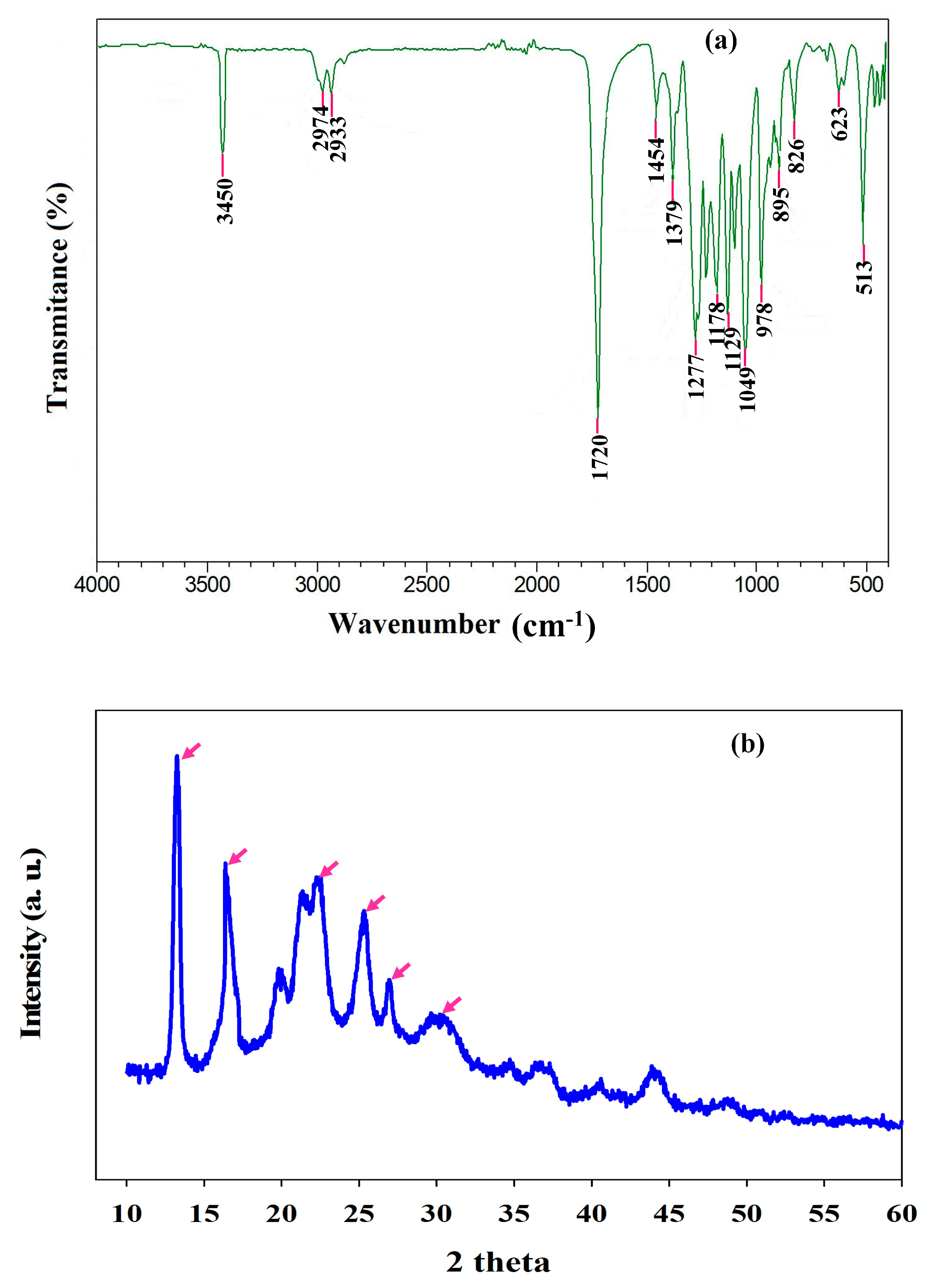
3.6. PHB Characterization
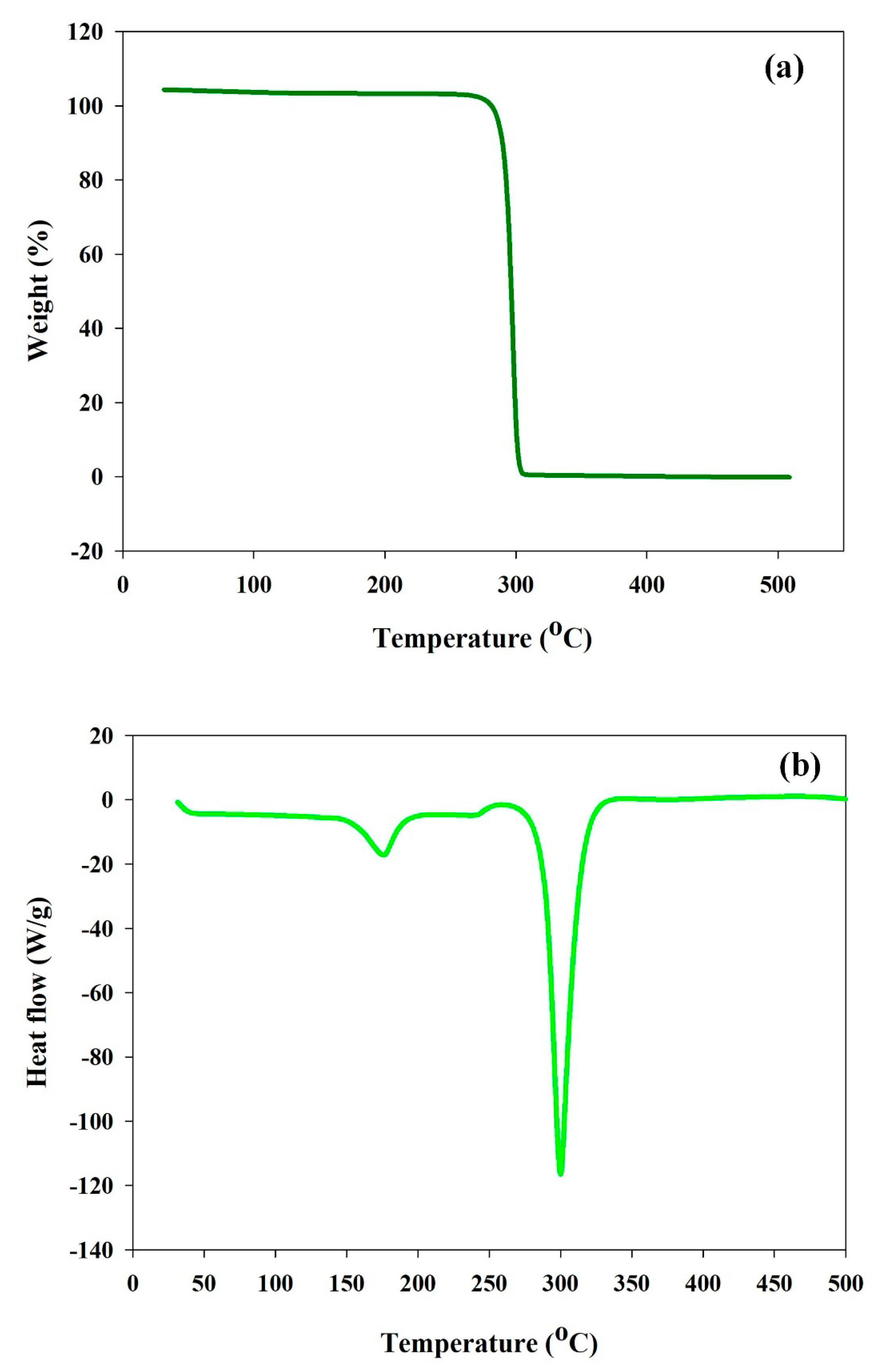
3.7. Thermal (TGA and DSC) Analysis of Produced PHB
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P.; Tarafdar, A.; Sindhu, R.; Parameswaran, B.; Pandey, A. Applications of poly-3-hydroxybutyrate based composite in advanced applications of polysaccharides and their composites. Mater. Res. Found. 2020, 68, 45–59. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rathour, R.; Singh, R.; Sun, Y.; Pandey, A.; Gnansounou, E.; Lin, K.-T.A.; Tsang, D.C.W.; Thakur, I.S. Bacterial polyhydroxyalkanoates: Opportunities, challenges, and prospects. J. Clean. Prod. 2020, 263, 121500. [Google Scholar] [CrossRef]
- Kaur, L.; Khajuria, R.; Parihar, L.; Dimpal Singh, G. Polyhydroxyalkanoates: Biosynthesis to commercial production—A review. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1098–1106. [Google Scholar] [CrossRef]
- Venkateswar Reddy, M.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Polyhydroxyalkanoates (PHA) production from synthetic waste using Pseudomonas pseudoflava: PHA synthase enzyme activity analysis from P. pseudoflava and P. palleronii. Bioresour. Technol. 2017, 234, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.K.; Ghodake, G.S.; Kadam, A.; Mulla, S.I.; Bharagava, R.N.; Kim, D.S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crops Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Pradhan, S.; Borah, A.J.; Poddar, M.K.; Dikshit, P.K.; Rohidas, L.; Moholkar, V.S. Microbial production, ultrasound-assisted extraction and characterization of biopolymer polyhydroxybutyrate (PHB) from terrestrial (P. hysterophorus) and aquatic (E. crassipes) invasive weeds. Bioresour. Technol. 2017, 242, 304–310. [Google Scholar] [CrossRef]
- Radhika, D.; Murugesan, A.G. Bioproduction, statistical optimization and characterization of microbial plastic (poly 3-hydroxy butyrate) employing various hydrolysates of water hyacinth (Eichhornia crassipes) as sole carbon source. Bioresour. Technol. 2012, 121, 83–92. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon sources for polyhydroxyalkanoates and an integrated biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Thi, B.T.N.; Ong, L.K.; Thi, D.T.N.; Ju, Y.H. Effect of subcritical water pretreatment on cellulose recovery of water hyacinth (Eichhornia crassipe). J. Taiwan Inst. Chem. Eng. 2017, 71, 55–61. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Y.; Han, H.; Weng, C. Enhancing bioethanol production from water hyacinth by new combined pretreatment methods. Bioresour. Technol. 2018, 251, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Binod, P.; Pandey, A.; Madhavan, A.; Alphonsa, J.A.; Vivek, N.; Gnansounou, E.; Castro, E.; Faraco, V. Water hyacinth a potential source for value addition: An overview. Bioresour. Technol. 2017, 230, 152–162. [Google Scholar] [CrossRef]
- Saratale, G.D.; Jung, M.Y.; Oh, M.K. Reutilization of green liquor chemicals for pretreatment of whole rice waste biomass and its application to 2, 3-butanediol production. Bioresour. Technol. 2016, 205, 90–96. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi-Bakó, K.; Yoon, J.J.; Kim, S.H. A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.K. Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef]
- Duncan, S.; Jing, Q.; Katona, A.; Kazlauskas, R.J.; Schilling, J.; Tschirner, U.; Aldajani, W.W. Increased saccharification yields from aspen biomass upon treatment with enzymatically generated peracetic acid. Appl. Biochem. Biotechnol. 2010, 160, 1637–1652. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Liu, D. Peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis: A continued work. J. Chem. Technol. Biotechnol. 2008, 83, 950–956. [Google Scholar] [CrossRef]
- Saratale, R.G.; Shin, H.S.; Ghodake, G.S.; Kumar, G.; Oh, M.K.; Saratale, G.D. Combined effect of inorganic salts with calcium peroxide pretreatment for kenaf core biomass and their utilization for 2, 3-butanediol production. Bioresour. Technol. 2018, 258, 26–32. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kim, S.; Holtzapple, M.T. Effect of structural features on enzyme digestibility of corn stover. Bioresour. Technol. 2006, 97, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Lu, J.; Tong, Y.; Li, H.; Chen, Q. Isolation and characterization of Poly(3-hydroxybutyrate)-producing bacteria from aerobic sludge. Appl. Biochem. Biotechnol. 2015, 175, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Cheng, J.; Song, W.; Yu, C.; Zhou, J.; Cen, K. Enhancing enzymatic saccharification of water hyacinth through microwave heating with dilute acid pretreatment for biomass energy utilization. Energy 2013, 61, 158–166. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bishnoi, N.R. Comparative study of various pretreatment techniques for ethanol production from water hyacinth. Ind. Crops Prod. 2013, 44, 283–289. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Chen, K.Y.; Chou, T.H. Concurrent calcium peroxide pretreatment and wet storage of water hyacinth for fermentable sugar production. Bioresour. Technol. 2015, 176, 267–272. [Google Scholar] [CrossRef]
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Hydrogen production from water hyacinth through dark- and photo-fermentation. Int. J. Hydrogen Energy 2010, 35, 8929–8937. [Google Scholar] [CrossRef]
- Abraham, M.; Kurup, G.M. Bioconversion of tapioca (Manihot esculenta) waste and water hyacinth (Eichhornia crassipes)—Influence of various physico-chemical factors. J. Ferment. Bioeng. 1996, 82, 259–263. [Google Scholar] [CrossRef]
- Zhang, Q.; Weng, C.; Huang, H.; Achal, V.; Wang, D. Optimization of bioethanol production using whole plant of water hyacinth as substrate in simultaneous saccharification and fermentation process. Front. Microbiol. 2016, 6, 1411. [Google Scholar] [CrossRef]
- Barua, V.B.; Kalamdhad, A.S. Effect of various types of thermal pretreatment techniques on the hydrolysis, compositional analysis and characterization of water hyacinth. Bioresour Technol. 2017, 227, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Ma, Y.L.; Wang, L.Q.; Wang, F.Z.; Wu, Q.Q.; Pan, G.Y. Physicochemical properties of corn stalk after treatment using steam explosion coupled with acid or alkali. Carbohyd. Polym. 2015, 117, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Song, W.; Ding, L.; Xie, B.; Zhou, J.; Cen, K. Characterisation of water hyacinth with microwave-heated alkali pretreatment for enhanced enzymatic digestibility and hydrogen/methane fermentation. Bioresour. Technol. 2015, 182, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, T.T.; Wang, Y.B.; Cao, X.H.; Wu, S.Q.; Zhao, M.L.; Wang, H.Y.; Xu, M.; Zheng, B.D.; Zheng, J.G.; et al. Pretreatments of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharya, A.; Haldar, S.; Ganguly, A.; Gu, S.; Ting, Y.P.; Chatterjee, P.K. Optimization of enzymatic saccharification of water hyacinth biomass for bio-ethanol: Comparison between artificial neural network and response surface methodology. Sustain. Mat. Technol. 2015, 3, 17–28. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; de Aragao, G.M.F.; Poletto, P. Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process. Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Upadhayay, V.; Verma, S.; Kuila, A. Production of poly hydroxy butyrate (PHB) from Eichhornia crassipes through microbial fermentation process. Plant. Sci. Today 2019, 6, 541–550. [Google Scholar] [CrossRef]
- Mehrabi, R.; Bagheri, G.; Alipour, F. Screening and optimization of Poly-Hydroxy Butyrate (PHB), using Eichhornia crassipes as substrate by Bacillus cereus and Bacillus subtilis. J. Entomol. Zool. Stud. 2015, 3, 33–37. [Google Scholar]
- Annamalai, N.; Al-Battashi, H.; Al-Bahry, S.; Sivakumar, N. Biorefinery production of poly-3-hydroxybutyrate using waste office paper hydrolysate as feedstock for microbial fermentation. J. Biotechnol. 2018, 265, 25–30. [Google Scholar] [CrossRef]
- Azizi, N.; Najafpour, G.; Younesi, H. Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int. J. Biol. Macromol. 2017, 101, 1029–1040. [Google Scholar] [CrossRef]
- Sandhya, M.; Aravind, J.; Kanmani, P. Production of polyhydroxyalkanoates from Ralstonia eutropha using paddy straw as cheap substrate. Int. J. Environ. Sci. Technol. 2013, 10, 47–54. [Google Scholar] [CrossRef]
- Yu, J.; Stahl, H. Microbial utilization and biopolyester synthesis of bagasse hydrolysates. Bioresour. Technol. 2008, 99, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.O.; Kanekar, P.P.; Nilegaonkar, S.S.; Sarnaik, S.S.; Jog, J.P. Production and characterization of a biodegradable poly (hydroxybutyrate-co-hydroxyvalerate) (PHB-co-PHV) copolymer by moderately haloalkalitolerant Halomonas campisalis MCM B-1027 isolated from Lonar Lake, India. Bioresour. Technol. 2010, 101, 9765–9771. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Ammu, B.; Binod, P.; Deepthi, S.K.; Ramachandran, K.B.; Soccol, C.R.; Pandey, A. Production and characterization of poly-3-hydroxybutyrate from crude glycerol by Bacillus sphaericus NII 0838 and improving its thermal properties by blending with other polymers. Braz. Arch. Biol.Technol. 2011, 54, 783–794. [Google Scholar] [CrossRef]
- Pan, W.; Perrotta, J.A.; Stipanovic, A.J.; Nomura, C.T.; Nakas, J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J. Ind. Microbiol. Biotechnol. 2011, 39, 459–469. [Google Scholar] [CrossRef]


| Name of Component | Concentration (g/L) |
|---|---|
| NaH2PO4 | 3.60 |
| Na2HPO4 | 2.84 |
| K2SO4 | 3.49 |
| NaOH | 0.40 |
| Yeast extract | 0.20 |
| MgSO4·7H2O | 0.39 |
| CaCl2 | 0.06 |
| (NH4)2SO4 | 0.10 |
| CuSO4·5H2O | 0.005 |
| ZnSO4·7H2O | 0.024 |
| MnSO4·H2O | 0.024 |
| FeSO4·7H2O | 0.15 |
| WH hydrolysates # | 20.0 |
| SH hydrolysates # | 20.0 |
| Type of Pretreatment | Pretreatment Conditions | WH Biochemicals Constituents (%) | Delignification (%) | TRS (mg/g of WH) | Hydrolysis Yield (%) | Glucose Yield (%) | ||
|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemi-cellulose | Lignin | ||||||
| Control | No pretreatment | 29.15 ± 1.25 | 32.66 ± 1.28 | 10.25 ± 0.68 | ND | 65.80 ± 1.50 | 10.65 ± 0.51 | 13.7 ± 0.25 |
| NaOH | 2% NaOH at 100 °C for 3 h | 42.25 ± 1.85 | 22.86 ± 0.89 | 5.11 ± 0.32 | 50.2 ± 1.25 | 418.0 ± 3.87 | 64.32 ± 0.68 | 75.0 ± 0.75 |
| Peracetic acid (PA) | 2% Peracetic acid at 100 °C for 3 h | 37.86 ± 1.95 | 24.50 ± 1.15 | 6.15 ± 0.30 | 40.3 ± 1.29 | 312.7 ± 3.56 | 50.12 ± 0.62 | 60.5 ± 0.65 |
| Parameters | NaOH | PA | SH |
|---|---|---|---|
| Fermentation time (h) | 36 | 36 | 36 |
| TRS (initial) (g/L) | 20 ± 0.55 | 20 ± 0.65 | 20 ± 0.66 |
| TRS (after) (g/L) | 6.0 ± 0.15 | 8.4 ± 0.14 | 4.2 ± 0.12 |
| Total Sugar consumption (%) | 70.4 ± 1.25 | 60.5 ± 0.98 | 80.0 ± 1.00 |
| DCW (g/L) | 8.40 ± 0.35 | 7.25 ± 0.35 | 9.72 ± 0.48 |
| PHB/DCW content (%) | 67.5 ± 1.88 | 62.5 ± 1.65 | 70.0 ± 1.45 |
| PHB (g/L) | 5.67 ± 0.25 | 4.53 ± 0.25 | 6.80 ± 0.22 |
| Qp gPHB/L/h | 0.157 ± 0.001 | 0.125 ± 0.001 | 0.188 ± 0.002 |
| PHB yield (g/g) | 0.405 ± 0.001 | 0.377 ± 0.001 | 0.425 ± 0.002 |
| Name of Substrate | Microorganism | Operation Mode | PHB Content (%) | PHB Concentration (g/L) | Reference |
|---|---|---|---|---|---|
| Water hyacinth | R. eutropha ATCC 17699 | Batch | 73 | 7.30 | This study |
| Water hyacinth | R. eutropha MTCC 1472 | Batch | 58.0 | 7.0 | [10] |
| Partheniumhysterophorus Pentose-rich hydrolysate Hexose-rich hydrolysate | R. eutropha MTCC 8320 | Batch | 8.03 17.93 | 0.24 0.60 | [9] |
| Eicchornia crassipes Pentose-rich hydrolysate Hexose-rich hydrolysate | R. eutropha MTCC 8320 | Batch | 8.11 21.62 | 0.30 0.96 | [9] |
| Waste office paper | R. eutropha NCIMB 11599 | Batch | 57.5 | 3.93 | [39] |
| Sargassum sp. seaweed hydrolysate | C. necator PTCC 1615 | Batch | 74.4 | 3.93 | [40] |
| Wheat waste biomass | R. eutropha ATCC 17699 | Batch | 74.0 | 7.85 | [8] |
| Paddy straw | R. eutropha MTCC 1472 | Batch | 37.55 | 5.19 | [41] |
| Rice paddy straw | R. eutropha ATCC 17699 | Batch | 75.45 | 11.42 | [17] |
| Bagasse hydrolysate | R. eutropha ATCC 17699 | Batch | 65 | 3.9 | [42] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saratale, R.G.; Cho, S.-K.; Ghodake, G.S.; Shin, H.-S.; Saratale, G.D.; Park, Y.; Lee, H.-S.; Bharagava, R.N.; Kim, D.-S. Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers 2020, 12, 1704. https://doi.org/10.3390/polym12081704
Saratale RG, Cho S-K, Ghodake GS, Shin H-S, Saratale GD, Park Y, Lee H-S, Bharagava RN, Kim D-S. Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers. 2020; 12(8):1704. https://doi.org/10.3390/polym12081704
Chicago/Turabian StyleSaratale, Rijuta Ganesh, Si-Kyung Cho, Gajanan S. Ghodake, Han-Seung Shin, Ganesh Dattatraya Saratale, Yooheon Park, Hee-Seok Lee, Ram Naresh Bharagava, and Dong-Su Kim. 2020. "Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach" Polymers 12, no. 8: 1704. https://doi.org/10.3390/polym12081704
APA StyleSaratale, R. G., Cho, S.-K., Ghodake, G. S., Shin, H.-S., Saratale, G. D., Park, Y., Lee, H.-S., Bharagava, R. N., & Kim, D.-S. (2020). Utilization of Noxious Weed Water Hyacinth Biomass as a Potential Feedstock for Biopolymers Production: A Novel Approach. Polymers, 12(8), 1704. https://doi.org/10.3390/polym12081704








