Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery
Abstract
1. Introduction
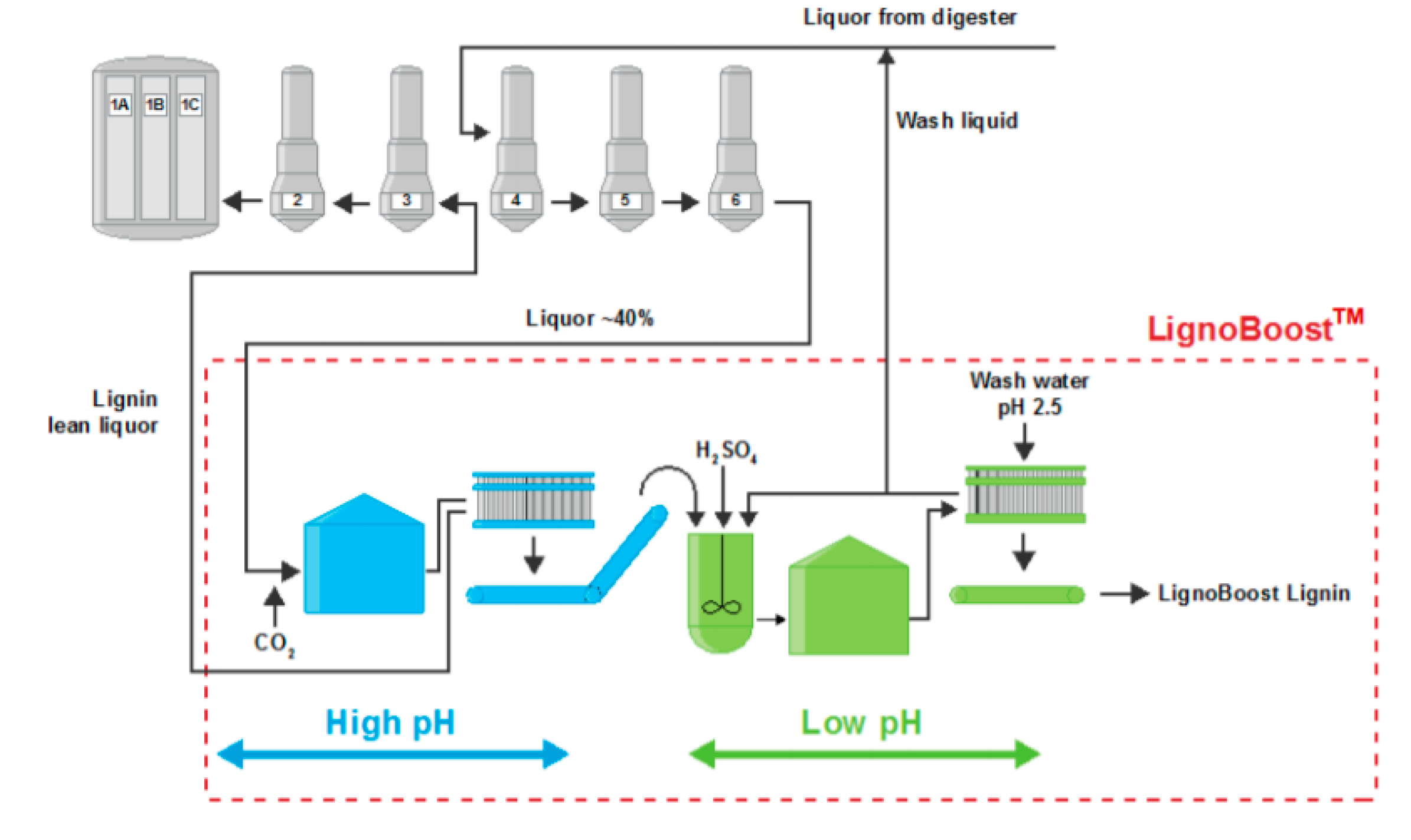
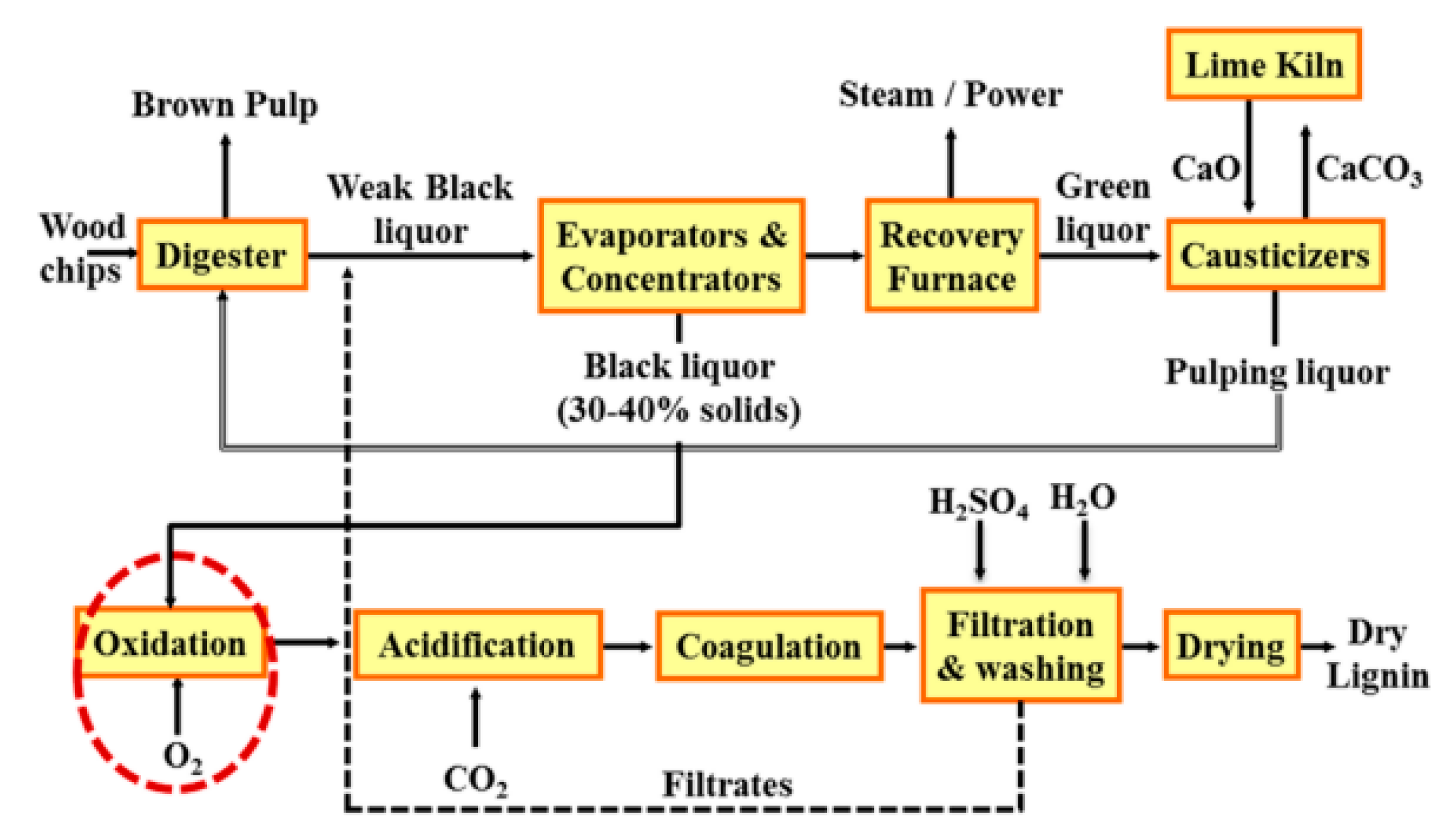
2. Lignin Extraction
3. Structure and Properties
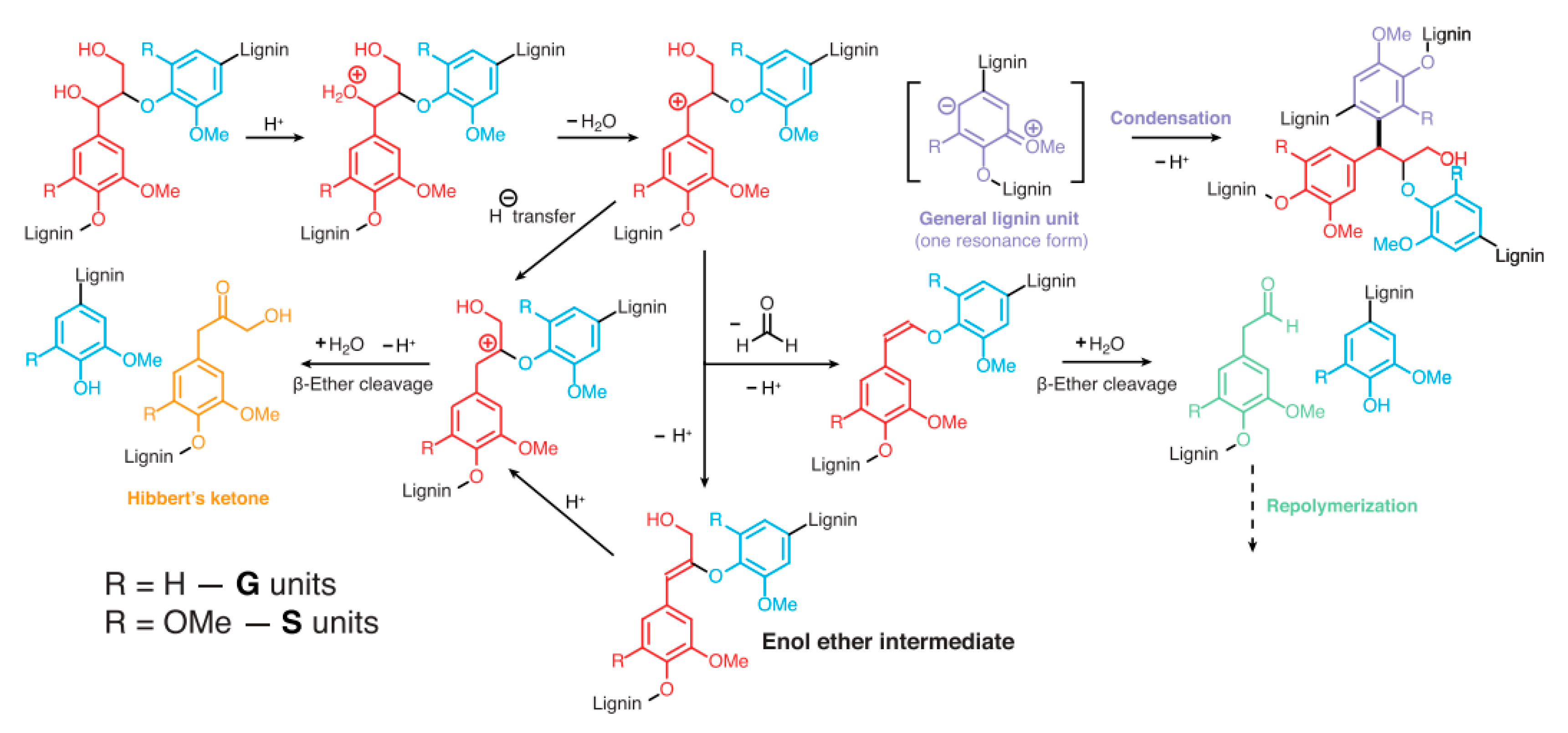
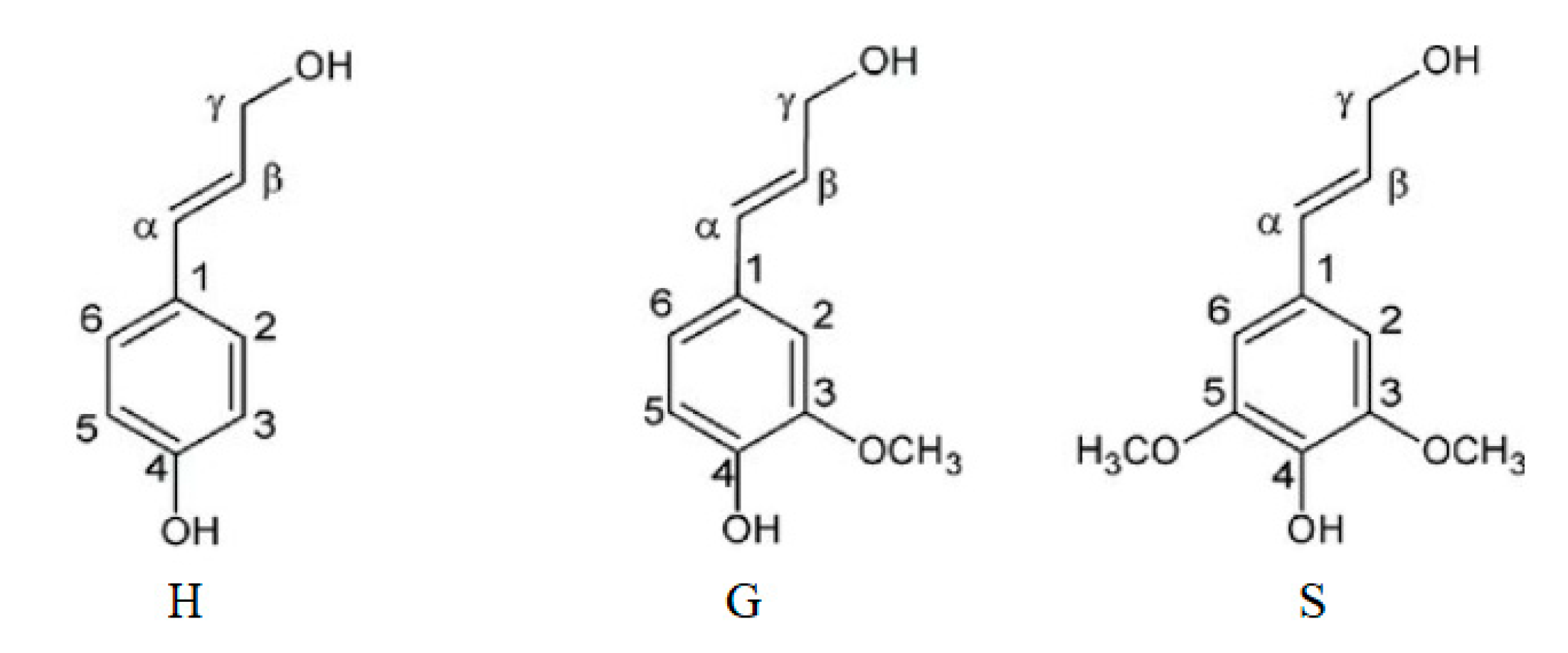
3.1. Linkages
3.2. Molecular Weight
3.3. Thermo-Mechanical Properties
3.4. Functional Groups
3.5. Reactivity
3.6. Elemental Composition
4. Lignin Fractionation
4.1. Fractionation with Organic Solvents
4.2. Fractionation with Ultrafiltration Membranes
4.3. Fractionation by Acid Precipitation
5. Lignin Modification
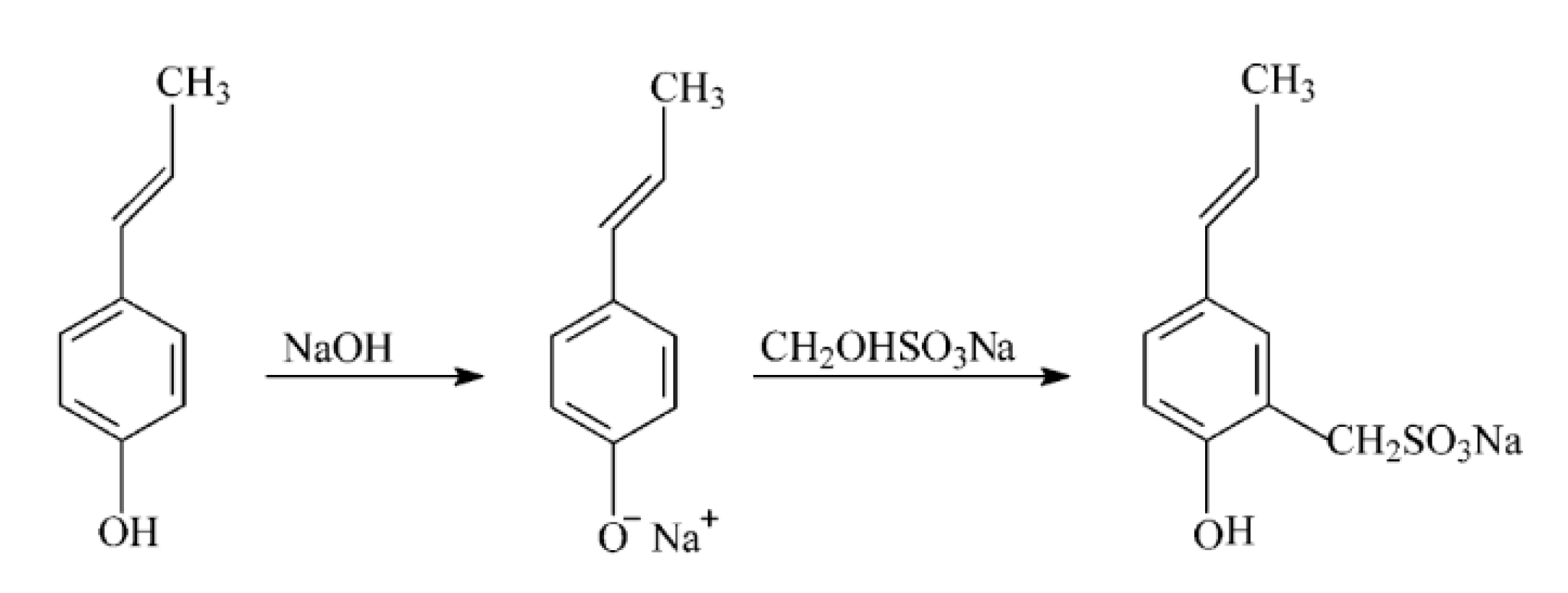
5.1. Sulfomethylation or Sulfonation
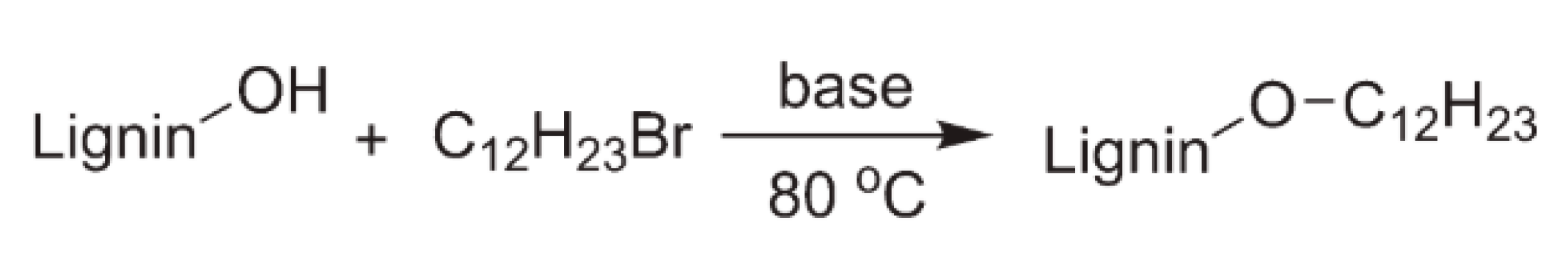
5.2. Alkylation
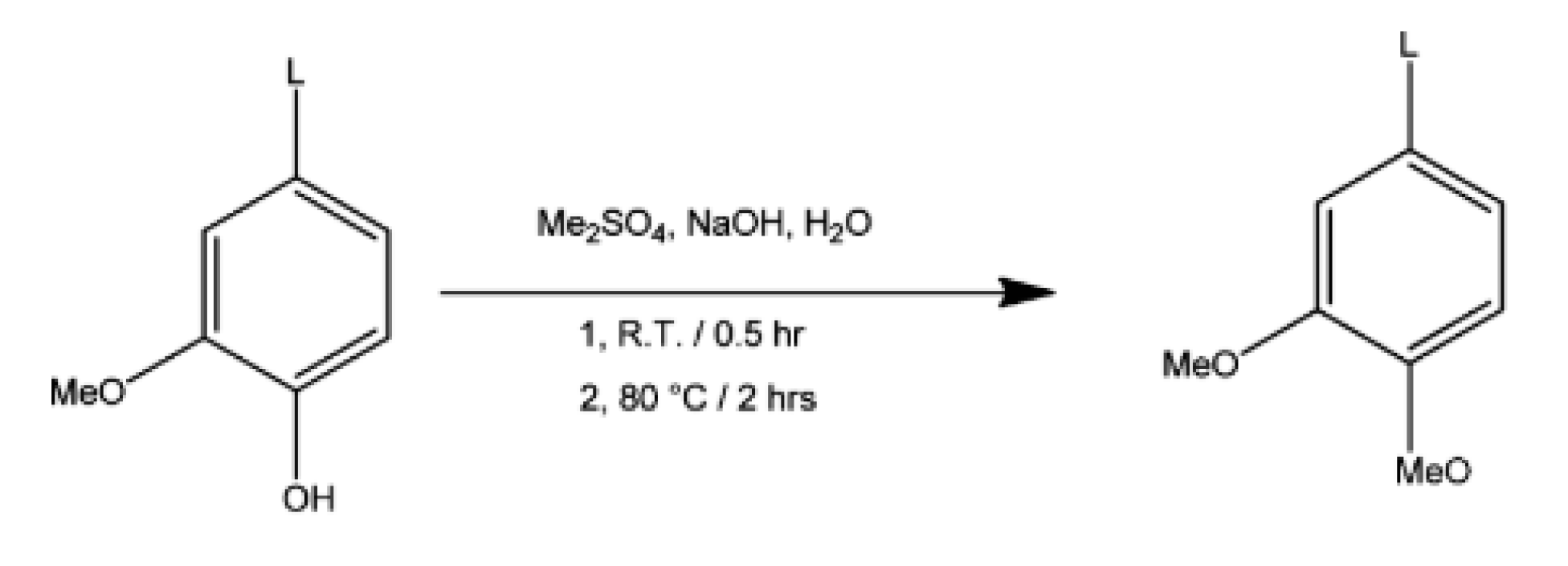
5.3. Methylation
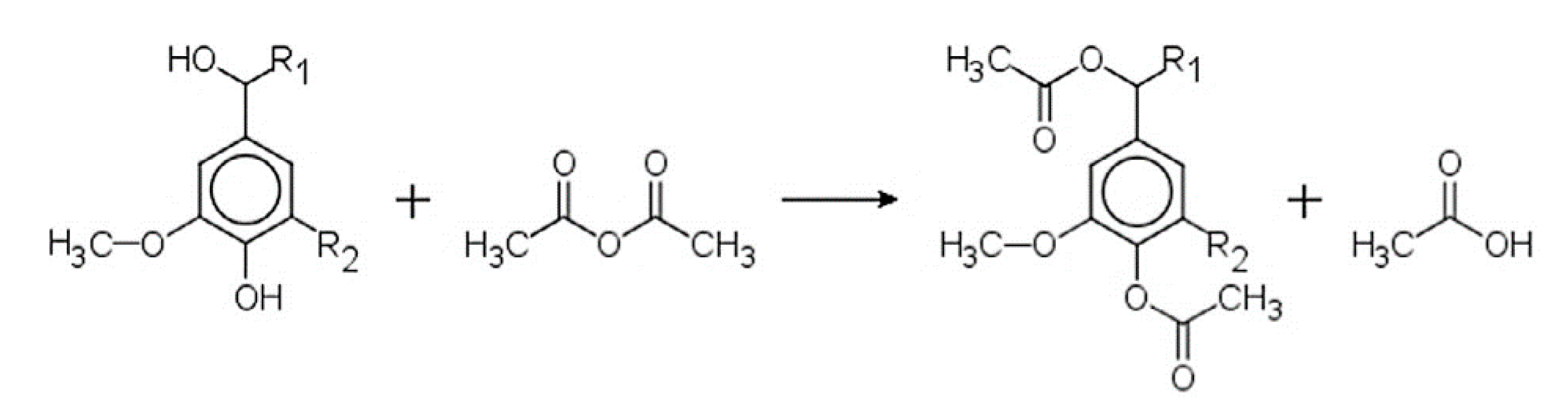
5.4. Esterification
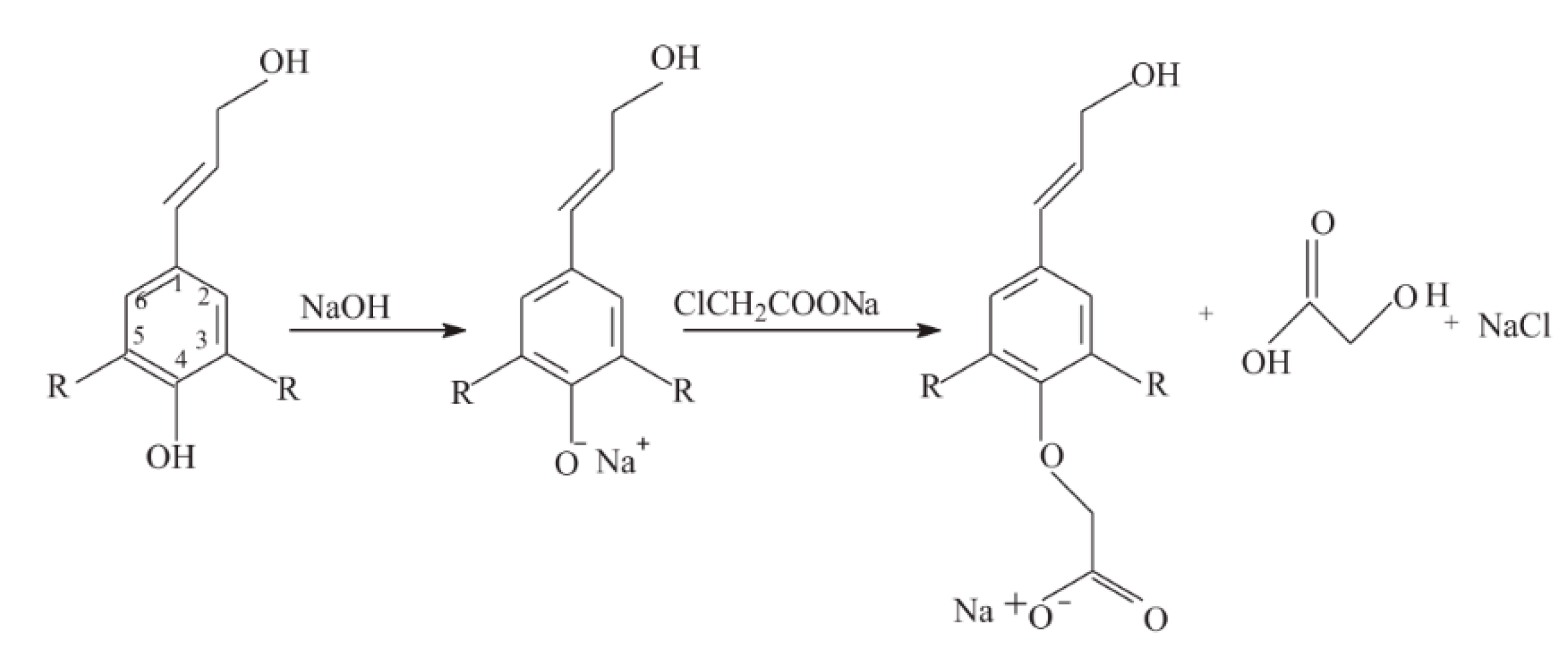
5.5. Carboxymethylation
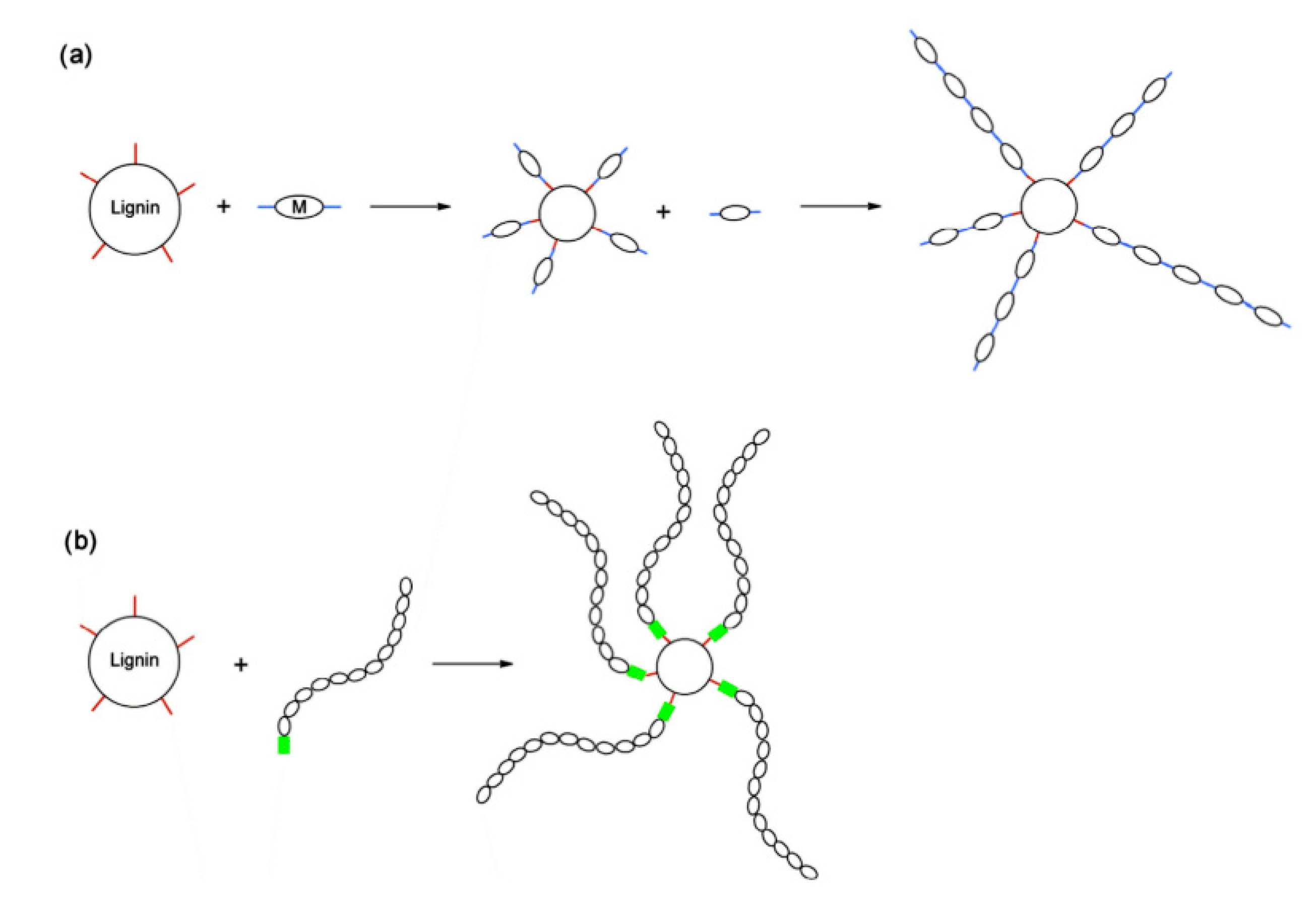
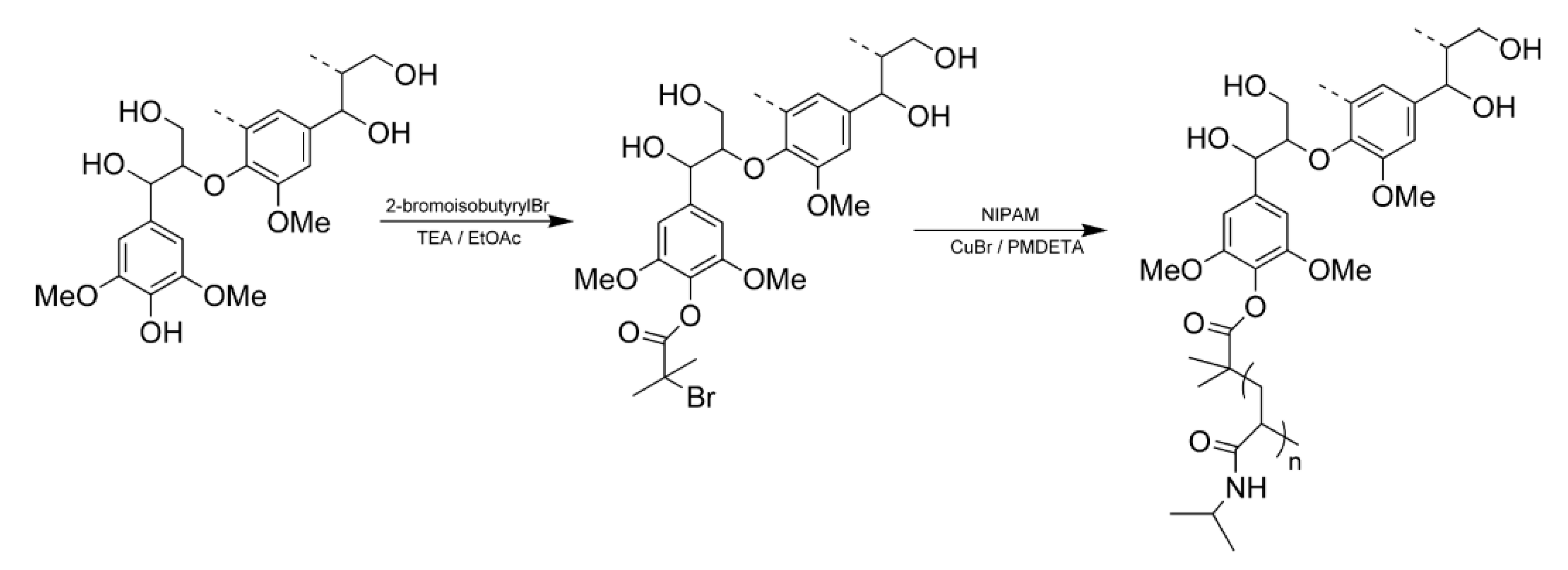
5.6. Grafting
6. Lignin Applications
6.1. Briquettes and Pellets
6.2. Dispersant
6.3. Adsorbents
6.4. Hydrogels
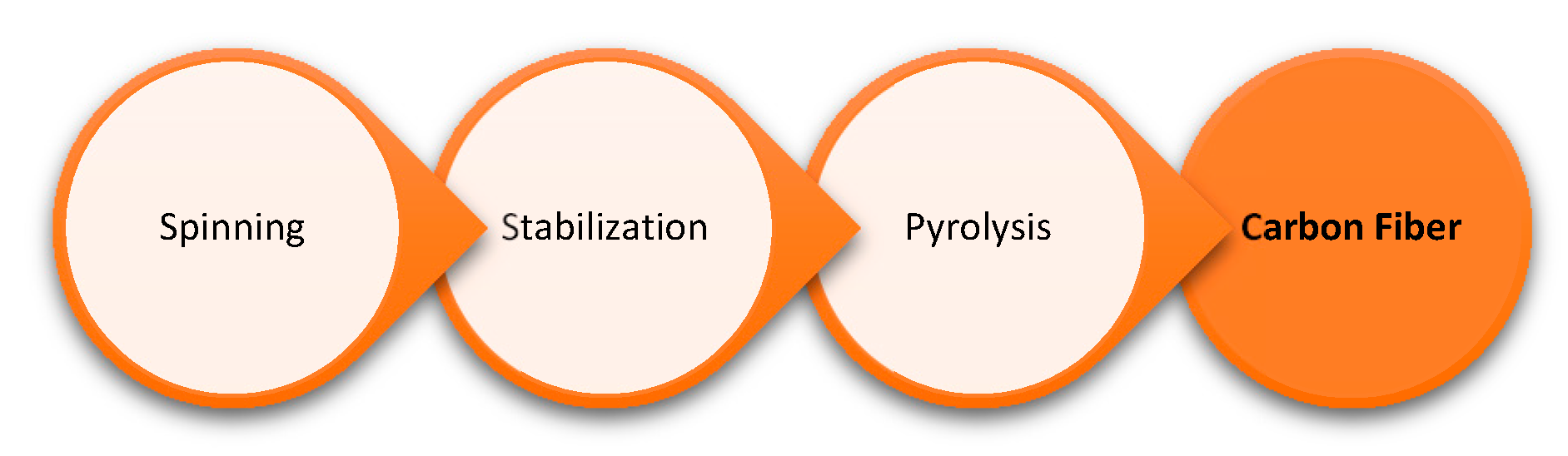
6.5. Carbon Fibers (CF)
6.6. Antioxidants
6.7. Aromatic Compounds–Chemicals
6.8. Polymer Blends and Composites
7. Conclusions
Funding
Conflicts of Interest
References
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S. Miscibility and Hydrogen Bonding in Blends of Poly (ethylene oxide) and Kraft Lignin. Macromolecules 2003, 7803–7811. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products–strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, A.L.; Mai, M.; Kadla, J.F. Bio-based chemicals from biorefining: Lignin conversion and utilisation. In Advances in Biorefineries; Woodhead Publishing: Cambridge, UK, 2014; ISBN 9780857095213. [Google Scholar]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. (Charles) Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Bajpai, P. Basic Overview of Pulp and Paper Manufacturing Process. In Green Chemistry and Sustainability in Pulp and Paper Industry; Springer: Cham, Switzerland, 2015; pp. 11–39. ISBN 9783319187440. [Google Scholar]
- Magdeldin, M.; Järvinen, M. Supercritical water gasification of Kraft black liquor: Process design, analysis, pulp mill integration and economic evaluation. Appl. Energy 2020, 262, 114558. [Google Scholar] [CrossRef]
- Miller, J.; Faleiros, M. Lignin: Technology, Applications, and Markets; RISI, Inc.: Cincinnati, OH, USA, 2016. [Google Scholar]
- Gellerstedt, G.; Sjöholm, E.; Brodin, I. The Wood-Based Biorefinery: A Source of carbon fiber? Open Agric. J. 2010, 3, 119–124. [Google Scholar] [CrossRef]
- Öhman, F.; Wallmo, H.; Theliander, H. Precipitation and filtration of lignin from black liquor of different origin. Nord. Pulp Pap. Res. J. 2007, 22, 188–193. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Pu, Y.; Ragauskas, A. Cellulosic biorefineries-unleashing lignin opportunities. Curr. Opin. Environ. Sustain. 2010, 2, 383–393. [Google Scholar] [CrossRef]
- Rodrigues Pinto, P.C.; Borges Da Silva, E.A.; Rodrigues, A.E. Insights into oxidative conversion of lignin to high-added-value phenolic aldehydes. Ind. Eng. Chem. Res. 2011, 50, 741–748. [Google Scholar] [CrossRef]
- Schlee, P.; Hosseinaei, O.; Baker, D.; Landmér, A.; Tomani, P.; Mostazo-López, M.J.; Cazorla-Amorós, D.; Herou, S.; Titirici, M.M. From Waste to Wealth: From Kraft Lignin to Free-standing Supercapacitors. Carbon N. Y. 2019, 145, 470–480. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. BioResources 2019, 14, 2300–2351. [Google Scholar]
- Wallmo, H.; Littorin, A.; Karlsson, H.; Lindholm, K.; Stern, R.; Christiansen, G. The Evolution of LignoBoost Technology and the Lignin BioProducts Market-Part 2. In Proceedings of the IBBC International Bioenergy & Bioproducts Conference, Jacksonville, FL, USA, 28–30 September 2016. [Google Scholar]
- Kouisni, L.; Gagné, A.; Maki, K.; Holt-Hindle, P.; Paleologou, M. LignoForce System for the Recovery of Lignin from Black Liquor: Feedstock Options, Odor Profile, and Product Characterization. ACS Sustain. Chem. Eng. 2016, 4, 5152–5159. [Google Scholar] [CrossRef]
- Fatehi, P.; Chen, J. Extraction of Technical Lignins from Pulping Spent Liquors, Challenges and Opportunities. In Production of Biofuels and Chemicals from Lignin; Springer: Singapore, 2016; pp. 35–54. ISBN 978-981-10-1964-7. [Google Scholar]
- Li, Y.; Shuai, L.; Kim, H.; Motagamwala, A.H.; Mobley, J.K.; Yue, F.; Tobimatsu, Y.; Havkin-Frenkel, D.; Chen, F.; Dixon, R.A.; et al. An “ideal lignin” facilitates full biomass utilization. Sci. Adv. 2018, 4, eaau2968. [Google Scholar] [CrossRef]
- Yinghuai, Z.; Yuanting, K.T.; Hosmane, N.S. Applications of Ionic Liquids in Lignin Chemistry. In Ionic Liquids—New Aspects for the Future; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Peng, J.; Yang, G.; Qi, L.; Liu, J.; Li, F.; Chen, J.; Lucia, L. Enhancement of Delignification by Ionic Liquids Pretreatment and Modification of Hardwood Kraft Pulp in Preparation for Bleaching. BioResources 2020, 15, 6299–6308. [Google Scholar]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechnol. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef]
- Malaeke, H.; Housaindokht, M.R.; Monhemi, H.; Izadyar, M. Deep eutectic solvent as an efficient molecular liquid for lignin solubilization and wood delignification. J. Mol. Liq. 2018, 263, 193–199. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Maugeri, Z. Ionic liquids in biotransformations: From proof-of-concept to emerging deep-eutectic-solvents. Curr. Opin. Chem. Biol. 2011, 15, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984; ISBN 3110084813. [Google Scholar]
- Alén, R. Structure and chemical composition of wood. In Forest Products Chemistry; Stenius, P., Ed.; Fapet Oy: Helsinki, Finland, 2000; pp. 11–57. [Google Scholar]
- Nordström, Y.; Norberg, I.; Sjöholm, E.; Drougge, R. A new softening agent for melt spinning of softwood kraft lignin. J. Appl. Polym. Sci. 2013, 129, 1274–1279. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Chang, H.M.; Allan, G.G. Species variation in lignins III. TAPPI J. 1967, 50, 587. [Google Scholar]
- Akiyama, T.; Goto, H.; Nawawi, D.S.; Syafii, W.; Matsumoto, Y.; Meshitsuka, G. Erythro/threo ratio of β-O-4-structures as an important structural characteristic of lignin. Part 4: Variation in the erythro/threo ratio in softwood and hardwood lignins and its relation to syringyl/guaiacyl ratio. Holzforschung 2005, 59, 276–281. [Google Scholar] [CrossRef]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.M.; Jameel, H. Lignin structural variation in hardwood species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Lindfors, E.-L.; Lapierre, C.; Monties, B. Structural changes in lignin during kraft cooking. Part 2. Characterization by acidolysis. Sven. Papp. 1984, 87, 61. [Google Scholar]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Ralph, J.; Brunow, G.; Boerjan, W. Lignins. Encycl. Life Sci. 2007, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of lignin: A sustainable and eco-friendly approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Berlin, A.; Balakshin, M. Industrial Lignins; Elsevier: Oxford, UK, 2014; ISBN 9780444595614. [Google Scholar]
- Wang, L.; Uraki, Y.; Koda, K.; Gele, A.; Zhou, X.; Chen, F. Determination of the absolute molar mass of acetylated eucalyptus kraft lignin by two types of size-exclusion chromatography combined with multi-angle laser light-scattering detectors. Holzforschung 2019, 73, 363–369. [Google Scholar] [CrossRef]
- Baumberger, S.; Abaecherli, A.; Fasching, M.; Gellerstedt, G.; Gosselink, R.; Hortling, B.; Li, J.; Saake, B.; De Jong, E. Molar mass determination of lignins by size-exclusion chromatography: Towards standardisation of the method. Holzforschung 2007, 61, 459–468. [Google Scholar] [CrossRef]
- Asikkala, J.; Tamminen, T.; Argyropoulos, D.S. Accurate and reproducible determination of lignin molar mass by acetobromination. J. Agric. Food Chem. 2012, 60, 8968–8973. [Google Scholar] [CrossRef]
- Glasser, W.G.; Davé, V.; Frazier, C.E. Molecular weight distribution of (semi-) commercial lignin derivatives. J. Wood Chem. Technol. 1993, 13, 545–559. [Google Scholar] [CrossRef]
- Torrezan, T. Avaliação do comportamento reológico, térmico e mecânico de misturas de PBAT com elevados teores de lignina. Master’s Thesis, Universidade Federal de São Carlos, São Paulo, Brazil, 2019; 106. [Google Scholar]
- Kadla, J.F.; Kubo, S. Lignin-based polymer blends: Analysis of intermolecular interactions in lignin-synthetic polymer blends. Compos. Part A Appl. Sci. Manuf. 2004, 35, 395–400. [Google Scholar] [CrossRef]
- Thielemans, W.; Wool, R.P. Lignin Esters for Use in Unsaturated Thermosets: Lignin Modification and Solubility Modeling. Biomacromolecules 2005, 6, 1895–1905. [Google Scholar] [CrossRef]
- Ház, A.; Jablonský, M.; Šurina, I.; Kačík, F.; Bubeníková, T.; Ďurkovič, J. Chemical composition and thermal behavior of kraft lignins. Forests 2019, 10, 483. [Google Scholar] [CrossRef]
- Dehne, L.; Babarro, C.V.; Saake, B.; Schwarz, K.U. Influence of lignin source and esterification on properties of lignin-polyethylene blends. Ind. Crops Prod. 2016, 86, 320–328. [Google Scholar] [CrossRef]
- Zhao, Y.; Tagami, A.; Dobele, G.; Lindström, M.E.; Sevastyanova, O. The impact of lignin structural diversity on performance of cellulose nanofiber (CNF)-starch composite films. Polymers (Basel) 2019, 11, 538. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefi ning studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Lange, H.; Rulli, F.; Crestini, C. Gel Permeation Chromatography in Determining Molecular Weights of Lignins: Critical Aspects Revisited for Improved Utility in the Development of Novel Materials. ACS Sustain. Chem. Eng. 2016, 4, 5167–5180. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin-A review. Cellul. Chem. Technol. 2009, 44, 353–363. [Google Scholar]
- Kubo, S.; Kadla, J.F. Thermal decomposition study of isolated lignin using temperature modulated TGA. J. Wood Chem. Technol. 2008, 28, 106–121. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Ludwig, C.H. Liguins. Occurrence, Formation, Structure, and Reactions; Wiley-Interscience: New York, NY, USA, 1971. [Google Scholar]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Glasser, W.G. Classification of Lignin According to Chemical and Molecular Structure. In Lignin: Historical, Biological, and Materials Perspectives; Glasser, W.G., Northey, R.A., Schultz, T.P., Eds.; American Chemical Society: Washington, DC, USA, 1999; pp. 216–238. ISBN 9780841236110. [Google Scholar]
- Ropponen, J.; Räsänen, L.; Rovio, S.; Ohra-Aho, T.; Liitiä, T.; Mikkonen, H.; Van De Pas, D.; Tamminen, T. Solvent extraction as a means of preparing homogeneous lignin fractions. Holzforschung 2011, 65, 543–549. [Google Scholar] [CrossRef]
- Jawerth, M.E.; Brett, C.J.; Terrier, C.; Larsson, P.T.; Lawoko, M.; Roth, S.V.; Lundmark, S.; Johansson, M. Mechanical and Morphological Properties of Lignin-Based Thermosets. ACS Appl. Polym. Mater. 2020, 2, 668–676. [Google Scholar] [CrossRef]
- Park, C.W.; Youe, W.J.; Kim, S.J.; Han, S.Y.; Park, J.S.; Lee, E.A.; Kwon, G.J.; Kim, Y.S.; Kim, N.H.; Lee, S.H. Effect of lignin plasticization on physico-mechanical properties of lignin/poly(lactic acid) composites. Polymers (Basel) 2019, 11, 2089. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Lignin-based carbon fibers: Effect of synthetic polymer blending on fiber properties. J. Polym. Environ. 2005, 13, 97–105. [Google Scholar] [CrossRef]
- Li, Y.; Sarkanen, S. Alkylated kraft lignin-based thermoplastic blends with aliphatic polyesters. Macromolecules 2002, 35, 9707–9715. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017. [Google Scholar] [CrossRef]
- Husson, E.; Hulin, L.; Hadad, C.; Boughanmi, C.; Stevanovic, T.; Sarazin, C. Acidic Ionic Liquid as Both Solvent and Catalyst for Fast Chemical Esterification of Industrial Lignins: Performances and Regioselectivity. Front. Chem. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Kadla, J.F. Kraft lignin/poly(ethylene oxide) blends: Effect of lignin structure on miscibility and hydrogen bonding. J. Appl. Polym. Sci. 2005, 98, 1437–1444. [Google Scholar] [CrossRef]
- Harmita, H.; Karthikeyan, K.G.; Pan, X.J. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef] [PubMed]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef]
- Goldmann, W.M.; Ahola, J.; Mankinen, O.; Kantola, A.M.; Komulainen, S.; Telkki, V.-V.; Tanskanen, J. Determination of Phenolic Hydroxyl Groups in Technical Lignins by Ionization Difference Ultraviolet Spectrophotometry (∆ε-IDUS method). Period. Polytech. Chem. Eng. 2016, 61, 93–101. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Pinto, P.C.R.; Rodrigues, A.E. Evaluation of chemical processing impact on E. globulus wood lignin and comparison with bark lignin. Ind. Crops Prod. 2014, 61, 479–491. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Zhang, H.; Xiao, R. Catalytic oxidation of lignin to valuable biomass-based platform chemicals: A review. Fuel Process. Technol. 2019, 191, 181–201. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H.; Chang, H. A Quantitative Comparison of the Precipitation Behavior of Lignin from Sweetgum and Pine Kraft Black Liquors. BioResources 2020, 15, 5464–5480. [Google Scholar] [CrossRef]
- Schorr, D.; Diouf, P.N.; Stevanovic, T. Evaluation of industrial lignins for biocomposites production. Ind. Crops Prod. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin-a biosorbent. J. Colloid Interface Sci. 2006, 297, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ragauskas, A.; Yuan, J. Lignin carbon fiber: The path for quality. TAPPI J. 2017, 16, 107–108. [Google Scholar] [CrossRef]
- Cui, C.; Sun, R.; Argyropoulos, D.S. Fractional precipitation of softwood kraft lignin: Isolation of narrow fractions common to a variety of lignins. ACS Sustain. Chem. Eng. 2014, 2, 959–968. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Dizhbite, T.; Lauberts, M.; Viksna, A.; Dobele, G.; Bikovens, O.; Telysheva, G. Characterization of softwood and hardwood lignoboost kraft lignins with emphasis on their antioxidant activity. BioResources 2014, 9, 2051–2068. [Google Scholar] [CrossRef]
- Sevastyanova, O.; Helander, M.; Chowdhury, S.; Lange, H.; Wedin, H.; Zhang, L.; Ek, M.; Kadla, J.F.; Crestini, C.; Lindström, M.E. Tailoring the molecular and thermo-mechanical properties of kraft lignin by ultrafiltration. J. Appl. Polym. Sci. 2014, 131, 9505–9515. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative study of lignin fractionation by ultrafiltration and selective precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Pinto, P.C.R.; Rodrigues, A.E. Lignin fractionation from E. Globulus kraft liquor by ultrafiltration in a three stage membrane sequence. Sep. Purif. Technol. 2018, 192, 140–151. [Google Scholar] [CrossRef]
- dos Santos, P.S.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- García, A.; Toledano, A.; Serrano, L.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Characterization of lignins obtained by selective precipitation. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Lourençon, T.V.; Hansel, F.A.; Thiago, A.; Ramos, L.P.; De Muniz, G.I.B.; Magalhães, W.L.E. Hardwood and softwood kraft lignins fractionation by simple sequential acid precipitation. Sep. Purif. Technol. 2015, 154, 82–88. [Google Scholar] [CrossRef]
- Sewring, T.; Durruty, J.; Schneider, L.; Schneider, H.; Mattsson, T.; Theliander, H. Acid Precipitation of Kraft Lignin from Aqueous Solutions: The Influence of pH, Temperature, and Xylan. J. Wood Chem. Technol. 2019, 39, 1–13. [Google Scholar] [CrossRef]
- Brodin, I.; Sjöholm, E.; Gellerstedt, G. The behavior of kraft lignin during thermal treatment. J. Anal. Appl. Pyrolysis 2010, 87, 70–77. [Google Scholar] [CrossRef]
- Konduri, M.K.R.; Fatehi, P. Production of water-soluble hardwood kraft lignin via sulfomethylation using formaldehyde and sodium sulfite. ACS Sustain. Chem. Eng. 2015, 3, 1172–1182. [Google Scholar] [CrossRef]
- Watanabe, M.; Meshitsuka, G.; Ishizu, A.R. Radical sulfonation of lignin II: Water solubilisation of acid hydrolysis lignin. J. Jpn. Wood Res. Soc. 1990, 36, 876–882. [Google Scholar]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Physical Properties of Lignin-Based Polypropylene Blends. Polym. Polym. Compos. 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Cui, C.; Sadeghifar, H.; Sen, S.; Argyropoulos, D.S. Toward thermoplastic lignin polymers; Part II: Thermal & polymer characteristics of kraft lignin & derivatives. BioResources 2013, 8, 864–886. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Cui, C.; Argyropoulos, D.S. Toward thermoplastic lignin polymers. Part 1. Selective masking of phenolic hydroxyl groups in kraft lignins via methylation and oxypropylation chemistries. Ind. Eng. Chem. Res. 2012, 51, 16713–16720. [Google Scholar] [CrossRef]
- Dehne, L.; Vila, C.; Saake, B.; Schwarz, K.U. Esterification of Kraft lignin as a method to improve structural and mechanical properties of lignin-polyethylene blends. J. Appl. Polym. Sci. 2017, 134, 1–8. [Google Scholar] [CrossRef]
- Kumar, H.; Alén, R. Recovery of aliphatic low-molecular-mass carboxylic acids from hardwood kraft black liquor. Sep. Purif. Technol. 2015, 142, 293–298. [Google Scholar] [CrossRef]
- Konduri, M.K.; Kong, F.; Fatehi, P. Production of carboxymethylated lignin and its application as a dispersant. Eur. Polym. J. 2015, 70, 371–383. [Google Scholar] [CrossRef]
- Jelonek, Z.; Drobniak, A.; Mastalerz, M.; Jelonek, I. Assessing pellet fuels quality: A novel application for reflected light microscopy. Int. J. Coal Geol. 2020, 222, 103433. [Google Scholar] [CrossRef]
- Boschetti, W.T.N.; Carvalho, A.M.M.L.; Carneiro, A.D.C.O.; Santos, L.C.; Poyares, L.D.B.Q. Potential of kraft lignin as an additive in briquette production. Nord. Pulp Pap. Res. J. 2019. [Google Scholar] [CrossRef]
- Pereira, B.L.C.; de Cássia Oliveira Carneiro, A.; Carvalho, A.M.M.L.; Vital, B.R.; Oliveira, A.C.; Canal, W.D. Influência da adição de lignina kraft nas propriedades de pellets de eucalipto. Floresta 2016, 46, 235–242. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Zerpa, A.; Pakzad, L.; Fatehi, P. Hardwood Kraft Lignin-Based Hydrogels: Production and Performance. ACS Omega 2018, 3, 8233–8242. [Google Scholar] [CrossRef]
- Fang, W.; Yang, S.; Wang, X.-L.; Yuan, T.-Q.; Sun, R.-C. Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefin. 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Baker, D.A.; Rials, T.G. Recent advances in low-cost carbon fiber manufacture from lignin. J. Appl. Polym. Sci. 2013, 130, 713–728. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon N. Y. 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Nordström, Y.; Joffe, R.; Sjöholm, E. Mechanical characterization and application of Weibull statistics to the strength of softwood lignin-based carbon fibers. J. Appl. Polym. Sci. 2013, 130, 3689–3697. [Google Scholar] [CrossRef]
- Maradur, S.P.; Kim, C.H.; Kim, S.Y.; Kim, B.H.; Kim, W.C.; Yang, K.S. Preparation of carbon fibers from a lignin copolymer with polyacrylonitrile. Synth. Met. 2012, 162, 453–459. [Google Scholar] [CrossRef]
- Brodin, I.; Ernstsson, M.; Gellerstedt, G.; Sjöholm, E. Oxidative stabilisation of kraft lignin for carbon fibre production. Holzforschung 2012, 66, 141–147. [Google Scholar] [CrossRef]
- Kubo, S.; Yoshida, T.; Kadla, J.F. Surface porosity of lignin/PP blend carbon fibers. J. Wood Chem. Technol. 2007, 27, 257–271. [Google Scholar] [CrossRef]
- Pakkang, N.; Kumar, M.; Taira, S.; Koda, K.; Shigetomi, K.; Uraki, Y. Preparation of kraft lignin-based activated carbon fiber electrodes for electric double layer capacitors using an ionic liquid electrolyte. Holzforschung 2020, 74, 577–588. [Google Scholar] [CrossRef]
- da Silva, S.H.; Gordobil, O.; Labidi, J. Organic acids as a greener alternative for the precipitation of hardwood kraft lignins from the industrial black liquor. Int. J. Biol. Macromol. 2020, 142, 583–591. [Google Scholar] [CrossRef]
- Ye, D.; Li, S.; Lu, X.; Zhang, X.; Rojas, O.J. Antioxidant and Thermal Stabilization of Polypropylene by Addition of Butylated Lignin at Low Loadings. ACS Sustain. Chem. Eng. 2016, 4, 5248–5257. [Google Scholar] [CrossRef]
- Widsten, P.; Tamminen, T. Natural Sunscreens Based on Nanoparticles of Modi fi ed Kraft Lignin (CatLignin). ACS Omega 2020. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Shen, D.; Xue, J.; Guan, S.; Gu, S.; Luo, K.H. Catalytic oxidation of lignin in solvent systems for production of renewable chemicals: A review. Polymers (Basel) 2017, 9, 240. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.C.R.; Costa, C.E.; Rodrigues, A.E. Oxidation of lignin from eucalyptus globulus pulping liquors to produce syringaldehyde and vanillin. Ind. Eng. Chem. Res. 2013, 52, 4421–4428. [Google Scholar] [CrossRef]
- Villar, J.C.; Caperos, A.; García-Ochoa, F. Oxidation of hardwood kraft-lignin to phenolic derivatives with oxygen as oxidant. Wood Sci. Technol. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Saito, T.; Brown, R.H.; Hunt, M.A.; Pickel, D.L.; Pickel, J.M.; Messman, J.M.; Baker, F.S.; Keller, M.; Naskar, A.K. Turning renewable resources into value-added polymer: Development of lignin-based thermoplastic. Green Chem. 2012, 14, 3295–3303. [Google Scholar] [CrossRef]
- Torrezan, T.; Agnelli, J.A.M.; Bettini, S.H.P. Polymeric Olefinic Composition, Lignin Use and Object. U.S. Patent Application No. 16/324,372, 6 June 2019. [Google Scholar]
- Kubo, S.; Kadla, J.F. The formation of strong intermolecular interactions in immiscible blends of poly(vinyl alcohol) (PVA) and lignin. Biomacromolecules 2003, 4, 561–567. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Rudnitskaya, A.; Fernando, F.A.; Evtuguin, D.V.; Maria, M.T.; Joaõ, J.A.; Costa, L.C. Electrochemical impedance study of the lignin-derived conducting polymer. Electrochim. Acta 2012, 76, 69–76. [Google Scholar] [CrossRef]
- Gonçalves, S.S.L.; Rudnitskaya, A.; Sales, A.J.M.; Costa, L.C.C.; Evtuguin, D.V. Nanocomposite Polymeric Materials Based on Eucalyptus Lignoboost® Kraft Lignin for Liquid Sensing Applications. Materials (Basel) 2020, 13, 1637. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Evtuguin, D.V.; Costa, L.C.; Pedro Graça, M.P.; Fernandes, A.J.S.; Rosario Correia, M.; Teresa Gomes, M.T.; Oliveira, J.A.B.P. Potentiometric chemical sensors from lignin-poly(propylene oxide) copolymers doped by carbon nanotubes. Analyst 2013, 138, 501–508. [Google Scholar] [CrossRef]
- Kim, Y.S.; Youe, W.J.; Kim, S.J.; Lee, O.K.; Lee, S.S. Preparation of a Thermoresponsive Lignin-Based Biomaterial through Atom Transfer Radical Polymerization. J. Wood Chem. Technol. 2010, 35, 251–259. [Google Scholar] [CrossRef]
- Webb, C.D.; Sanford, W.M.; McCall, A.A. Acrylonitrile Butadiene Styrene Copolymer/Lignin Blends. U.S. Patent Application No. 16/663,497, 30 April 2020. [Google Scholar]



| Biomass | Linkages (%) | References | |||||
|---|---|---|---|---|---|---|---|
| β-O-4 | β-5 | α-O-4 | β-β | 5-5 | 4-O-5 | ||
| Hardwood | 60–62% | 3–11% | 3–12% | <1% | 2% | [38] | |
| Lignin | Linkage (per 100 Ar) | |||
|---|---|---|---|---|
| β-O-4 | β-5 | β-β | References | |
| Birch | 2 | 2 | 3 | [39] |
| E. globulus | 12 | 1 | 2 | [39] |
| E. grandis | 5 | 2 | 3 | [39] |
| Sample | Mw (g/mol) | Mn (g/mol) | PDI | Method | Ref. |
|---|---|---|---|---|---|
| HWKL | 3900 | 1000 | 3.9 | HPLC | [42] |
| HWKL | 3300 | 1000 | 3.3 | HPLC | [42] |
| Eucalyptus exserta | 55,500 | 31,700 | 1.8 | MALLS | [40] |
| Indulin | 3700 | 1300 | 2.3 | GPC | [43] |
| Eucalin | 3900 | 1700 | 2.3 | GPC | [43] |
| Ligflow 401 | 2042 | 719 | 2.8 | GPC | [44] |
| HWKL | 2400 | 1263 | 1.9 | GPC | [45] |
| HWKL | 3290 | 1793 | 1.8 | GPC | [46] |
| European beech | 1711 | 1044 | 1.6 | GPC | [47] |
| Eucalyptus | 4200 | 1273 | 3.3 | HPLC | [48] |
| Eucalyptus grandis | 1740 | 910 | 1.9 | GPC | [49] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery. Polymers 2020, 12, 1795. https://doi.org/10.3390/polym12081795
Jardim JM, Hart PW, Lucia L, Jameel H. Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery. Polymers. 2020; 12(8):1795. https://doi.org/10.3390/polym12081795
Chicago/Turabian StyleJardim, Juliana M., Peter W. Hart, Lucian Lucia, and Hasan Jameel. 2020. "Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery" Polymers 12, no. 8: 1795. https://doi.org/10.3390/polym12081795
APA StyleJardim, J. M., Hart, P. W., Lucia, L., & Jameel, H. (2020). Insights into the Potential of Hardwood Kraft Lignin to Be a Green Platform Material for Emergence of the Biorefinery. Polymers, 12(8), 1795. https://doi.org/10.3390/polym12081795








