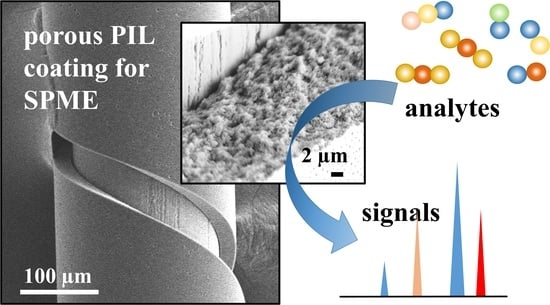
Thin Porous Poly(ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.1.1. Chemicals
2.1.2. Instruments
2.2. Experimental Methods
2.2.1. Synthesis of Poly(1-Cyanomethyl-3-Vinylimidazolium Bis(Trifluoromethane Sulfonyl)imide) (PCMVImTFSI)
2.2.2. Preparation and Characterization of the Porous material.
2.2.3. Analytes
2.2.4. Chromatographic Conditions
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Patinha, D.J.D.; Silvestre, A.J.D.; Marrucho, I.M. Poly(ionic liquids) in solid phase microextraction: Recent advances and perspectives. Prog. Polym. Sci. 2019, 98, 101148. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Ohtani, N.; Iwatsuki, K.; Sotoyama, K.; Wada, A.; Tokuno, K.; Iwabuchi, H.; Tanaka, K. Analysis of volatile compounds released during the grinding of roasted coffee beans using solid-phase microextraction. J. Agric. Food Chem. 2003, 51, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Helin, A.; Rönkkö, T.; Parshintsev, J.; Hartonen, K.; Schilling, B.; Läubli, T.; Riekkola, M.-L. Solid phase microextraction Arrow for the sampling of volatile amines in wastewater and atmosphere. J. Chromatogr. A 2015, 1426, 56–63. [Google Scholar] [CrossRef]
- Matin, A.A.; Biparva, P.; Gheshlaghi, M. Gas chromatographic determination of polycyclic aromatic hydrocarbons in water and smoked rice samples after solid-phase microextraction using multiwalled carbon nanotube loaded hollow fiber. J. Chromatogr. A 2014, 1374, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Del Castillo, M.L.R.; Blanch, G.P.; Herraiz, M. Detection of the adulteration of olive oils by solid phase microextraction and multidimensional gas chromatography. Food Chem. 2006, 97, 336–342. [Google Scholar] [CrossRef]
- Bessonneau, V.; Zhan, Y.; De Lannoy, I.A.M.; Saldivia, V.; Pawliszyn, J. In vivo solid-phase microextraction liquid chromatography-tandem mass spectrometry for monitoring blood eicosanoids time profile after lipopolysaccharide-induced inflammation in Sprague-Dawley rats. J. Chromatogr. A 2015, 1424, 134–138. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef]
- Souza Silva, E.A.; Pawliszyn, J. Optimization of fiber coating structure enables direct immersion solid phase microextraction and high-throughput determination of complex samples. Anal. Chem. 2012, 84, 6933–6938. [Google Scholar] [CrossRef]
- Naccarato, A.; Gionfriddo, E.; Elliani, R.; Pawliszyn, J.; Sindona, G.; Tagarelli, A. Investigating the robustness and extraction performance of a matrix-compatible solid-phase microextraction coating in human urine and its application to assess 2–6-ring polycyclic aromatic hydrocarbons using GC–MS/MS. J. Sep. Sci. 2018, 41, 929–939. [Google Scholar] [CrossRef]
- Hou, X.; Wang, L.; Guo, Y. Recent developments in solid-phase microextraction coatings for Environmental and biological analysis. Chem. Lett. 2017, 46, 1444–1455. [Google Scholar] [CrossRef]
- Feng, J.; Qiu, H.; Liu, X.; Jiang, S.; Feng, J. The development of solid-phase microextraction fibers with metal wires as supporting substrates. TrACs Trends Anal. Chem. 2013, 46, 44–58. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Sun, J.-K.; Kochovski, Z.; Zhang, W.-Y.; Kirmse, H.; Lu, Y.; Antonietti, M.; Yuan, J. General synthetic route toward highly dispersed metal clusters enabled by poly(ionic liquid)s. J. Am. Chem. Soc. 2017, 139, 8971–8976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Meng, Y.; Anderson, J.L. Polymeric ionic liquids as selective coatings for the extraction of esters using solid-phase microextraction. J. Chromatogr. A 2008, 1208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; Zhang, C.; Devasurendra, A.M.; Tillekeratne, L.M.V.; Anderson, J.L.; Kirchhoff, J.R. Conductive polymeric ionic liquids for electroanalysis and solid-phase microextraction. Anal. Chim. Acta 2016, 910, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Patinha, D.J.S.; Tomé, L.C.; Isik, M.; Mecerreyes, D.; Silvestre, A.J.D.; Marrucho, I.M. Expanding the applicability of poly(Ionic Liquids) in solid phase microextraction: Pyrrolidinium coatings. Materials 2017, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Patinha, D.J.S.; Pothanagandhi, N.; Vijayakrishna, K.; Silvestre, A.J.D.; Marrucho, I.M. Layer-by-layer coated imidazolium–Styrene copolymers fibers for improved headspace-solid phase microextraction analysis of aromatic compounds. React. Funct. Polym. 2018, 125, 93–100. [Google Scholar] [CrossRef]
- Yu, H.; Ho, T.D.; Anderson, J.L. Ionic liquid and polymeric ionic liquid coatings in solid-phase microextraction. TrAC Trends Anal. Chem. 2013, 45, 219–232. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X.; Chen, L. Recent development and applications of poly (ionic liquid)s in microextraction techniques. TrAC Trends Anal. Chem. 2019, 112, 123–134. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Xu, L.; Wang, S.; Liu, X.; Jiang, S. Novel double-confined polymeric ionic liquids as sorbents for solid-phase microextraction with enhanced stability and durability in high-ionic-strength solution. J. Chromatogr. A 2012, 1268, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, J.F. Development of a solid-phase microextraction fiber by chemical binding of polymeric ionic liquid on a silica coated stainless steel wire. J. Chromatogr. A 2012, 1230, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Lin, C.; Fang, G.; Wang, S. A novel SPME fiber chemically linked with 1-vinyl-3-hexadecylimidazolium hexafluorophosphate ionic liquid coupled with GC for the simultaneous determination of pyrethroids in vegetables. Chromatographia 2012, 75, 789–797. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Yu, H.; Cole, W.T.S.; Ho, T.D.; Pino, V.; Anderson, J.L.; Afonso, A.M. Polymeric ionic liquid coatings versus commercial solid-phase microextraction coatings for the determination of volatile compounds in cheeses. Talanta 2014, 121, 153–162. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Li, L.; Wang, X.; Duan, H.; Luo, C. Multiwalled carbon nanotubes-doped polymeric ionic liquids coating for multiple headspace solid-phase microextraction. Talanta 2014, 123, 18–24. [Google Scholar] [CrossRef]
- Mei, M.; Yu, J.; Huang, X.; Li, H.; Lin, L.; Yuan, D. Monitoring of selected estrogen mimics in complicated samples using polymeric ionic liquid-based multiple monolithic fiber solid-phase microextraction combined with high-performance liquid chromatography. J. Chromatogr. A 2015, 1385, 12–19. [Google Scholar] [CrossRef]
- Wu, M.; Wang, L.; Zeng, B.; Zhao, F. Ionic liquid polymer functionalized carbon nanotubes-doped poly(3,4-ethylenedioxythiophene) for highly-efficient solid-phase microextraction of carbamate pesticides. J. Chromatogr. A 2016, 1444, 42–49. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.; Sun, J.-K.; Antonietti, M.; Yuan, J. Poly(ionic liquid)s with engineered nanopores for energy and environmental applications. Polymer 2020, 202, 122640. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, M.; Zhang, A.P.; Prescher, S.; Antonietti, M.; Yuan, J. Hierarchically structured nanoporous poly(ionic liquid) membranes: Facile preparation and application in fiber-optic pH sensing. J. Am. Chem. Soc. 2013, 135, 5549–5552. [Google Scholar] [CrossRef]
- Zhao, Q.; Fellinger, T.-P.; Antonietti, M.; Yuan, J. A novel polymeric precursor for micro/mesoporous nitrogen-doped carbons. J. Mater. Chem. A 2013, 1, 5113–5120. [Google Scholar] [CrossRef]
- Levkin, P.A.; Svec, F.; Fréchet, J.M.J. Porous polymer coatings: A versatile approach to superhydrophobic surfaces. Adv. Funct. Mater. 2009, 19, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Escalé, P.; Rubatat, L.; Billon, L.; Save, M. Recent advances in honeycomb-structured porous polymer films prepared via breath figures. Eur. Polym. J. 2012, 48, 1001–1025. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. Porous polymer films with size-tunable surface pores. Chem. Mater. 2007, 19, 2581–2584. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Valdés-Bango, F.; Losada-Ambrinos, R.; Martín, J.I.; Vélez, M.; Alameda, J.M.; Alonso, F.J.G. Polymer porous thin films obtained by direct spin coating. Polym. Int. 2018, 67, 393–398. [Google Scholar] [CrossRef]
- Wang, H.; Min, S.; Ma, C.; Liu, Z.; Zhang, W.; Wang, Q.; Li, D.; Li, Y.; Turner, S.; Han, Y.; et al. Synthesis of single-crystal-like nanoporous carbon membranes and their application in overall water splitting. Nat. Commun. 2017, 8, 13592. [Google Scholar] [CrossRef]
- Feng, J.; Sun, M.; Wang, X.; Liu, X.; Jiang, S. Ionic liquids-based crosslinked copolymer sorbents for headspace solid-phase microextraction of polar alcohols. J. Chromatogr. A 2012, 1245, 32–38. [Google Scholar] [CrossRef]
- Silva, C.; Cavaco, C.; Perestrelo, R.; Pereira, J.; Câmara, J.S. Microextraction by packed Sorbent (MEPS) and solid-phase microextraction (SPME) as sample preparation procedures for the metabolomic profiling of urine. Metabolites 2014, 4, 71–97. [Google Scholar] [CrossRef]
- Jiang, R.; Pawliszyn, J. Thin-film microextraction offers another geometry for solid-phase microextraction. TrAC Trends Anal. Chem. 2012, 39, 245–253. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patinha, D.J.S.; Wang, H.; Yuan, J.; Rocha, S.M.; Silvestre, A.J.D.; Marrucho, I.M. Thin Porous Poly(ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction. Polymers 2020, 12, 1909. https://doi.org/10.3390/polym12091909
Patinha DJS, Wang H, Yuan J, Rocha SM, Silvestre AJD, Marrucho IM. Thin Porous Poly(ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction. Polymers. 2020; 12(9):1909. https://doi.org/10.3390/polym12091909
Chicago/Turabian StylePatinha, David J. S., Hong Wang, Jiayin Yuan, Sílvia M. Rocha, Armando J. D. Silvestre, and Isabel M. Marrucho. 2020. "Thin Porous Poly(ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction" Polymers 12, no. 9: 1909. https://doi.org/10.3390/polym12091909
APA StylePatinha, D. J. S., Wang, H., Yuan, J., Rocha, S. M., Silvestre, A. J. D., & Marrucho, I. M. (2020). Thin Porous Poly(ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction. Polymers, 12(9), 1909. https://doi.org/10.3390/polym12091909