Effect of Organic Cage Nucleating Agent Structure on Nucleating Efficiency and the Structure-Property Relationship
Abstract
1. Introduction
2. Experimental Setup
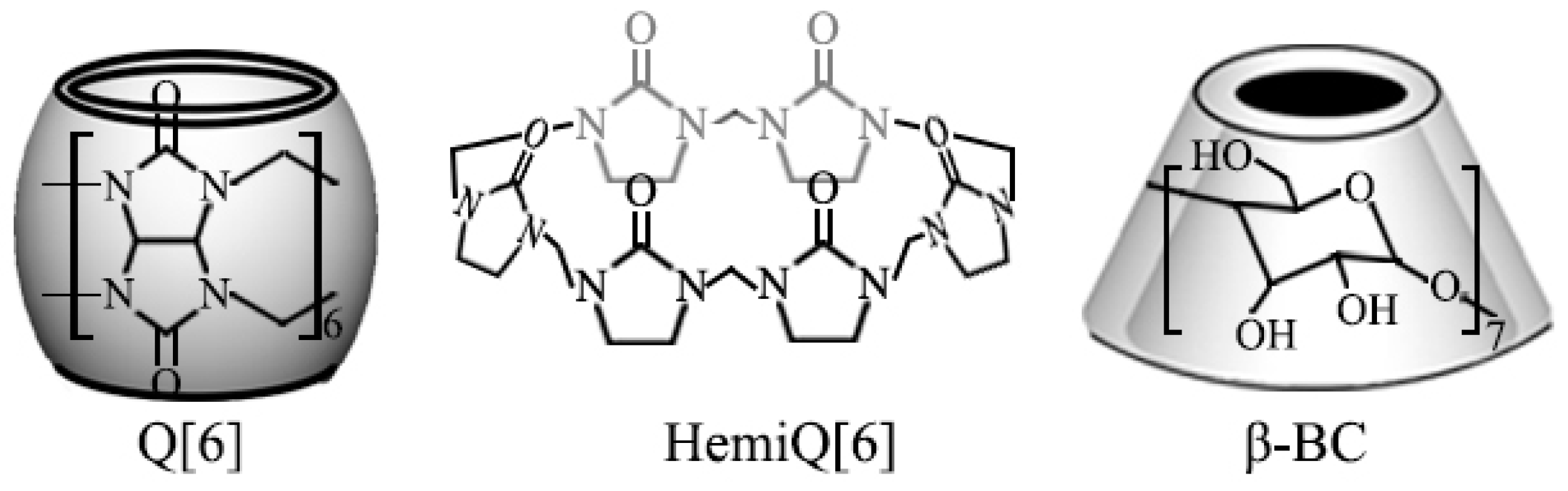
2.1. Materials
2.2. Nanocomposite Preparation
2.3. Injection Molding Foaming
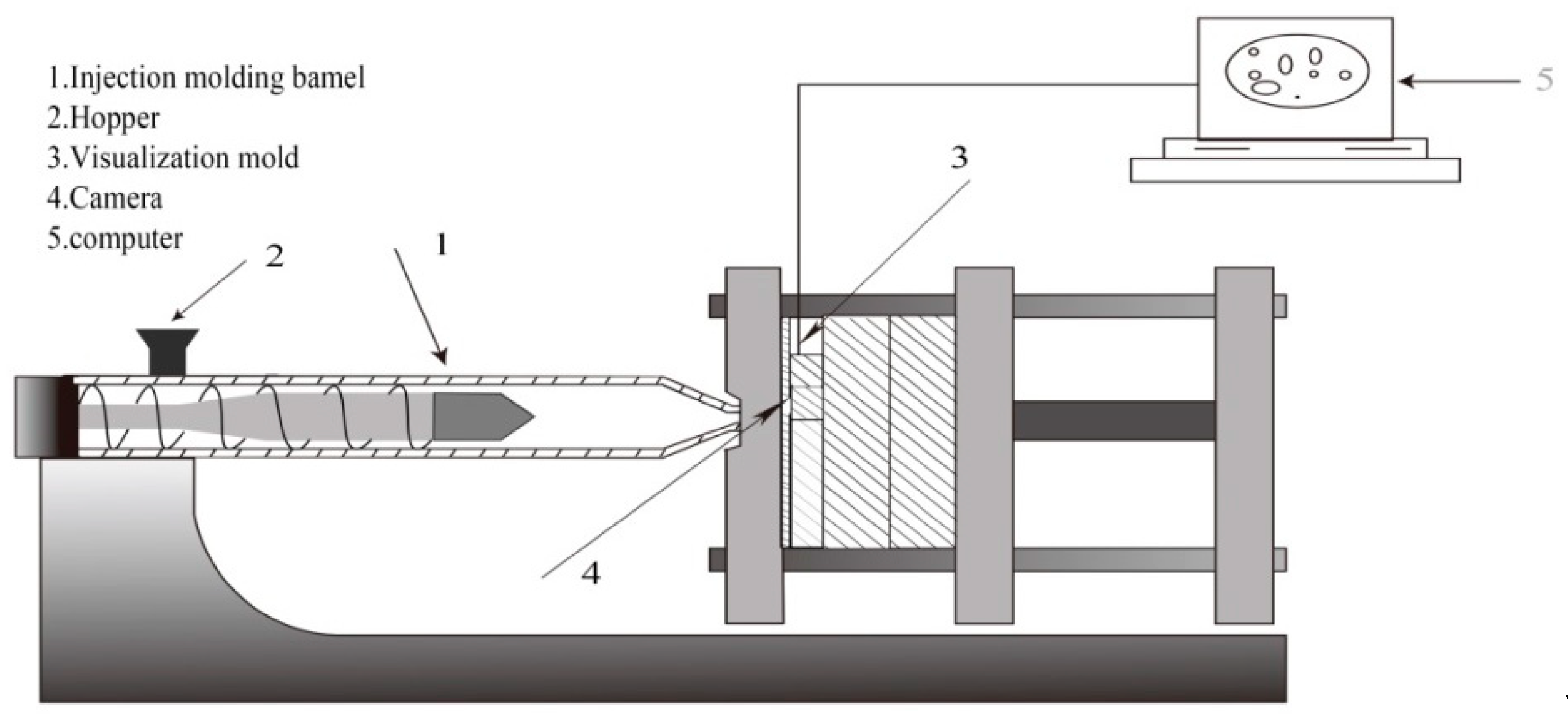
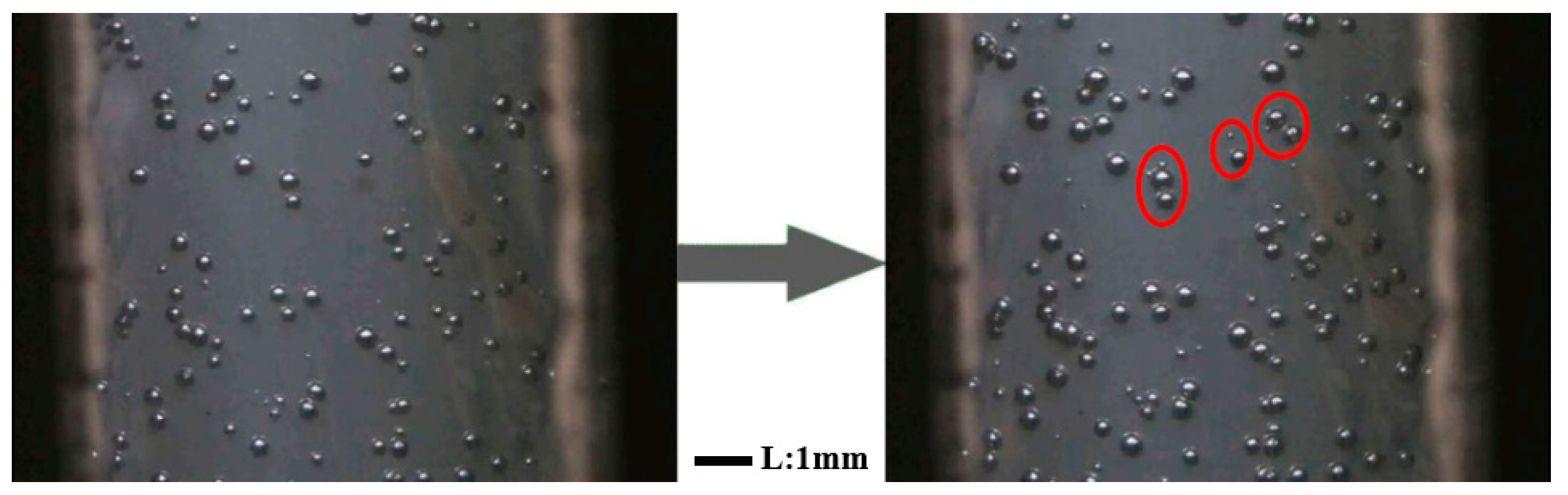
2.4. In Situ Foaming Visualization
2.5. Morphological Analysis
2.6. Transmission Electron Microscope (TEM) Analysis
3. Results and Discussion
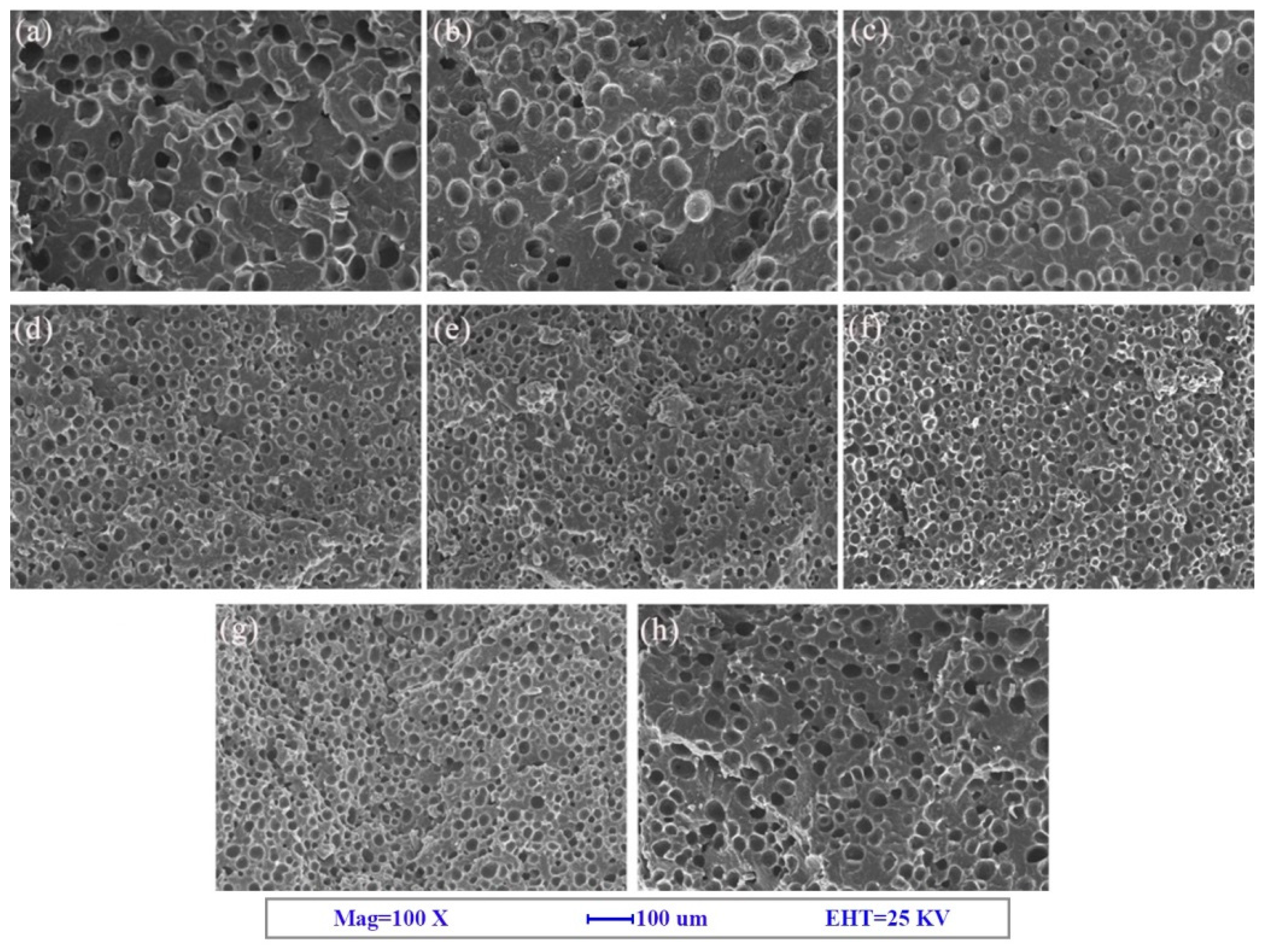
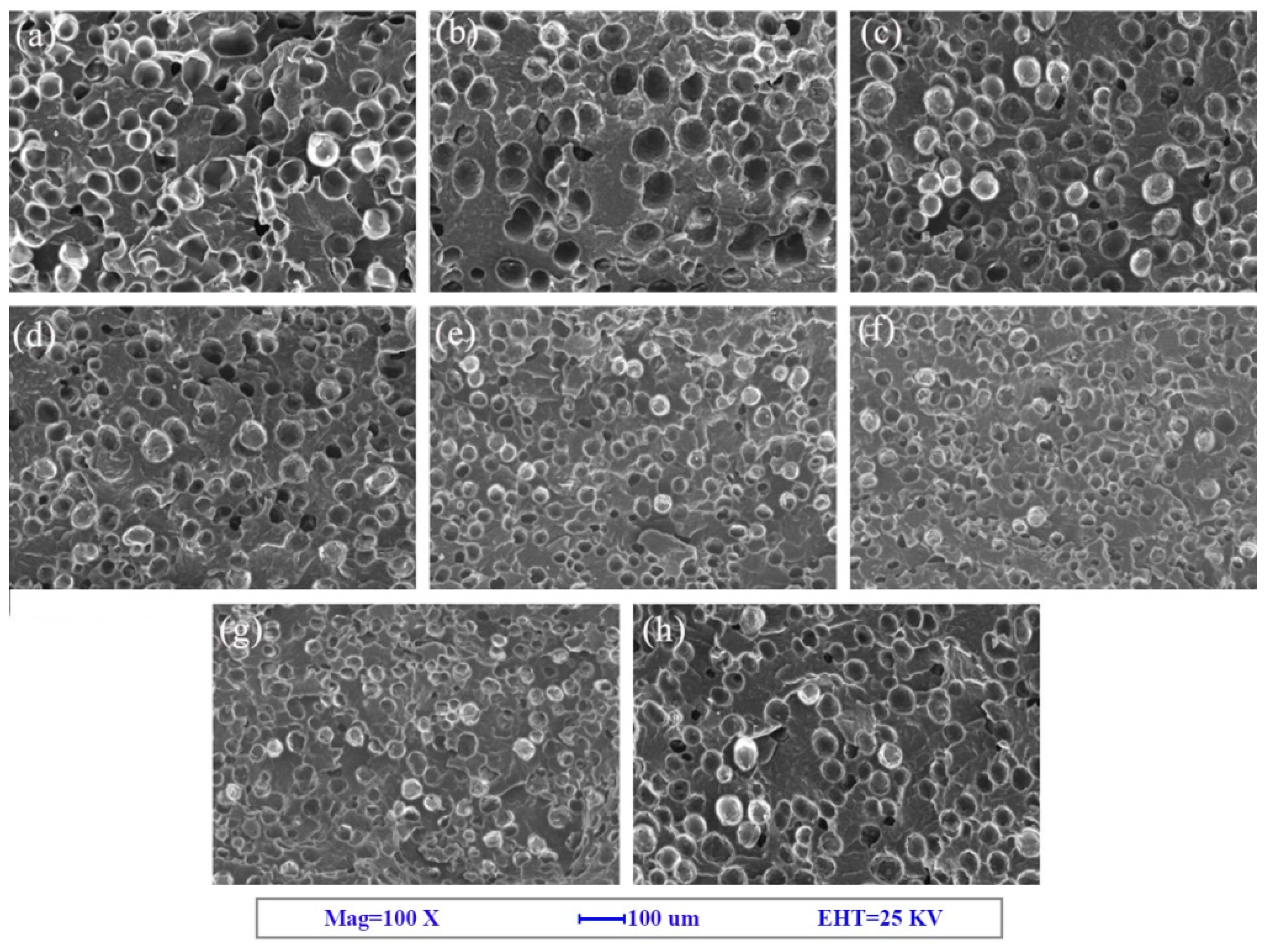
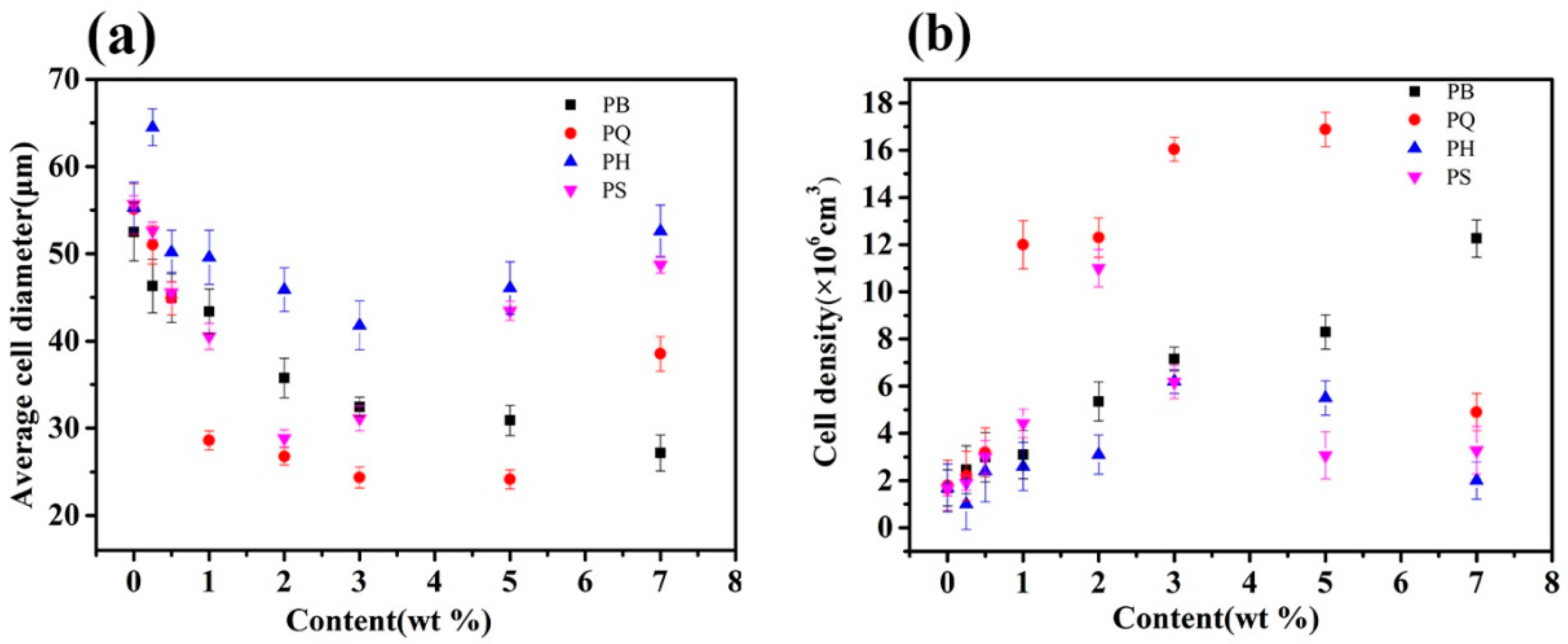
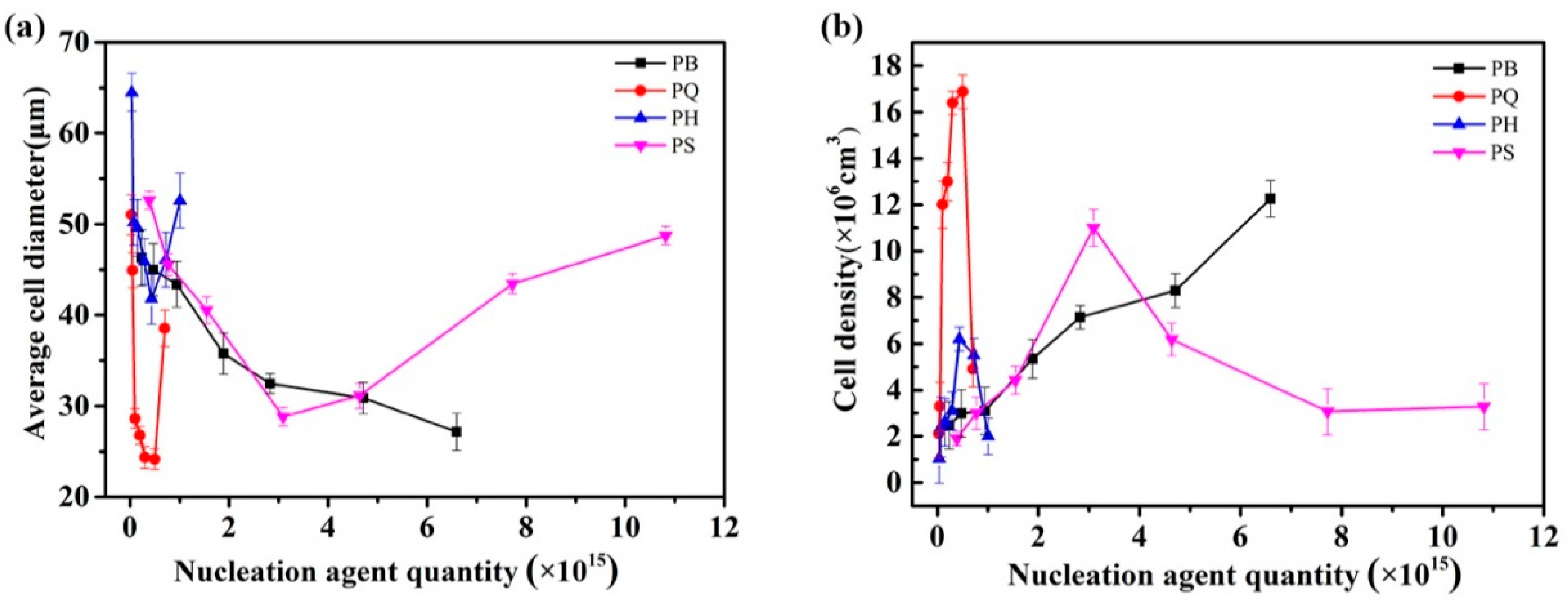
3.1. Cell Morphology
3.2. Discussion on Nucleating Efficiency and Structure-Property Relationship
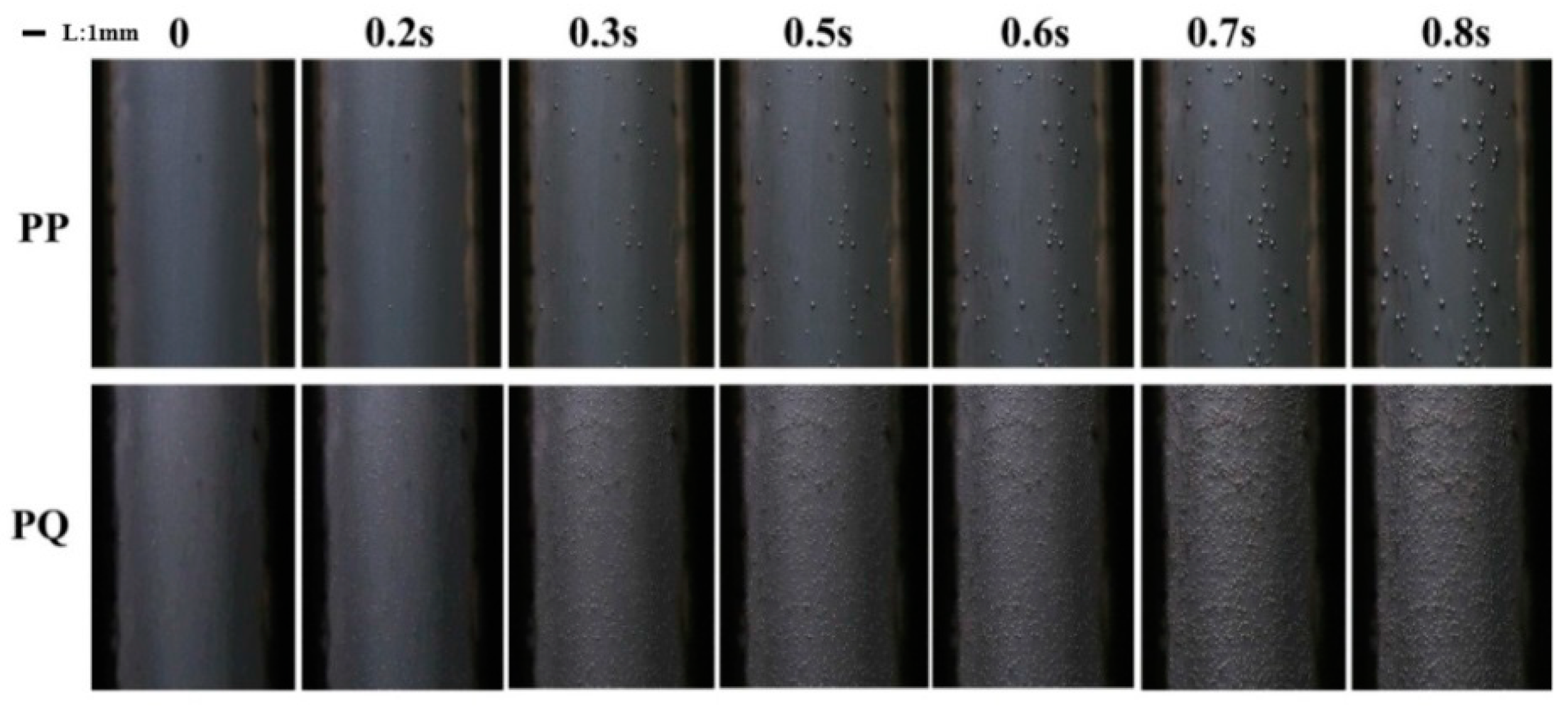
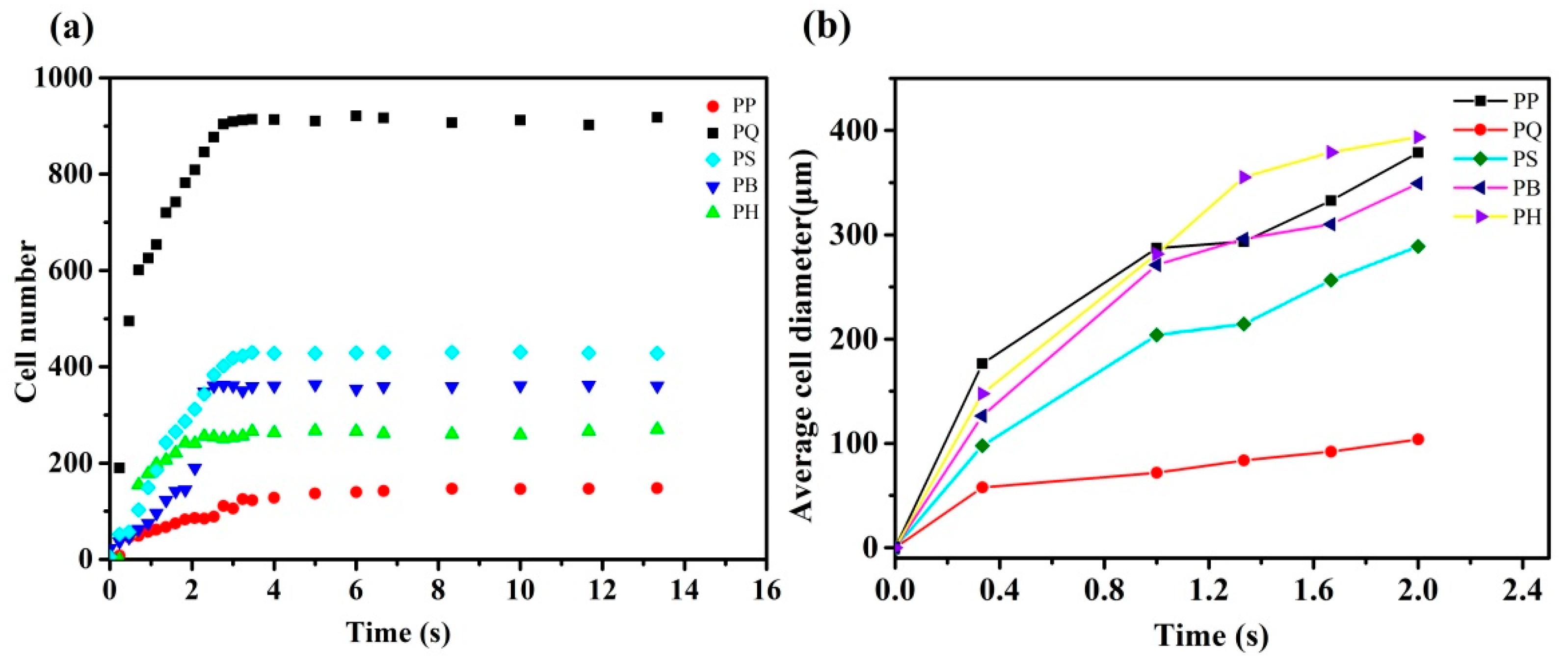
3.3. In Situ Foaming Visualisation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nam, P.H.; Maiti, P.; Okamoto, M. Foam processing and cellular structure of polypropylene/clay nanocomposites. Polym. Eng. Sci. 2002, 9, 1907–1918. [Google Scholar] [CrossRef]
- Suh, K.W.; Park, C.P.; Maurer, M.; Tusim, M.H.; Genova, R.D.; Broos, R.; Sophiea, D.P. Lightweight cellular plastics. Adv. Mater. 2000, 12, 1779–1789. [Google Scholar] [CrossRef]
- Kumar, V. Microcellular polymers: Novel materials for the 21st century. Cell. Polym. 1993, 12, 207–223. [Google Scholar]
- Ma, X.; Tu, R.; Cheng, X.; Zhu, S.; Ma, J.; Fang, T. Experimental study of thermal behavior of insulation material rigid polyurethane in parallel, symmetric, and adjacent building facade constructions. Polymers 2018, 10, 1104. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.J.; He, B.; Guo, W.; Hua, L.; Yang, Q. Effects of nano-CaCO3 content on the crystallization, mechanical properties, and cell structure of PP nanocomposites in microcellular injection molding. Polymers 2018, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Spina, R. Technological characterization of PE/EVA blends for foam injection molding. Mater. Design 2015, 84, 64–71. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part B 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Zhang, J.; Sui, W.; Jang, J.; Si, C. One-pot lignin depolymerization and activation by solid acid catalytic phenolation for lightweight phenolic foam preparation. Ind. Crops. Prod. 2018, 124, 216–225. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, X.; Liao, C.; Geng, X.; Wang, C.; Chu, F. Preparation and characterization of DOPO-ITA modified ethyl cellulose and its application in phenolic foams. Polymers 2018, 10, 1049. [Google Scholar] [CrossRef]
- Yang, J.; Ye, Y.; Li, X.; Lu, X.; Chen, R. Flexible, conductive, and highly pressure-sensitive graphene-polyimide foam for pressure sensor application. Compos. Sci. Technol. 2018, 164, 187–194. [Google Scholar] [CrossRef]
- Sun, G.; Wang, W.; Zhang, C.; Liu, L.; Wei, H.; Han, S. Fabrication of isocyanate-based polyimide foam by a post grafting method. J. Appl. Polym. Sci. 2017, 134, 44240. [Google Scholar] [CrossRef]
- Sun, G.; Wang, W.; Wang, L.; Yang, Z.; Liu, L.; Wang, J.; Ma, N.; Wei, H.; Han, S. Effects of aramid honeycomb core on the flame retardance and mechanical property for isocyanate-based polyimide foams. J. Appl. Polym. Sci. 2017, 134, 45041. [Google Scholar] [CrossRef]
- Tucker, A.S.; Ward, C.A. Critical state of bubbles in liquid-gas solutions. J. Appl. Phys. 1975, 46, 4801–4806. [Google Scholar] [CrossRef]
- Forest, T.W.; Ward, C.A. Effect of a dissolved gas on the homogeneous nucleation pressure of a liquid. J. Chem. Phys. 1977, 66, 2322–2330. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. Nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part II: Experimental results and discussion. Polym. Eng. Sci. 1987, 27, 493–499. [Google Scholar] [CrossRef]
- Nofar, M.; Majithiya, K.; Kuboki, T.; Park, C.B. The foamability of low-melt-strength linear polypropylene with nanoclay and coupling agent. J. Cell. Plast. 2012, 48, 271–287. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Li, J.; Zhou, L.; Jing, Z.; Ma, Z. Preparation and properties of polyimide/chopped carbon fiber composite foams. Polym. Adv. Technol. 2017, 28, 28–34. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Effects of carbon nanotubes/graphene nanoplatelets hybrid systems on the structure and properties of polyetherimide-based foams. Polymers 2018, 10, 348. [Google Scholar] [CrossRef]
- Collais, D.I.; Baird, D.G. Tensile toughness of microcellular foams of polystyrene, styrene-acrylonitrile copolymer and polycarbonate, and the effect of dissolved gas on the tensile toughness of the same polymer matrices and microcellular foams. Polym. Eng. Sci. 2010, 35, 1167–1177. [Google Scholar] [CrossRef]
- Yang, J.T.; Huang, L.Q.; Zhang, Y.F.; Chen, F.; Fan, P.; Zhong, M.Q.; Yeh, S. A new promising nucleating agent for polymer foaming: Applications of ordered Mesoporous silica particles in polymethyl methacrylate supercritical carbon dioxide microcellular foaming. Ind. Eng. Chem. Res. 2013, 52, 14169–14178. [Google Scholar] [CrossRef]
- Zhai, W.T.; Wang, J.; Chen, N.; Naguib, H.E.; Park, C.B. The orientation of carbon nanotubes in poly(ethylene-co-octene) microcellular foaming and its suppression effect on cell coalescence. Polym. Eng. Sci. 2012, 52, 2078–2089. [Google Scholar] [CrossRef]
- Miyahara, Y.; Goto, K.; Oka, M.; Inazu, T. Remarkably Facile Ring-Size Control in Macrocyclization: Synthesis of Hemicucurbit[6]uril and Hemicucurbit[12]uril. Angew. Chem. Int. Ed. 2004, 43, 5019–5022. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, L.H.; Xiao, X.; Zhang, Y.Q.; Xue, S.F.; Tao, Z.; Day, A. Locating the Cyclopentano Cousins of the Cucurbit[n]uril Family. J. Org. Chem. 2012, 77, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Mhlanga, S.D.; Mamba, B.B.; Krause, R.W.; Malefetse, T.J. Removal of organic contaminants from water using nanosponge cyclodextrin polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar] [CrossRef]
- Alongi, J.; Poskovic, M.P.; Visakh, M.; Frache, A.; Malucelli, G. Cyclodextrin nanosponges as novel green flame retardants for PP, LLDPE and PA6. Carbohydr. Polym. 2012, 88, 1387–1394. [Google Scholar] [CrossRef]
- Leung, S.N.; Wong, A.; Wang, L.C.; Park, C.B. Mechanism of extensional stress-induced cell formation in polymeric foaming processes with the presence of nucleating agents. J. Supercrit. Fluids 2012, 63, 187–198. [Google Scholar] [CrossRef]
- Leung, S.N.; Leung, S.N.; Li, H. Numerical simulation of polymeric foaming processes using modified nucleation theory. Plast., Rubber Compos. 2006, 35, 93–100. [Google Scholar] [CrossRef]
- Leung, S.N.; Wong, A.; Park, C.B.; Zong, J.H. Ideal surface geometry of nucleating agents to enhance cell nucleation in polymer foaming. J. Appl. Polym. Sci. 2008, 108, 3997–4003. [Google Scholar] [CrossRef]






| BC | Q[6] | HQ[6] | SiO2 | |
|---|---|---|---|---|
| density (g/cm3) | 1.37 | 1.44 | 1.23 | 1.12 |
| particle size (nm) | 114 | 204 | 326 | 104 |
| single particle volume (×10−15 cm3) | 0.775 | 4.443 | 5.774 | 0.589 |
| single particle weight (×10−15 g) | 1.062 | 6.220 | 6.929 | 0.647 |
| add weight per 100 g PP (g) | 0.085 | 0.5 | 0.55 | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; He, L.; Gong, W. Effect of Organic Cage Nucleating Agent Structure on Nucleating Efficiency and the Structure-Property Relationship. Polymers 2020, 12, 1975. https://doi.org/10.3390/polym12091975
Zhou Y, He L, Gong W. Effect of Organic Cage Nucleating Agent Structure on Nucleating Efficiency and the Structure-Property Relationship. Polymers. 2020; 12(9):1975. https://doi.org/10.3390/polym12091975
Chicago/Turabian StyleZhou, Yuhui, Li He, and Wei Gong. 2020. "Effect of Organic Cage Nucleating Agent Structure on Nucleating Efficiency and the Structure-Property Relationship" Polymers 12, no. 9: 1975. https://doi.org/10.3390/polym12091975
APA StyleZhou, Y., He, L., & Gong, W. (2020). Effect of Organic Cage Nucleating Agent Structure on Nucleating Efficiency and the Structure-Property Relationship. Polymers, 12(9), 1975. https://doi.org/10.3390/polym12091975





