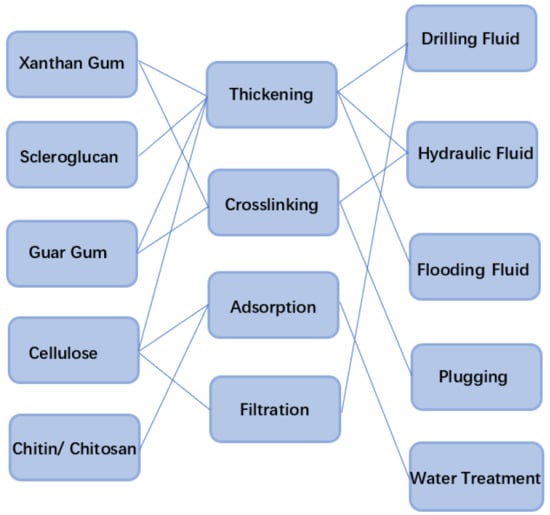
Application of Polysaccharide Biopolymer in Petroleum Recovery
Abstract
:1. Introduction
2. Polysaccharide Biopolymer
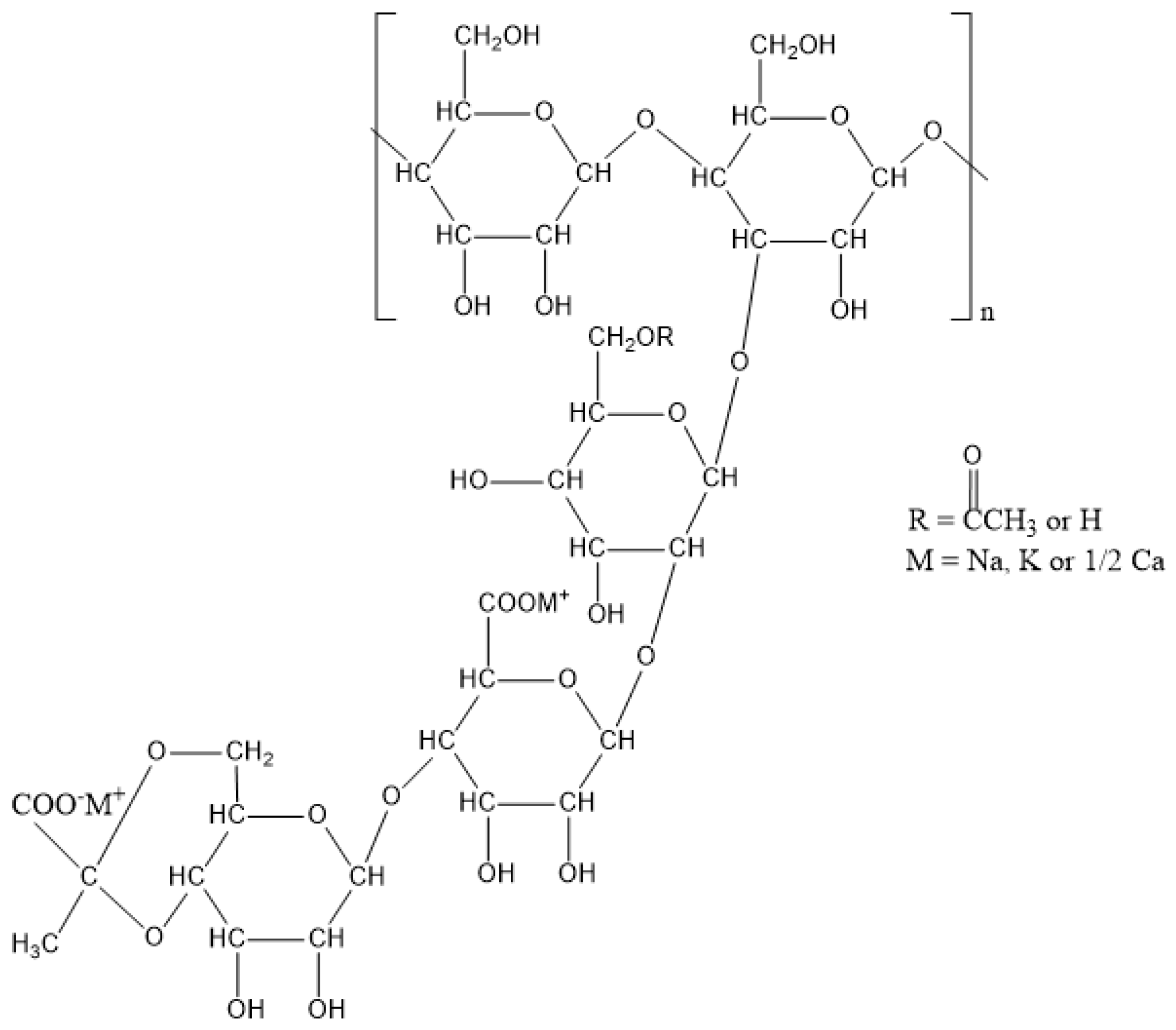
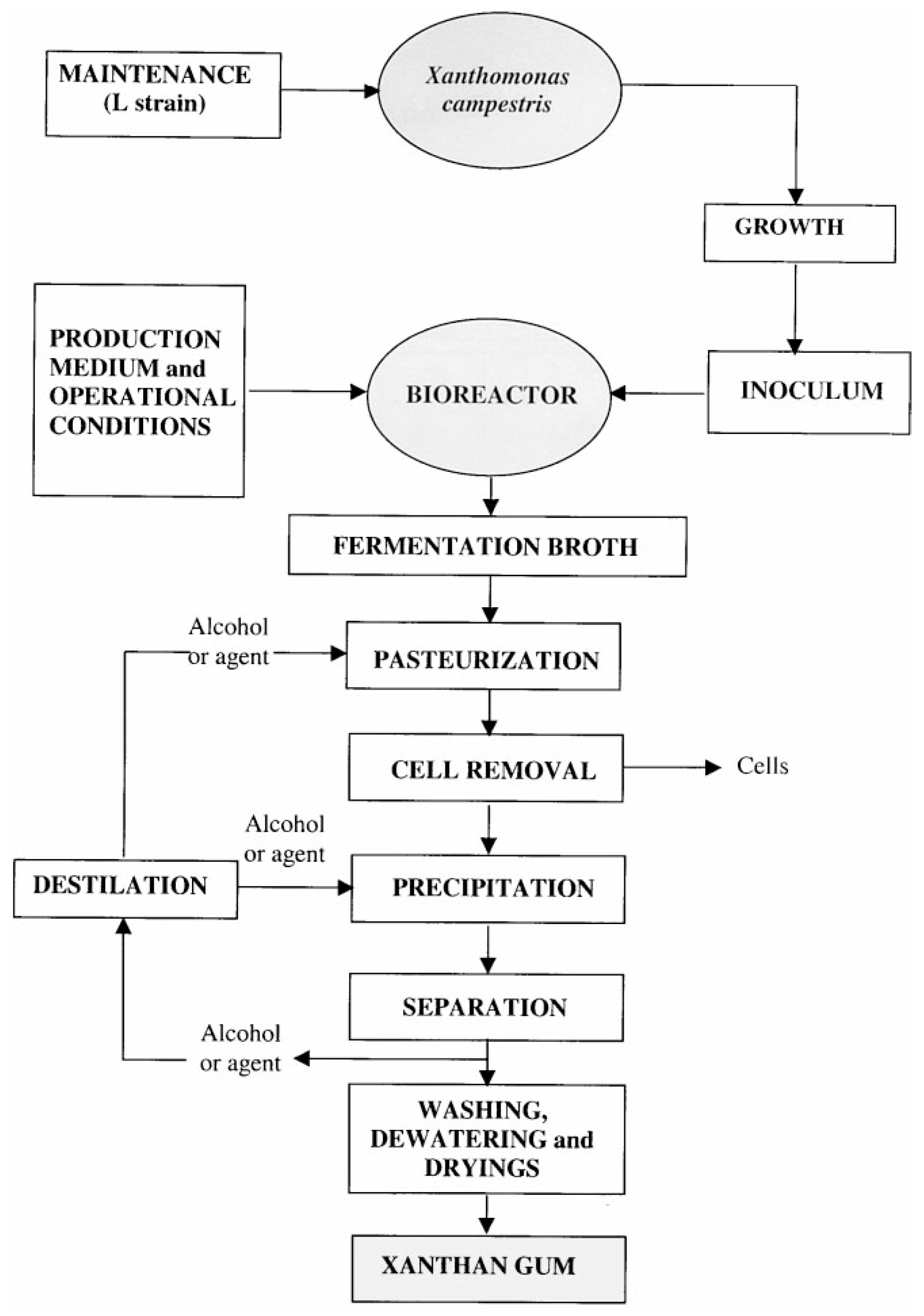
2.1. Xanthan Gum
2.2. Scleroglucan
2.3. Guar Gum
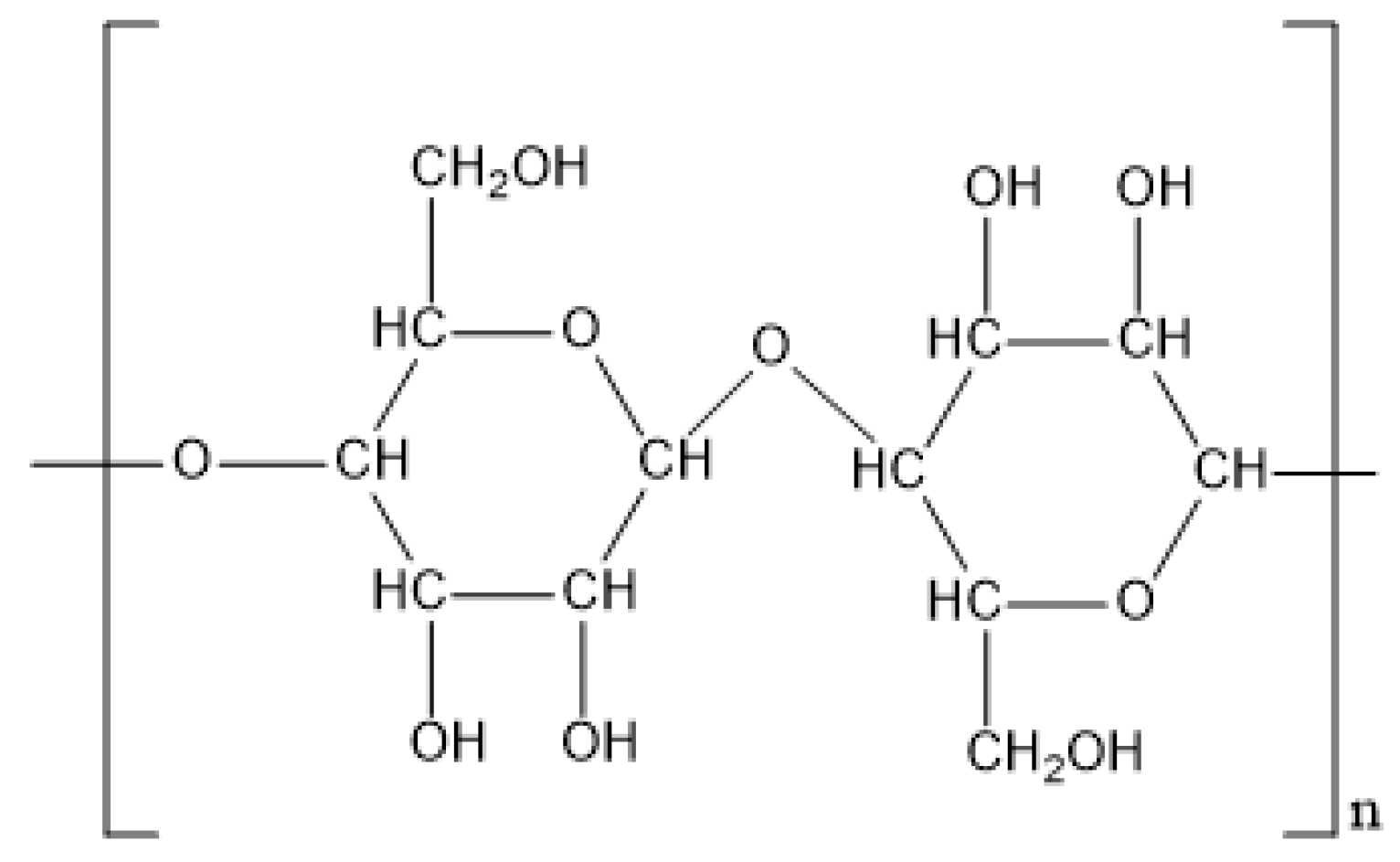
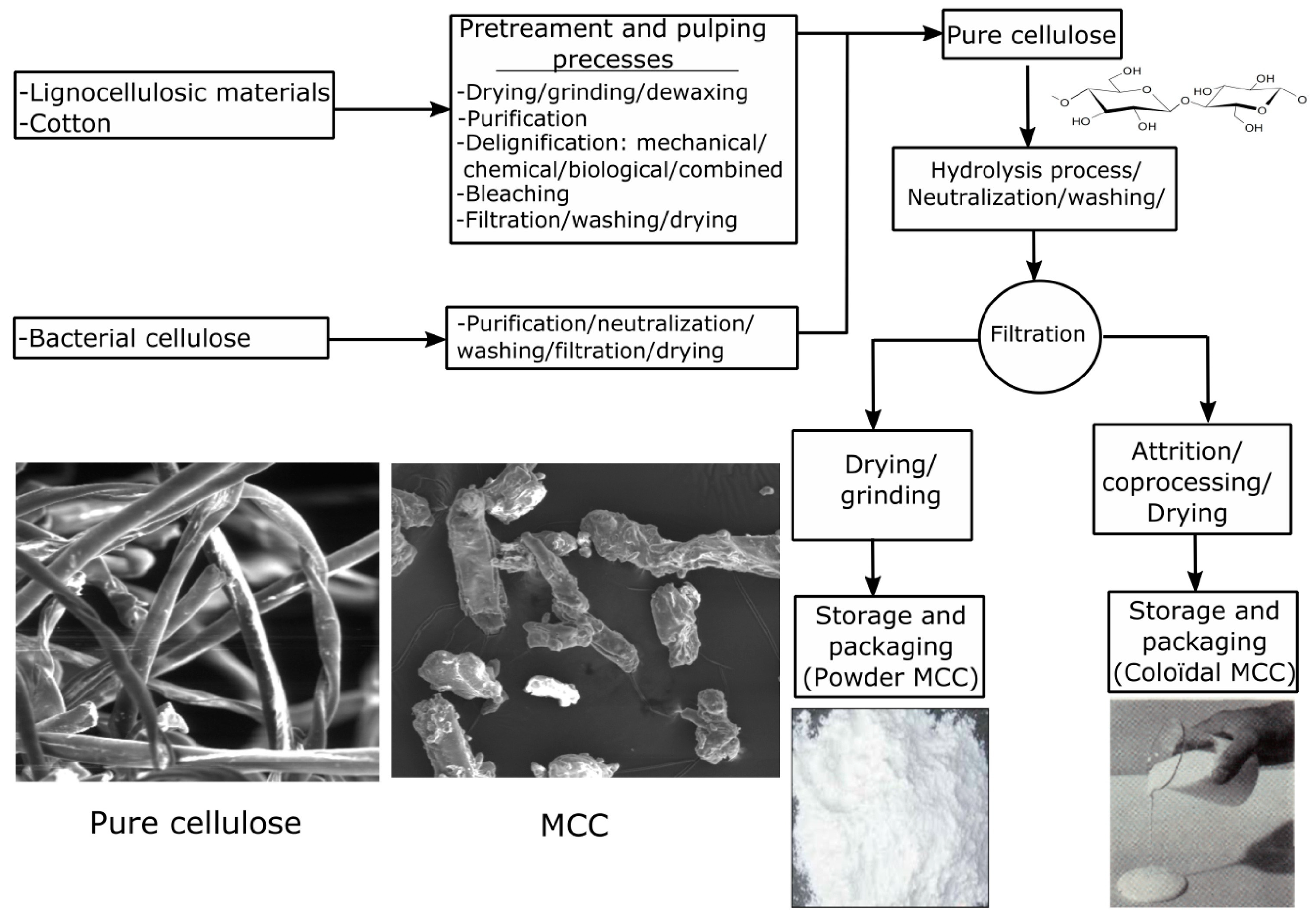
2.4. Cellulose
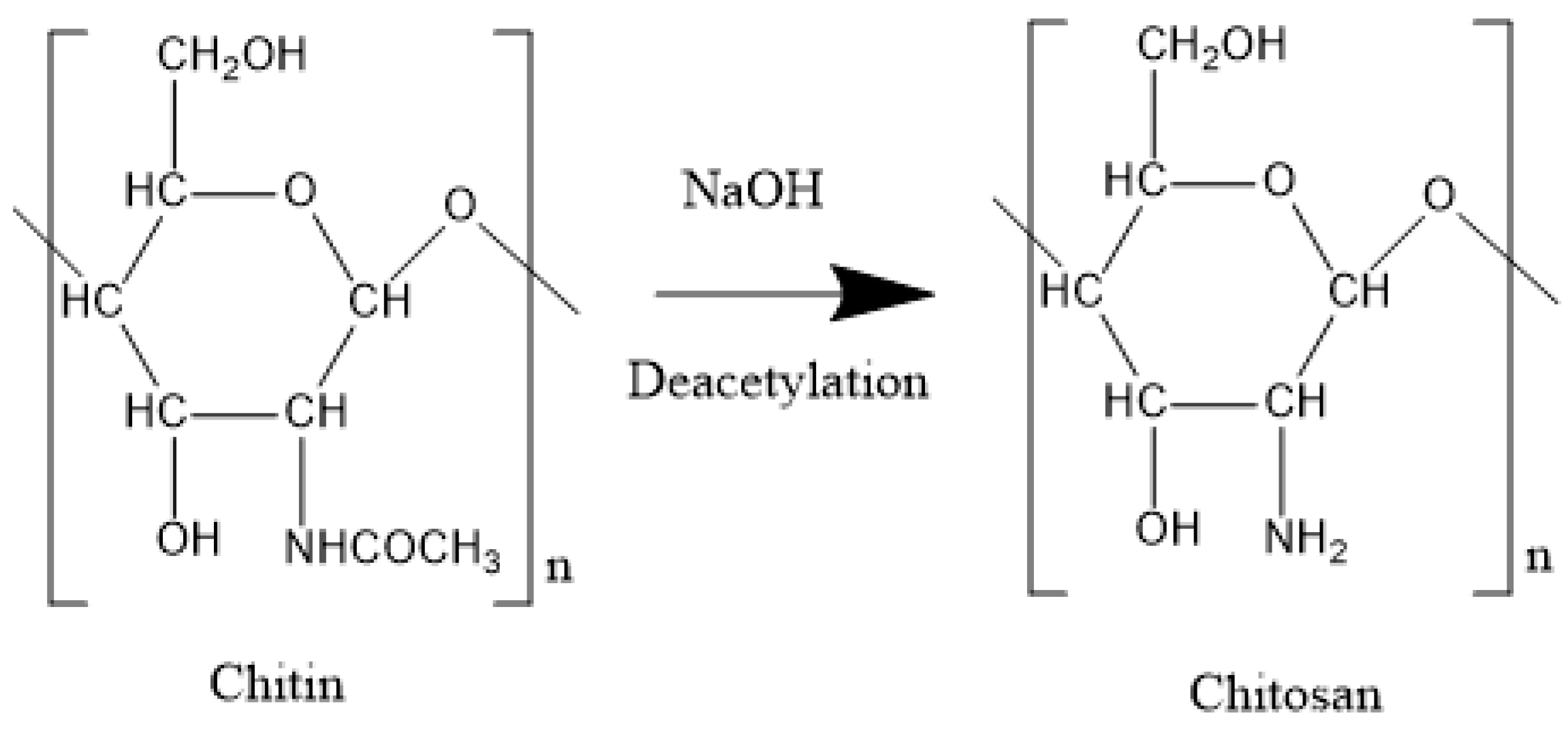
2.5. Chitin and Chitosan
3. Evaluation of Biopolymer
3.1. Rheological Analysis
3.2. Filtration Test
3.3. Surfactant–Polymer Compatibility Test
3.4. Core Flooding
4. Application of Polysaccharide Biopolymer in Petroleum Recovery
4.1. Drilling Fluid
4.1.1. Rheological Properties of Drilling Fluid
4.1.2. Fluid Loss Prevention
4.1.3. Drilling Fluid Stability
4.2. Hydraulic Fluid
4.2.1. Rheology Properties of Linear Biopolymer
4.2.2. Biopolymer Crosslinking
4.2.3. Biopolymer Breaking
4.3. Enhance Oil Recovery
4.3.1. Rheology Properties of Polymer Flooding Solution
4.3.2. Filtration Properties
4.3.3. Polymer Flooding Compatibility
4.3.4. Polymer Flooding Stability
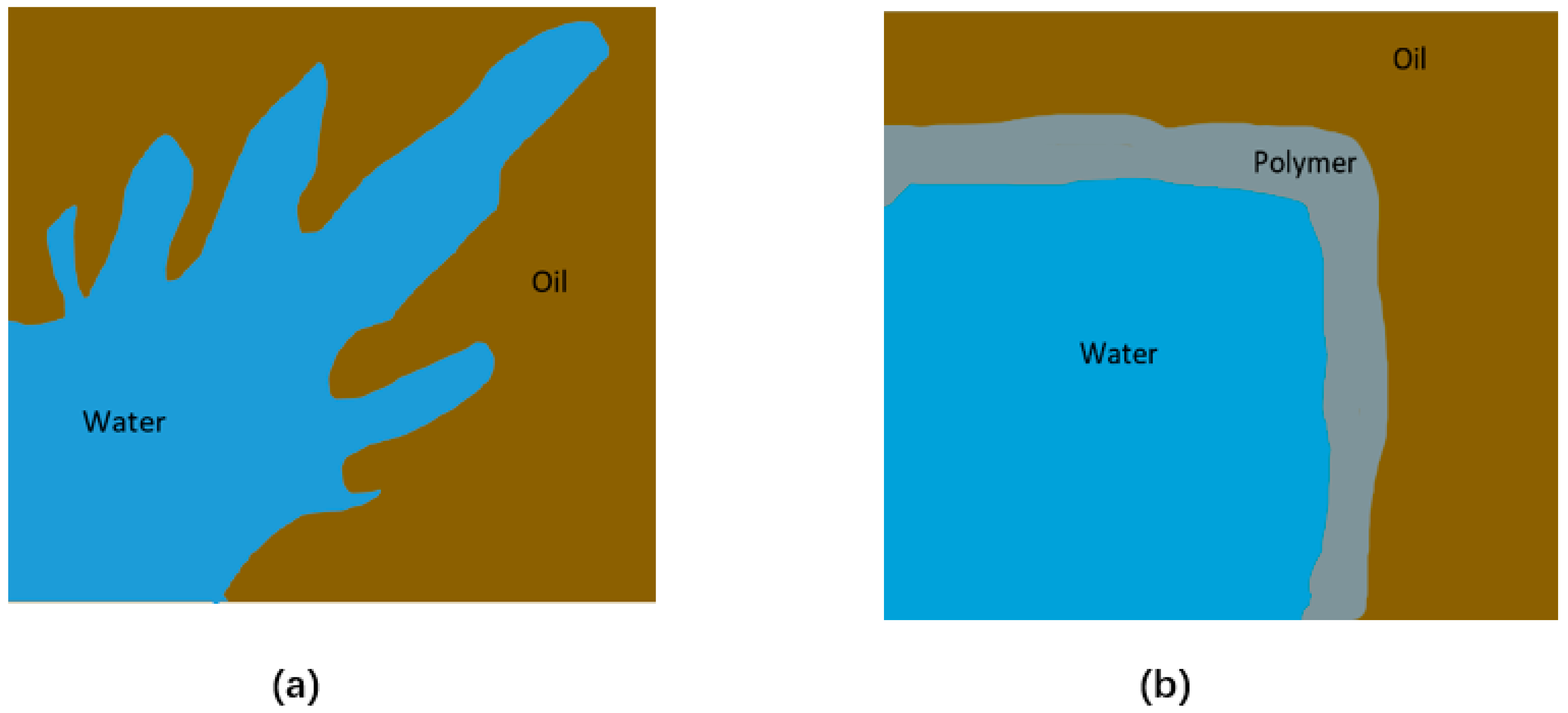
4.4. Bio-Plugging
4.4.1. Gelled Biopolymer
4.4.2. Microbial Plugging
4.5. Wastewater Treatment
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogovina, S.; Prut, E.; Berlin, A. Composite Materials Based on Synthetic Polymers Reinforced with Natural Fibers. Polym. Sci. Ser. A 2019, 61, 417–438. [Google Scholar] [CrossRef]
- Tang, X.; Kumar, P.; Alavi, S.; Sandeep, K. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef]
- Buescher, J.M.; Margaritis, A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit. Rev. Biotechnol. 2007, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of Chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Kim, S.; Selling, G.W.; Cheng, H. Conversion of agricultural residues to carboxymethylcellulose and carboxymethylcellulose acetate. Ind. Crops Prod. 2014, 60, 259–265. [Google Scholar] [CrossRef]
- LoPachin, R.M. The changing view of acrylamide neurotoxicity. Neurotoxicology 2004, 25, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 1–9. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of polymer-flooding technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Glaser, L.; Beach, D. Industrial Uses of Agricultural Materials Situation and Outlook Report; IUS-2; United States Department of Agriculture: Washington, DC, USA, 1993.
- Garcıa-Ochoa, F.; Santos, V.; Casas, J.; Gómez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Ivanov, V.; Karak, N.; Jonkers, H. Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bergmann, D.; Furth, G.; Mayer, C. Binding of bivalent cations by Xanthan in aqueous solution. Int. J. Biol. Macromol. 2008, 43, 245–251. [Google Scholar] [CrossRef]
- Bejenariu, A.; Popa, M.; Dulong, V.; Picton, L.; Le Cerf, D. Trisodium trimetaphosphate crosslinked Xanthan networks: Synthesis, swelling, loading and releasing behaviour. Polym. Bull. 2009, 62, 525–538. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Casas, J.; Mohedano, A. Xanthan precipitation from solutions and fermentation broths. Sep. Sci. Technol. 1993, 28, 1303–1313. [Google Scholar] [CrossRef]
- Reddy, B. Viscosification-on-demand: Chemical modification of biopolymers to control their activation by triggers in aqueous solutions. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011. [Google Scholar]
- Tian, M.; Fang, B.; Jin, L.; Lu, Y.; Qiu, X.; Jin, H.; Li, K. Rheological and drag reduction properties of hydroxypropyl Xanthan gum solutions. Chin. J. Chem. Eng. 2015, 23, 1440–1446. [Google Scholar] [CrossRef]
- Su, L.; Ji, W.; Lan, W.; Dong, X. Chemical modification of Xanthan gum to increase dissolution rate. Carbohydr. Polym. 2003, 53, 497–499. [Google Scholar] [CrossRef]
- Farina, J.; Sineriz, F.; Molina, O.; Perotti, N. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 2001, 44, 41–50. [Google Scholar] [CrossRef]
- Sandford, P.A. Advances in Carbohydrate Chemistry and Biochemistry; Exocellular, Microbial Polysaccharides; Elsevier: Amsterdam, The Netherlands, 1979; Volume 36, pp. 265–313. [Google Scholar]
- Kalpakci, B.; Jeans, Y.; Magri, N.; Padolewski, J. Thermal stability of scleroglucan at realistic reservoir conditions. In Proceedings of the SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, OK, USA, 22–25 April 1990. [Google Scholar]
- Leonhardt, B.; Visser, F.; Lessner, E.; Wenzke, B.; Schmidt, J. From flask to field—The long road to development of a new polymer. In Proceedings of the IOR 2011-16th European Symposium on Improved Oil Recovery, Cambridge, UK, 12–14 April 2011; p. cp-230-00031. [Google Scholar]
- Kashiwagi, Y.; Norisuye, T.; Fujita, H. Triple helix of Schizophyllum commune polysaccharide in dilute solution. 4. Light scattering and viscosity in dilute aqueous sodium hydroxide. Macromolecules 1981, 14, 1220–1225. [Google Scholar] [CrossRef]
- Bakhshi, M.; Ozeiri, M.; Sharif, A.; Aalaie, J. Effect of hydrophobic modification on the structure and rheology of aqueous and brine solutions of scleroglucan polymer. Korean J. Chem. Eng. 2017, 34, 903–912. [Google Scholar] [CrossRef]
- Chudzikowski, R. Guar gum and its applications. J. Soc. Cosmet. Chem. 1971, 22, 43. [Google Scholar]
- Grasdalen, H.; Painter, T. NMR studies of composition and sequence in legume-seed galactomannans. Carbohydr. Res. 1980, 81, 59–66. [Google Scholar] [CrossRef]
- Garti, N.; Leser, M.E. Emulsification properties of hydrocolloids. Polym. Adv. Technol. 2001, 12, 123–135. [Google Scholar] [CrossRef]
- Parija, S.; Misra, M.; Mohanty, A. Studies of natural gum adhesive extracts: An overview. J. Macromol. Sci. Part C Polym. Rev. 2001, 41, 175–197. [Google Scholar] [CrossRef]
- Wise, L.E. The Chemistry of Plant Gums and Mucilages and Some Related Polysaccharides. ACS Monograph No. 141. J. Am. Chem. Soc. 1960, 82, 3232. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Yang, Y.-Y.; Chung, T.-S. The influence of cold treatment on properties of temperature-sensitive poly (N-isopropylacrylamide) hydrogels. J. Colloid Interface Sci. 2002, 246, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Singh, R. Synthesis and characterization of grafted hydroxypropyl guar gum by ceric ion induced initiation. Eur. Polym. J. 2001, 37, 1655–1666. [Google Scholar] [CrossRef]
- Lawal, O.S.; Lechner, M.D.; Hartmann, B.; Kulicke, W.M. Carboxymethyl cocoyam starch: Synthesis, characterisation and influence of reaction parameters. Starch-Stärke 2007, 59, 224–233. [Google Scholar] [CrossRef]
- Pasha, M.; Ngn, S. Derivatization of guar to sodium carboxymethyl hydroxypropyl derivative; characterization and evaluation. Pak. J. Pharm. Sci 2008, 21, 40–44. [Google Scholar]
- Lei, C.; Clark, P.E. Crosslinking of guar and guar derivatives. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 26–29 September 2004. [Google Scholar]
- Masaoka, S.; Ohe, T.; Sakota, N. Production of cellulose from glucose by Acetobacter xylinum. J. Ferment. Bioeng. 1993, 75, 18–22. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Gupta, P.K.; Raghunath, S.S.; Prasanna, D.V.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. Cellulose; An Update on Overview of Cellulose, Its Structure and Applications; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Purves, C.B. Chemical nature of cellulose and its derivatives. Cellul. Cellul. Deriv. Part 1954, 1, 29–98. [Google Scholar]
- Abdel-Halim, E. Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cellulose. Arab. J. Chem. 2014, 7, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Liebert, T.; Meister, F.; Heinze, T. Homogenous carboxymethylation of cellulose in the NaOH/urea aqueous solution. React. Funct. Polym. 2009, 69, 779–784. [Google Scholar] [CrossRef]
- Labille, J.; Thomas, F.; Milas, M.; Vanhaverbeke, C. Flocculation of colloidal clay by bacterial polysaccharides: Effect of macromolecule charge and structure. J. Colloid Interface Sci. 2005, 284, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, A.; Jin, Y.; Cheng, Q. Synthesis of amphoteric cellulose in aqueous NaOH–urea solution in one pot and its application in paper strength enhancement. RSC Adv. 2013, 3, 24586–24592. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Songkroah, C.; Nakbanpote, W.; Thiravetyan, P. Recovery of silver-thiosulphate complexes with Chitin. Process Biochem. 2004, 39, 1553–1559. [Google Scholar] [CrossRef]
- Hattori, H.; Ishihara, M. Changes in blood aggregation with differences in molecular weight and degree of deacetylation of Chitosan. Biomed. Mater. 2015, 10, 015014. [Google Scholar] [CrossRef]
- Tsaih, M.L.; Chen, R.H. Effect of molecular weight and urea on the conformation of Chitosan molecules in dilute solutions. Int. J. Biol. Macromol. 1997, 20, 233–240. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Chang, H.-W.; Yu, H.-C.; Lin, Y.-S.; Tsai, Y.-D. Effect of Chitosan characteristics and solution conditions on gelation temperatures of Chitosan/2-glycerophosphate/nanosilver hydrogels. Carbohydr. Polym. 2011, 84, 1337–1343. [Google Scholar] [CrossRef]
- Abraham, S.; Rajamanickam, D.; Srinivasan, B. Research Article Preparation, Characterization and Cross-Linking of Chitosan by Microwave Assisted Synthesis. Sci. Int. 2018, 6, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.R.; Huang, C.; Chen, S.; Chung, Y.-C. Evaluation of a modified Chitosan biopolymer for coagulation of colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1999, 147, 359–364. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and Chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, J.A.; Bumgardner, J.D. Chitosan Based Biomaterials Volume 1: Fundamentals; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Chang, K.L.B.; Tai, M.-C.; Cheng, F.-H. Kinetics and products of the degradation of Chitosan by hydrogen peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.M.; Liang, H.B.; Yao, P.J.; Wei, Y.A. Effect of immobilized neutral protease on the preparation and physicochemical properties of low molecular weight Chitosan and chito-oligomers. J. Appl. Polym. Sci. 2006, 102, 4185–4193. [Google Scholar] [CrossRef]
- Tsaih, M.L.; Tseng, L.Z.; Chen, R.H. Effects of removing small fragments with ultrafiltration treatment and ultrasonic conditions on the degradation kinetics of Chitosan. Polym. Degrad. Stab. 2004, 86, 25–32. [Google Scholar] [CrossRef]
- Tsai, M.L.; Tseng, L.Z.; Chen, R.H. Two-stage microfluidization combined with ultrafiltration treatment for Chitosan mass production and molecular weight manipulation. Carbohydr. Polym. 2009, 77, 767–772. [Google Scholar] [CrossRef]
- Tsai, M.L.; Tseng, L.Z.; Chang, H.W.; Hsu, C.H.; Chen, R.H. Facile method to manipulate the molecular weight and practical mass production of Chitosan by mechanical shearing and concurrent ultrafiltration treatment. J. Appl. Polym. Sci. 2010, 118, 1442–1449. [Google Scholar] [CrossRef]
- Margaritis, A.; Zajic, J.E. Mixing, mass transfer, and scale-up of polysaccharide fermentations. Biotechnol. Bioeng. 1978, 20, 939–1001. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of Xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzym. Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Souw, P.; Demain, A.L. Role of citrate in Xanthan production by Xanthomonas campestris. J. Ferment. Technol. 1980, 58, 411–416. [Google Scholar]
- Souw, P.; Demain, A.L. Nutritional studies on Xanthan production by Xanthomonas campestris NRRL B1459. Appl. Environ. Microbiol. 1979, 37, 1186–1192. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.H.; Yang, S.T. Effects of temperature on cell growth and Xanthan production in batch cultures of Xanthomonas campestris. Biotechnol. Bioeng. 1990, 35, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Santos, V.; Alcon, A. Simulation of Xanthan gum production by a chemically structured kinetic model. Math. Comput. Simul. 1996, 42, 187–195. [Google Scholar] [CrossRef]
- Tako, M.; Nakamura, S. Rheological properties of deacetylated Xanthan in aqueous media. Agric. Biol. Chem. 1984, 48, 2987–2993. [Google Scholar]
- Khouryieh, H.; Herald, T.; Aramouni, F.; Bean, S.; Alavi, S. Influence of deacetylation on the rheological properties of Xanthan–guar interactions in dilute aqueous solutions. J. Food Sci. 2007, 72, C173–C181. [Google Scholar] [CrossRef] [PubMed]
- Halleck, F.E. Polysaccharides and Methods for Production Thereof. U.S. Patent No. 3,301,848, 31 January 1967. [Google Scholar]
- Cottrell, I. Industrial Potential of Fungal and Bacterial Polysaccharides; ACS Publications: Washington, DC, USA, 1980. [Google Scholar]
- Valdez, A.L.; Babot, J.D.; Schmid, J.; Delgado, O.D.; Fariña, J.I. Scleroglucan Production by Sclerotium rolfsii ATCC 201126 from Amylaceous and Sugarcane Molasses-Based Media: Promising Insights for Sustainable and Ecofriendly Scaling-Up. J. Polym. Environ. 2019, 27, 2804–2818. [Google Scholar] [CrossRef]
- Survase, S.A.; Saudagar, P.S.; Bajaj, I.B.; Singhal, R.S. Scleroglucan: Fermentative production, downstream processing and applications. Food Technol. Biotechnol. 2007, 45, 107–118. [Google Scholar]
- Johal, S.S.; Coleman, G.M. Recovery of Glucan by Employing a Divalent Cation at An Alkaline pH. U.S. Patent 4,950,749, 21 August 1990. [Google Scholar]
- DES. Directorate of Economics and Statistics, Ministry of Agriculture and Farmers Welfare, Government of India. 2015. Available online: https://eands.dacnet.nic.in/ (accessed on 21 May 2020).
- Sabahelkheir Murwan, K.; Abdalla Abdelwahab, H.; Nouri Sulafa, H. Quality assessment of guar gum (endosperm) of guar (Cyamopsis tetragonoloba). ISCA J. Biol. Sci. 2012, 1, 67–70. [Google Scholar]
- John, H.; William, G.; Herman, F. The role of the Endosperm in the germination of legumes: Gallactomannas, Nitrogen, Phosphorous change in the Germination of Guar (Cyamopsis tetragonolobus; Leguminosae). Am. J. Bot. 1976, 63, 790–797. [Google Scholar]
- Trivedi, J.H.; Kalia, K.; Patel, N.K.; Trivedi, H. Ceric-induced grafting of acrylonitrile onto sodium salt of partially carboxymethylated guar gum. Carbohydr. Polym. 2005, 60, 117–125. [Google Scholar] [CrossRef]
- Simon, J.; Müller, H.; Koch, R.; Müller, V. Thermoplastic and biodegradable polymers of cellulose. Polym. Degrad. Stab. 1998, 59, 107–115. [Google Scholar] [CrossRef]
- Mosier, N.S.; Ladisch, C.M.; Ladisch, M.R. Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnol. Bioeng. 2002, 79, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Rozet, E.; Carlin, B.; Evrard, B. Understanding the impact of microcrystalline cellulose physicochemical properties on tabletability. Int. J. Pharm. 2015, 490, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Power, D.; Zamora, M. Drilling fluid yield stress: Measurement techniques for improved understanding of critical drilling fluid parameters. In Proceedings of the AADE Technical Conference, Houston, TX, USA, 1–3 April 2003; pp. 1–3. [Google Scholar]
- Kulawardana, E.U.; Koh, H.; Kim, D.H.; Liyanage, P.J.; Upamali, K.; Huh, C.; Weerasooriya, U.; Pope, G.A. Rheology and transport of improved EOR polymers under harsh reservoir conditions. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Chauveteau, G.; Kohler, N. Influence of microgels in polysaccharide solutions on their flow behavior through porous media. Soc. Pet. Eng. J. 1984, 24, 361–368. [Google Scholar] [CrossRef]
- Shedid, S.A. Influences of fracture orientation on oil recovery by water and polymer flooding processes: An experimental approach. J. Pet. Sci. Eng. 2006, 50, 285–292. [Google Scholar] [CrossRef]
- Hamed, S.B.; Belhadri, M. Rheological properties of biopolymers drilling fluids. J. Pet. Sci. Eng. 2009, 67, 84–90. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M. Herschel-Bulkley rheological parameters of a novel environmentally friendly lightweight biopolymer drilling fluid from Xanthan gum and starch. J. Appl. Polym. Sci. 2012, 124, 595–606. [Google Scholar] [CrossRef]
- Song, K.; Wu, Q.; Li, M.-C.; Wojtanowicz, A.K.; Dong, L.; Zhang, X.; Ren, S.; Lei, T. Performance of low solid bentonite drilling fluids modified by cellulose nanoparticles. J. Nat. Gas Sci. Eng. 2016, 34, 1403–1411. [Google Scholar] [CrossRef]
- Silva, Í.G.; Lucas, E.F. Rheological properties of Xanthan gum, hydroxypropyl starch, cashew gum and their binary mixtures in aqueous solutions. In Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2018; p. 1800070. [Google Scholar]
- Li, M.-C.; Wu, Q.; Song, K.; De Hoop, C.F.; Lee, S.; Qing, Y.; Wu, Y. Cellulose nanocrystals and polyanionic cellulose as additives in bentonite water-based drilling fluids: Rheological modeling and filtration mechanisms. Ind. Eng. Chem. Res. 2016, 55, 133–143. [Google Scholar] [CrossRef]
- Koocheki, A.; Taherian, A.R.; Bostan, A. Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Res. Int. 2013, 50, 446–456. [Google Scholar] [CrossRef]
- Talabani, S.; Hatzignatiou, D.; Chukwu, G. Comprehensive description and evaluation of polymers as drilling fluids. In Proceedings of the SPE Western Regional Meeting, Anchorage, AK, USA, 26–28 May 1993. [Google Scholar]
- Kafashi, S.; Rasaei, M.; Karimi, G. Effects of sugarcane and polyanionic cellulose on rheological properties of drilling mud: An experimental approach. Egypt. J. Pet. 2017, 26, 371–374. [Google Scholar] [CrossRef] [Green Version]
- Ademiluyi Taiwo, J.O.; Kazeem, A.A. Investigation of local polymer (cassava starches) as a substitute for imported sample in viscosity and fluid loss control of water based drilling mud. ARPN J. Eng. Appl. Sci. 2006, 6, 43–48. [Google Scholar]
- Mahto, V.; Sharma, V. Rheological study of a water based oil well drilling fluid. J. Pet. Sci. Eng. 2004, 45, 123–128. [Google Scholar] [CrossRef]
- Rommetveit, R.; Bjorkevoll, K. Temperature and pressure effects on drilling fluid rheology and ECD in very deep wells. In Proceedings of the SPE/IADC Middle East Drilling Technology Conference, Manama, Bahrain, 23–25 November 1997. [Google Scholar]
- Meng, X.; Zhang, Y.; Zhou, F.; Chu, P.K. Effects of carbon ash on rheological properties of water-based drilling fluids. J. Pet. Sci. Eng. 2012, 100, 1–8. [Google Scholar] [CrossRef]
- Chenu, C.; Pons, C.; Robert, M. Interaction of kaolinite and montmorillonite with neutral polysaccharides. In Proceedings of the the International Clay Conference, Denver, CO, USA, 1–3 July 1985; pp. 375–381. [Google Scholar]
- Browning, W.C. Hydrosols and Rheology; Colloid Chemical Aspects of Drilling Fluid Rheology; Elsevier: Amsterdam, The Netherlands, 1976; pp. 563–581. [Google Scholar]
- Plank, J.; Gossen, F. Visualization of fluid-loss polymers in drilling-mud filter cakes. SPE Drill. Eng. 1991, 6, 203–208. [Google Scholar] [CrossRef]
- Fereydouni, M.; Sabbaghi, S.; Saboori, R.; Zeinali, S. Effect of polyanionic cellulose polymer nanoparticles on rheological properties of drilling mud. Int. J. Nanosci. Nanotechnol. 2012, 8, 171–174. [Google Scholar]
- Li, M.-C.; Wu, Q.; Song, K.; Lee, S.; Jin, C.; Ren, S.; Lei, T. Soy protein isolate as fluid loss additive in bentonite–water-based drilling fluids. ACS Appl. Mater. Interfaces 2015, 7, 24799–24809. [Google Scholar] [CrossRef]
- Adewole, J.K.; Najimu, M.O. A study on the effects of date pit-based additive on the performance of water-based drilling fluid. J. Energy Resour. Technol. 2018, 140. [Google Scholar] [CrossRef]
- Ash, S.; Clarke-Sturman, A.; Calvert, R.; Nisbet, T. Chemical stability of biopolymer solutions. In Proceedings of the the SPE Annual Technical Conference and Exhibition, San Francisco, CA, USA, 5–8 October 1983. [Google Scholar]
- Zou, Z.; Zhao, Q.; Wang, Q.; Zhou, F. Thermal stability of Xanthan gum biopolymer and its application in salt-tolerant bentonite water-based mud. J. Polym. Eng. 2019, 39, 501–507. [Google Scholar] [CrossRef]
- Howard, S.; Kaminski, L.; Downs, J. Xanthan Stability in Formate Brines-Formulating Non-damaging Fluids for High Temperature Applications. In Proceedings of the SPE European Formation Damage Conference and Exhibition, Budapest, Hungary, 3–5 June 2015. [Google Scholar]
- Akpan, E.; Enyi, G.; Nasr, G.; Yahaya, A. Stabilizing biopolymers in water-based drilling fluids at high temperature using antioxidants, a formate salt, and polyglycol. J. Eng. Technol. 2018, 7, 469–486. [Google Scholar]
- Clark, J. A hydraulic process for increasing the productivity of wells. J. Pet. Technol. 1949, 1, 1–8. [Google Scholar] [CrossRef]
- Hurst, W. Establishment of the skin effect and its impediment to fluid flow into a well bore. Pet. Eng. 1953, 25, B6–B16. [Google Scholar]
- Ely, J.W. Fracturing fluids and additives. Recent Adv. Hydraul. Fract. 1989, 12, 196–211. [Google Scholar]
- Gidley, J.L. Recent Advances in Hydraulic Fracturing. 1989. Available online: https://www.osti.gov/biblio/6558063-recent-advances-hydraulic-fracturing (accessed on 28 May 2020).
- Ihejirika, B.; Dosunmu, A.; Eme, C. Performance evaluation of guar gum as a carrier fluid for hydraulic fracturing. In Proceedings of the SPE Nigeria Annual International Conference and Exhibition, Lagos, Nigeria, 4–6 August 2015. [Google Scholar]
- Casas, J.A.; Mohedano, A.F.; García-Ochoa, F. Viscosity of guar gum and Xanthan/guar gum mixture solutions. J. Sci. Food Agric. 2000, 80, 1722–1727. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Sokhanvarian, K.; AlKhaldi, M.; Nasr-El-Din, H. Improving Hydraulic Fracturing Fluids Through Dual Polymer Technology. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 24–26 September 2018. [Google Scholar]
- Sarwar, M.U.; Cawiezel, K.E.; Nasr-El-Din, H.A. Gel degradation studies of oxidative and enzyme breakers to optimize breaker type and concentration for effective break profiles at low and medium temperature ranges. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 24–26 January 2011. [Google Scholar]
- Esmaeilirad, N.; Terry, C.; Kennedy, H.; Prior, A.; Carlson, K. Recycling fracturing flowback water for use in hydraulic fracturing: Influence of organic matter on stability of carboxyl-methyl-cellulose-based fracturing fluids. SPE J. 2016, 21, 1358–1369. [Google Scholar] [CrossRef]
- Esmaeilirad, N.; Terry, C.; Carlson, K. Optimization of carboxylmethyl cellulose frac fluid in low TDS water sources based on pH and crosslinker concentrations. Fuel 2016, 185, 211–218. [Google Scholar] [CrossRef]
- Fan, H.; Gong, Z.; Wei, Z.; Chen, H.; Fan, H.; Geng, J.; Kang, W.; Dai, C. Understanding the temperature–resistance performance of a borate cross-linked hydroxypropyl guar gum fracturing fluid based on a facile evaluation method. RSC Adv. 2017, 7, 53290–53300. [Google Scholar] [CrossRef] [Green Version]
- Almubarak, T.; Ng, J.H.; Sokhanvarian, K.; Khaldi, M.; Nasr-El-Din, H.A. Development of a Mixed Polymer Hydraulic Fracturing Fluid for High Temperature Applications. In Proceedings of the Unconventional Resources Technology Conference, Houston, TX, USA, 23–25 July 2018; pp. 4145–4162. [Google Scholar]
- Harris, P.C. Chemistry and rheology of borate-crosslinked fluids at temperatures to 300F. J. Pet. Technol. 1993, 45, 264–269. [Google Scholar] [CrossRef]
- Rae, P.; Di Lullo, G. Fracturing fluids and breaker systems—A review of the State-of-the-Art. In Proceedings of the SPE Eastern Regional Meeting, Columbus, OH, USA, 23–25 October 1996. [Google Scholar]
- Sun, H.; Qu, Q. High-efficiency boron crosslinkers for low-polymer fracturing fluids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011. [Google Scholar]
- Chauhan, G.; Verma, A.; Doley, A.; Ojha, K. Rheological and breaking characteristics of Zr-crosslinked gum karaya gels for high-temperature hydraulic fracturing application. J. Pet. Sci. Eng. 2019, 172, 327–339. [Google Scholar] [CrossRef]
- Legemah, M.; Guerin, M.; Sun, H.; Qu, Q. Novel high-efficiency boron crosslinkers for low-polymer-loading fracturing fluids. SPE J. 2014, 19, 737–743. [Google Scholar] [CrossRef]
- Kalgaonkar, R.A.; Patil, P.R. Performance Enhancements in Metal-Crosslinked Fracturing Fluids. In Proceedings of the North Africa Technical Conference and Exhibition, Cairo, Egypt, 20–22 February 2012. [Google Scholar]
- Zhang, B.; Davenport, A.H.; Whipple, L.; Urbina, H.; Barrett, K.; Wall, M.; Hutchins, R.; Mirakyan, A. A Superior, high-performance enzyme for breaking borate crosslinked fracturing fluids under extreme well conditions. SPE Prod. Oper. 2013, 28, 210–216. [Google Scholar] [CrossRef]
- Barati, R.; Johnson, S.J.; McCool, S.; Green, D.W.; Willhite, G.P.; Liang, J.T. Fracturing fluid cleanup by controlled release of enzymes from polyelectrolyte complex nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Al-Muntasheri, G.A. A Critical Review of Hydraulic-Fracturing Fluids for Moderate-to Ultralow-Permeability Formations Over the Last Decade. SPE Prod. Oper. 2014, 29, 243–260. [Google Scholar] [CrossRef]
- Pye, D.J. Improved secondary recovery by control of water mobility. J. Pet. Technol. 1964, 16, 911–916. [Google Scholar] [CrossRef]
- Sandiford, B. Laboratory and field studies of water floods using polymer solutions to increase oil recoveries. J. Pet. Technol. 1964, 16, 917–922. [Google Scholar] [CrossRef]
- Thomas, A. Polymer flooding. InTech 2016. [Google Scholar] [CrossRef] [Green Version]
- Samanta, A.; Bera, A.; Ojha, K.; Mandal, A. Effects of alkali, salts, and surfactant on rheological behavior of partially hydrolyzed polyacrylamide solutions. J. Chem. Eng. Data 2010, 55, 4315–4322. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of Xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244, 160–170. [Google Scholar] [CrossRef]
- Benchabane, A.; Bekkour, K. Rheological properties of carboxymethyl cellulose (CMC) solutions. Colloid Polym. Sci. 2008, 286, 1173. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Lu, J.-P.; Li, X.-H.; Tong, Z.-F. Effect of mechanical activation on physico-chemical properties and structure of cassava starch. Carbohydr. Polym. 2007, 68, 128–135. [Google Scholar] [CrossRef]
- Alamri, M.S.; Mohamed, A.; Hussain, S.; Xu, J. Effect of Okra extract on properties of wheat, corn and rice starches. J. Food Agric. Environ. 2012, 10, 217–222. [Google Scholar]
- Sengkhamparn, N.; Sagis, L.M.; De Vries, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G. Physicochemical properties of pectins from okra (Abelmoschus esculentus (L.) Moench). Food Hydrocoll. 2010, 24, 35–41. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Gbonhinbor, J.; Onyekonwu, M. Natural polymer flow behaviour in porous media for enhanced oil recovery applications: A review. J. Pet. Explor. Prod. Technol. 2018, 8, 1349–1362. [Google Scholar] [CrossRef] [Green Version]
- Zaharuddin, N.D.; Noordin, M.I.; Kadivar, A. The use of Hibiscus esculentus (Okra) gum in sustaining the release of propranolol hydrochloride in a solid oral dosage form. BioMed Res. Int. 2014, 2014, 735891. [Google Scholar] [CrossRef] [Green Version]
- Bashir, M.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Ojedokun, O.S.; Fasogbon, B.M. Physico-chemical properties of instant ogbono (Irvingia gabonensis) mix powder. Food Sci. Nutr. 2015, 3, 313–318. [Google Scholar] [CrossRef]
- Abuajah, C.I.; Alonge, A.F. Physical Properties of African Kidney Bean (Phaseolus vulgaris) Relevant to its Processing. Food Biol. 2013, 2, 18–23. [Google Scholar]
- Levitt, D.; Pope, G. Selection and screening of polymers for enhanced-oil recovery. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 20–23 April 2008. [Google Scholar]
- Kohler, N.; Chauveteau, G. Xanthan polysaccharide plugging behavior in porous media-preferential use of fermentation broth. J. Pet. Technol. 1981, 33, 349–358. [Google Scholar] [CrossRef]
- Philips, J.; Miller, J.; Wernau, W.; Tate, B.; Auerbach, M. A high-pyruvate Xanthan for EOR. Soc. Pet. Eng. J. 1985, 25, 594–602. [Google Scholar] [CrossRef]
- Jewett, R.; Schurz, G. Polymer flooding-a current appraisal. J. Pet. Technol. 1970, 22, 675–684. [Google Scholar] [CrossRef]
- Selby, R.; Alikhan, A.; Ali, S. Potential of non-thermal methods for heavy oil recovery. J. Can. Pet. Technol. 1989, 28. [Google Scholar] [CrossRef]
- Rellegadla, S.; Prajapat, G.; Agrawal, A. Polymers for enhanced oil recovery: Fundamentals and selection criteria. Appl. Microbiol. Biotechnol. 2017, 101, 4387–4402. [Google Scholar] [CrossRef] [PubMed]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Du, Y.; Guan, L. Field-scale polymer flooding: Lessons learnt and experiences gained during past 40 years. In Proceedings of the SPE International Petroleum Conference in Mexico, Puebla, Mexico, 7–9 November 2004. [Google Scholar]
- Reed, R.L.; Healy, R.N. Some physicochemical aspects of microemulsion flooding: A review. Improv. Oil Recovery Surfactant Polym. Flooding 1977, 383–437. [Google Scholar] [CrossRef]
- Goddard, E. Interactions of Surfactants with Polymers and Proteins; Polymer-Surfactant Interaction: Part I. Uncharged Water-Soluble Polymers and Charged Surfactants; CRC Press: Boca Raton, FL, USA, 2018; pp. 123–202. [Google Scholar]
- Lachke, A. Xanthan—A versatile gum. Resonance 2004, 9, 25–33. [Google Scholar] [CrossRef]
- Alquraishi, A.A.; Alsewailem, F.D. Xanthan and guar polymer solutions for water shut off in high salinity reservoirs. Carbohydr. Polym. 2012, 88, 859–863. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and Xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef]
- Lund, T.; Lecourtier, J.; Müller, G. Properties of Xanthan solutions after long-term heat treatment at 90 C. Polym. Degrad. Stab. 1990, 27, 211–225. [Google Scholar] [CrossRef]
- Lai, N.; Wen, Y.; Yang, Z.; Chen, J.; Zhao, X.; Wang, D.; He, W.; Chen, Y. Polymer flooding in high-temperature and high-salinity heterogeneous reservoir by using diutan gum. J. Pet. Sci. Eng. 2020, 188, 106902. [Google Scholar] [CrossRef]
- Quadri, S.M.R.; Jiran, L.; Shoaib, M.; Hashmet, M.R.; AlSumaiti, A.M.; Alhassan, S.M. Application of biopolymer to improve oil recovery in high temperature high salinity carbonate reservoirs. In Proceedings of the Abu Dhabi international petroleum exhibition and conference, Abu Dhabi, UAE, 9–12 November 2015. [Google Scholar]
- Chang, H.L. Polymer flooding technology yesterday, today, and tomorrow. J. Pet. Technol. 1978, 30, 1113–1128. [Google Scholar] [CrossRef]
- Celik, M.; Ahmad, S.; Al-Hashim, H. Adsorption/desorption of polymers from Saudi Arabian limestone. J. Pet. Sci. Eng. 1991, 6, 213–223. [Google Scholar] [CrossRef]
- Stewart, T.L.; Fogler, H.S. Biomass plug development and propagation in porous media. Biotechnol. Bioeng. 2001, 72, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Etemadi, O.; Petrisor, I.G.; Kim, D.; Wan, M.-W.; Yen, T.F. Stabilization of metals in subsurface by biopolymers: Laboratory drainage flow studies. Soil Sediment Contam. 2003, 12, 647–661. [Google Scholar] [CrossRef]
- McCool, C.; Green, D.; Willhite, G.P. Permeability reduction mechanisms involved in in-situ gelation of a polyacrylamide/chromium (VI)/thiourea system. SPE Reserv. Eng. 1991, 6, 77–83. [Google Scholar] [CrossRef]
- Vossoughi, S.; Buller, C. Permeability modification by in-situ gelation with a newly discovered biopolymer. SPE Reserv. Eng. 1991, 6, 485–489. [Google Scholar] [CrossRef]
- Eggert, R., Jr.; Willhite, G.; Green, D. Experimental measurement of the persistence of permeability reduction in porous media treated with Xanthan/Cr (III) gel systems. SPE Reserv. Eng. 1992, 7, 29–35. [Google Scholar] [CrossRef]
- Fletcher, A.; Lamb, S.; Clifford, P. Formation damage from polymer solutions: Factors governing injectivity. SPE Reserv. Eng. 1992, 7, 237–246. [Google Scholar] [CrossRef]
- Hermans, J. Statistical thermodynamics of swollen polymer networks. J. Polym. Sci. 1962, 59, 191–208. [Google Scholar] [CrossRef]
- Hoefner, M.L.; Seetharam, R.V.; Shu, P.; Phelps, C.H. Selective penetration of biopolymer profile-control gels: Experiment and model. J. Pet. Sci. Eng. 1992, 7, 53–66. [Google Scholar] [CrossRef]
- Khachatoorian, R.; Petrisor, I.G.; Kwan, C.-C.; Yen, T.F. Biopolymer plugging effect: Laboratory-pressurized pumping flow studies. J. Pet. Sci. Eng. 2003, 38, 13–21. [Google Scholar] [CrossRef]
- Bao, M.; Kong, X.; Jiang, G.; Wang, X.; Li, X. Laboratory study on activating indigenous microorganisms to enhance oil recovery in Shengli Oilfield. J. Pet. Sci. Eng. 2009, 66, 42–46. [Google Scholar] [CrossRef]
- Reksidler, R.; Volpon, A.G.; Barbosa, L.C.F.; Brasileiro, C.G.; Nicolau, H.C.C.; Calasans, J.A. A microbial enhanced oil recovery field pilot in a Brazilian Onshore Oilfield. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Le, J.-J.; Wu, X.-L.; Wang, R.; Zhang, J.-Y.; Bai, L.-L.; Hou, Z.-W. Progress in pilot testing of microbial-enhanced oil recovery in the Daqing oilfield of north China. Int. Biodeterior. Biodegrad. 2015, 97, 188–194. [Google Scholar] [CrossRef]
- Jeong, M.S.; Noh, D.-H.; Hong, E.; Lee, K.S.; Kwon, T.-H. Systematic modeling approach to selective plugging using in situ bacterial biopolymer production and its potential for microbial-enhanced oil recovery. Geomicrobiol. J. 2019, 36, 468–481. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Amro, M.M.; Bock, M.; Boseker, K.; Fredrickson, H.L.; Kessel, D.G.; Timmis, K.N. The potential of Bacillus licheniformis strains for in situ enhanced oil recovery. J. Pet. Sci. Eng. 1997, 18, 147–160. [Google Scholar] [CrossRef]
- Nagase, K.; Zhang, S.; Asami, H.; Yazawa, N.; Fujiwara, K.; Enomoto, H.; Hong, C.; Liang, C. A successful field test of microbial EOR process in Fuyu Oilfield, China. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 13–17 April 2002. [Google Scholar]
- Halim, A.Y.; Nielsen, S.M.; Lantz, A.E.; Suicmez, V.S.; Lindeloff, N.; Shapiro, A. Investigation of spore forming bacterial flooding for enhanced oil recovery in a North Sea chalk Reservoir. J. Pet. Sci. Eng. 2015, 133, 444–454. [Google Scholar] [CrossRef]
- Shaw, J.C.; Bramhill, B.; Wardlaw, N.; Costerton, J. Bacterial fouling in a model core system. Appl. Environ. Microbiol. 1985, 49, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Suthar, H.; Hingurao, K.; Desai, A.; Nerurkar, A. Selective plugging strategy-based microbial-enhanced oil recovery using Bacillus licheniformis TT33. J. Microbiol. Biotechnol. 2009, 19, 1230–1237. [Google Scholar]
- Volpon, G.; de Melo, A. Laboratory testing of a microbial enhanced oil recovery process by produced biopolymer in situ. In Proceedings of the 16th World Petroleum Congress, Calgary, AB, Canada, 11–15 June 2000. [Google Scholar]
- Armstrong, R.T.; Wildenschild, D. Investigating the pore-scale mechanisms of microbial enhanced oil recovery. J. Pet. Sci. Eng. 2012, 94, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Sabooniha, E.; Rokhforouz, M.-R.; Ayatollahi, S. Pore-scale investigation of selective plugging mechanism in immiscible two-phase flow using phase-field method. Oil Gas Sci. Technol. Rev. d’IFP Energy Nouv. 2019, 74, 78. [Google Scholar] [CrossRef] [Green Version]
- Neff, J.M. Bioaccumulation in Marine Organisms: Effect of Contaminants from Oil Well Produced Water; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Lucas, E.F.; Mansur, C.R.; Spinelli, L.; Queirós, Y.G. Polymer science applied to petroleum production. Pure Appl. Chem. 2009, 81, 473–494. [Google Scholar] [CrossRef]
- Ahmad, A.; Sumathi, S.; Hameed, B. Chitosan: A natural biopolymer for the adsorption of residue oil from oily wastewater. Adsorpt. Sci. Technol. 2004, 22, 75–88. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Xi, X.; Nie, S.; Ke, H.; Liu, Y.; Tong, S.; Chen, Z. A versatile TOCN/CGG self-assembling hydrogel for integrated wastewater treatment. Cellulose 2020, 27, 915–925. [Google Scholar] [CrossRef]
- Wilton, N.; Lyon-Marion, B.A.; Kamath, R.; McVey, K.; Pennell, K.D.; Robbat, A., Jr. Remediation of heavy hydrocarbon impacted soil using biopolymer and polystyrene foam beads. J. Hazard. Mater. 2018, 349, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lü, T.; Qi, D.; Zhao, H. Flocculation performance and mechanism of Chitosan-based flocculants in the treatment of emulsified oily wastewater. J. Dispers. Sci. Technol. 2017, 38, 1049–1054. [Google Scholar] [CrossRef]
- Paixão, M.V.G.; de Carvalho Balaban, R. Application of guar gum in brine clarification and oily water treatment. Int. J. Biol. Macromol. 2018, 108, 119–126. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Santos, M.V.; Zaritzky, N. Optimal clarification of emulsified oily wastewater using a surfactant/Chitosan biopolymer. J. Environ. Chem. Eng. 2018, 6, 3808–3818. [Google Scholar] [CrossRef]


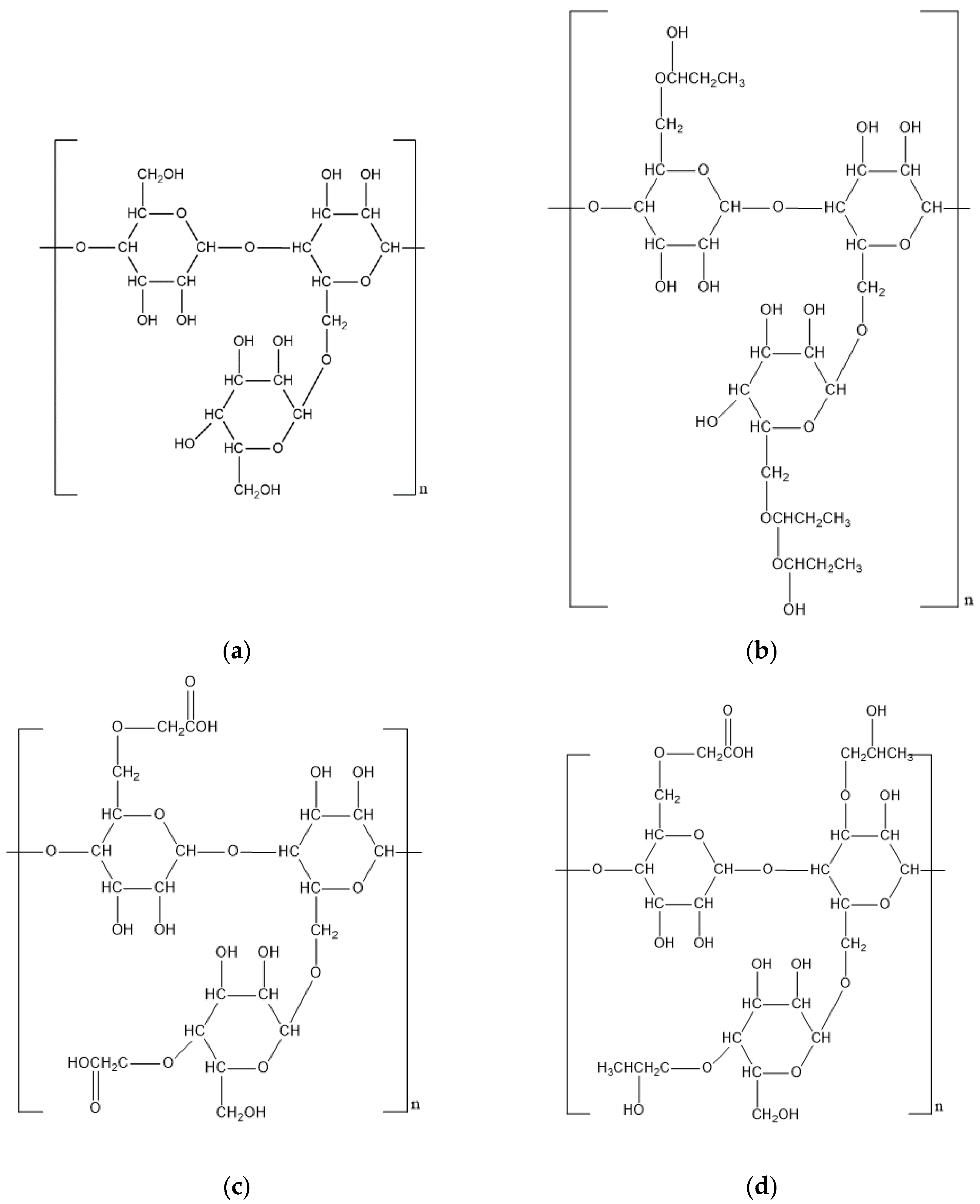


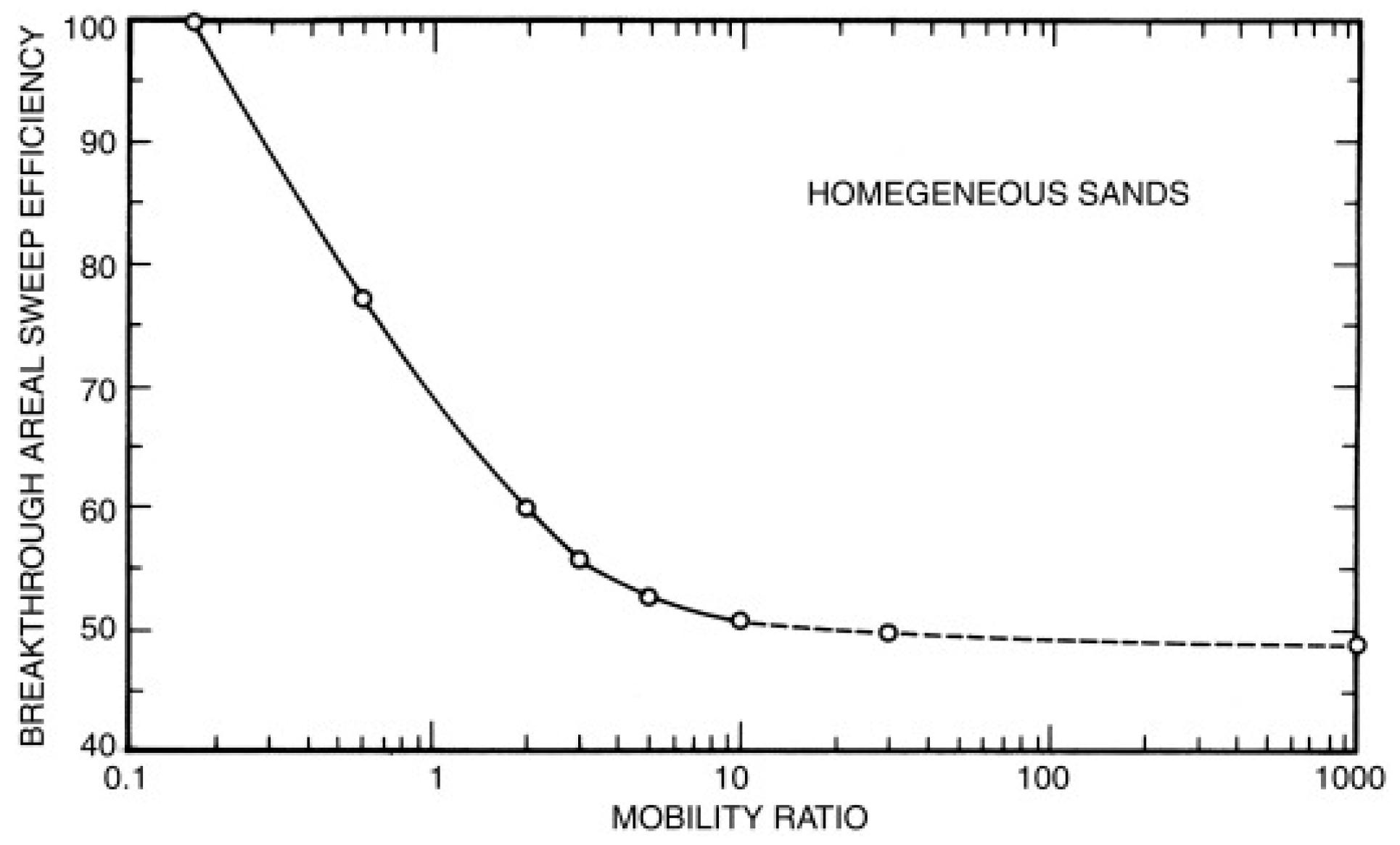

| Biopolymer | Source | Monomers | Molecular Weight | Properties | Price (USD) | Modification | Ref. |
|---|---|---|---|---|---|---|---|
| Xanthan gum | Fermentation product of Xanthomonas campestris | D-mannose, D-glucose, Pyruvic acid, D-glucuronic acid | 2 × 106 to 2 × 107 Da | Thickening Crosslinking | 12/kg | Carbonate modified Formaldehyde modified Propylene oxide modified | [9,10,11,12,13,14,15,16,17,18] |
| Scleroglucan | Fermentation product of Sclerotium rolfsii | D-glucose | 1.3 × 105 to 6 × 106 Da | Thickening | 50/kg | Hydrophobic modified | [19,20,21,22,23,24] |
| Guar gum | Endosperm component of Cyamopsis tetragonolobus | D-mannose, D-galactose | 106 to 2 × 106 Da | Thickening Crosslinking | 2/kg | Hydroxypropyl modified Carboxymethyl modified Carboxymethyl hydroxypropyl modified | [25,26,27,28,29,30,31,32,33,34] |
| Cellulose | Lignocellulose of plants Fermentation product of Acetobacter Xylinam | D-glucose | 2 × 106 Da | Thickening Filtration Adsorption | 4/kg | Hydroxyethyl modified Carboxymethyl modified Amphoteric modified | [35,36,37,38,39,40,41,42] |
| Chitin/Chitosan | Shells of crustaceans, exoskeletons of insects and cell walls of fungi | D-glucosamine, N-acetyl-D-glucosamine | 2 × 103 Da to 106 Da | Adsorption | 220/kg | Modification of MW | [43,44,45,46,47,48,49,50,51,52,53,54,55,56] |
| Modification | Polymer: Carbonate Ratio | Viscosity (mPa·s) 1 | MW (Da) | Polydispersity Index |
|---|---|---|---|---|
| Control | NA | 680 | 3.98 × 106 | 1.25 |
| Ethylene Carbonate | 1:0.132 | 1640 | 5.89 × 106 | 1.06 |
| Propylene Carbonate | 1:0.132 | 1040 | 7.0 × 106 | 1.32 |
| Butylene Carbonate | 1:0.122 | 1040 | 4.44 × 106 | 1.26 |
| Diethyl Carbonate | 1:0.122 | 1000 | 5.67 × 106 | 1.24 |
| Glycerine Carbonate | 1:0.130 | 2200 | 6.93 × 106 | 1.50 |
| Recipe | Model | Parameters | Ref. |
|---|---|---|---|
| Xanthan gum, starch and bactericide and clay were 5.75, 11.5, and 1.72 g/L, respectively, then 10 wt% clay was added. | Herschel–Bulkley | : 3.78 (Pa) K: 3.22 (Pa·s n) n: 0.31 | [83] |
| Scleroglucan, starch and bactericide were 5.75, 11.5, and 1.72 g/L, respectively, then 10 w/w % clay was added. | Herschel–Bulkley | : 3.36 (Pa) K: 0.79 (Pa·s n) n: 0.72 | [83] |
| Xanthan gum, starch, NaCl, paraformaldehyde and clay were 0.5, 1.5, 0.75, 0.125 and 2.5 wt%, respectively. | Herschel–Bulkley | : 3.88 (Pa) K: 0.46 (Pa·s n) n: 0.64 | [84] |
| Cellulose nanoparticles, bentonite and polyanionic cellulose were 3.05, 10.15, and 0.87 g/L, respectively. | Herschel–Bulkley | : 0.41 (Pa) K: 0.44 (Pa·s n) n: 0.53 | [85] |
| Water contains 5 g/L of Xanthan gum. | Casson | : 6.32 (Pa) : 0.58 (10−3 mPa·s) | [86] |
| Cellulose nanoparticles, bentonite and polyanionic cellulose were 0.5, 4.5, and 0.05 g/L, respectively. | Casson | : 3.43 (Pa) : 0.13 (10−3 mPa·s) | [87] |
| Water contains 1 g/L Lepidium perfoliatum seed gum. | Casson | : 10.31 (Pa) : 0.23 (10−3 mPa·s) | [88] |
| Polymer Type | Concentration (wt%) | Temperature (°C) | Shear Rate (s−1) | Viscosity (mPa·s) | Ref. |
|---|---|---|---|---|---|
| Guar gum | 0.24 | 25 | 511 | 10 | [108] |
| 0.54 | 25 | 511 | 42 | ||
| 0.95 | 25 | 511 | 103 | ||
| 0.48 | 25 | 10 | 250 | [109] | |
| 0.48 | 25 | 100 | 88 | ||
| 0.48 | 25 | 1000 | 24 | ||
| 1 | 25 | 15 | 225 | [110] | |
| 1 | 40 | 15 | 160 | ||
| 1 | 60 | 15 | 120 | ||
| 1 | 80 | 15 | 80 | ||
| CMHPG | 0.48 | 25 | 170 | 58 | [111] |
| 0.48 | 25 | 511 | 35 |
| Type | Form | Bond | pH | Temperature (°C) |
|---|---|---|---|---|
| Borate | Borax; Boric acid | Hydrogen; Ionic | 8–11 | 38–107 |
| Ti4+ | Titanium acetylacetonate Titanium mono-triethanolamine chelate | Covalent bond | 3–11 | 38–163 |
| Zr4+ | Zirconium ammonium lactate Zirconium tetra-acetate | Covalent bond | 3–11 | 38–177 |
| Al3+ | Aluminum phosphate | Covalent bond | 3–5 | 38–94 |
| Category | Form | Disadvantages | Advantages |
|---|---|---|---|
| Enzymes | Hemicellulose | Unstable when T > 135 °C or pH > 10.5 | Environmentally benign Reaction specific Effective Leave less residue |
| Oxidizers | Ammonium, potassium sodium peroxydisulfate | Slow when T < 52 °C Harm to equipment | Tolerance of high temperature |
| Polymer Type | Concentration (%) | n | K (m Pa·s n) | Ref. |
|---|---|---|---|---|
| HPAM | 1 | 0.28 | 1080 | [129] |
| 2 | 0.26 | 2050 | ||
| 5 | 0.25 | 5770 | ||
| Xanthan gum | 0.5 | 0.58 | 1190 | [130] |
| 1 | 0.65 | 3163 | ||
| 2 | 0.71 | 6526 | ||
| Scleroglucan | 0.5 | 0.49 | 55 | [19] |
| 1 | 0.31 | 272 | ||
| 2 | 0.20 | 1073 | ||
| CMC | 1 | 0.95 | 50 | [131] |
| 2 | 0.85 | 450 | ||
| 4 | 0.61 | 830 |
| Microbial Species | Plugging Agents | Results | Ref. |
|---|---|---|---|
| Leuconostoc mesenteroides | Bacterial dextran | Permeability decreased from 4.08 μm2 to 0.17 μm2 and a 36.7% improvement of the oil recovery in lab scale | [170] |
| Bacillus licheniformis BNP29 | Biomass, Extracellular polymer | A 9.3–22.1% additional recovery of the residual oil after water flooding | [171] |
| Enterobacter sp. CJF002 | Insoluble biopolymer | A 260% increase in oil production in field test | [172] |
| B. licheniformis 421 | Spore | Additional 1.0–2.3% and 6.9–8.8% oil recovery in homogenous and heterogeneous reservoir chalk cores, respectively | [173] |
| Pseudomonas sp. | Exopolysaccharides, biofilm | A more than 99% decrease in core permeability | [174] |
| Bacillus licheniformis TT33 | Biofilm, Biopolymer | A 20–30% additional oil recovery in a sand pack column | [175] |
| B3 bacterium isolated from reservoirs of Carmopólis field | Biopolymer | A 20% additional oil recovery in the laboratory test | [176] |
| Shewanella oneidensis MR-1 | Biofilm | A 7.1% additional oil recovery after water flooding in microfluidic channels | [177] |
| Acinetobacter RAG-1 | Biofilm | A 18% additional oil recovery after a 41% oil recovery from water flooding in micromodel | [178] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. https://doi.org/10.3390/polym12091860
Xia S, Zhang L, Davletshin A, Li Z, You J, Tan S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers. 2020; 12(9):1860. https://doi.org/10.3390/polym12091860
Chicago/Turabian StyleXia, Shunxiang, Laibao Zhang, Artur Davletshin, Zhuoran Li, Jiahui You, and Siyuan Tan. 2020. "Application of Polysaccharide Biopolymer in Petroleum Recovery" Polymers 12, no. 9: 1860. https://doi.org/10.3390/polym12091860
APA StyleXia, S., Zhang, L., Davletshin, A., Li, Z., You, J., & Tan, S. (2020). Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers, 12(9), 1860. https://doi.org/10.3390/polym12091860