Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Epoxy Resin System Selection
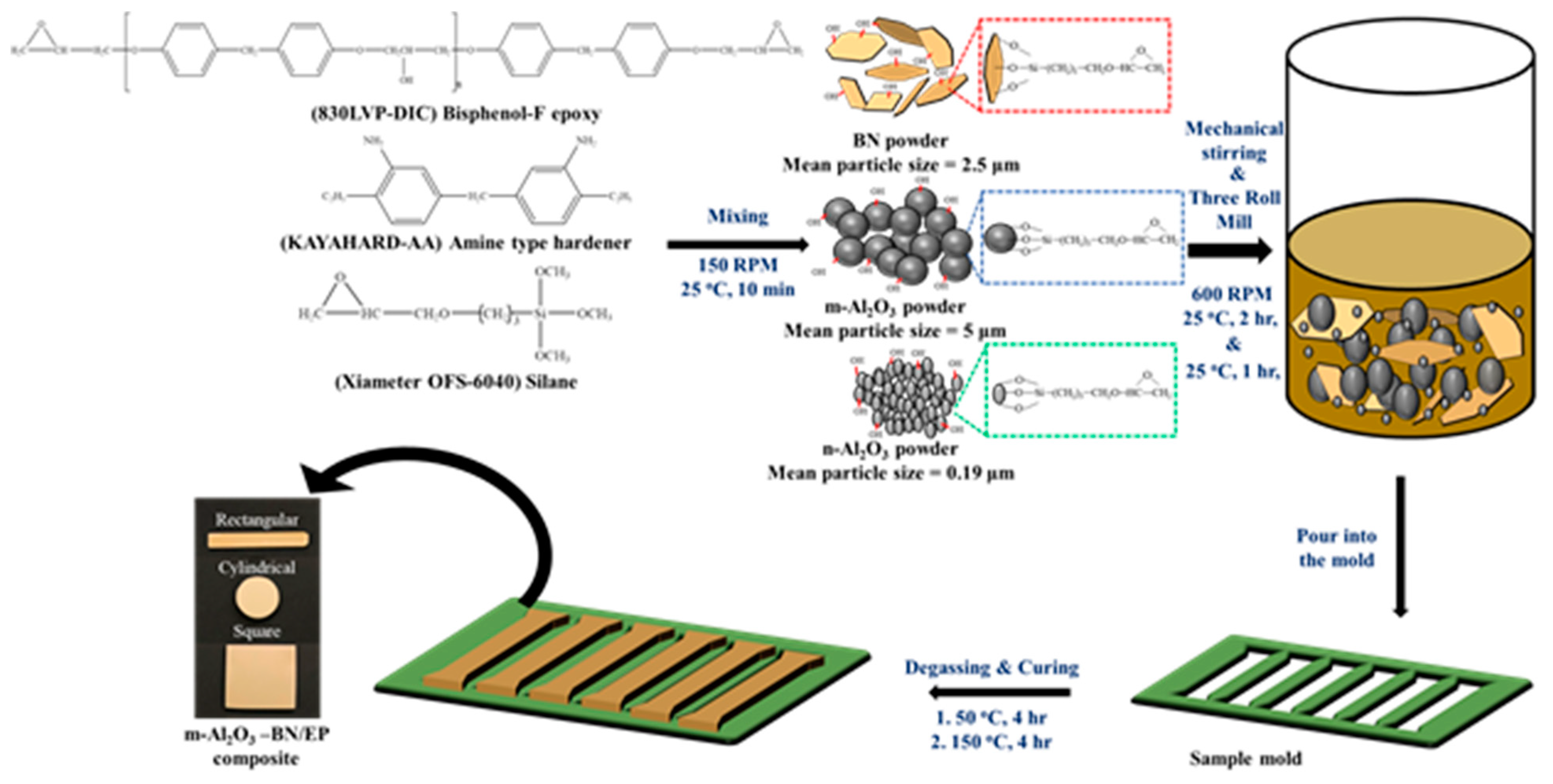
2.3. Fabrication of m-Al2O3-BN/EP Composites
2.4. Characterization
3. Results and Discussion
3.1. Underfill Technique
3.2. FTIR
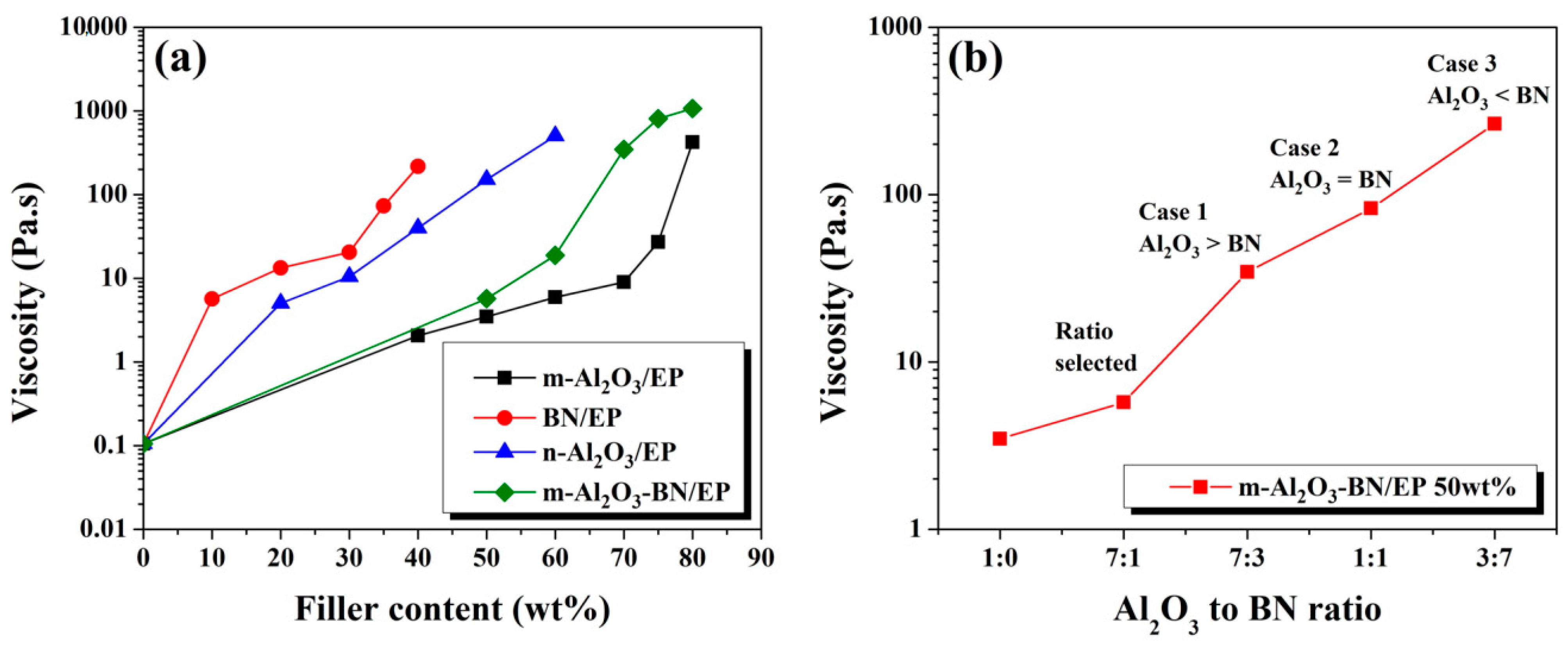
3.3. Rheological Study
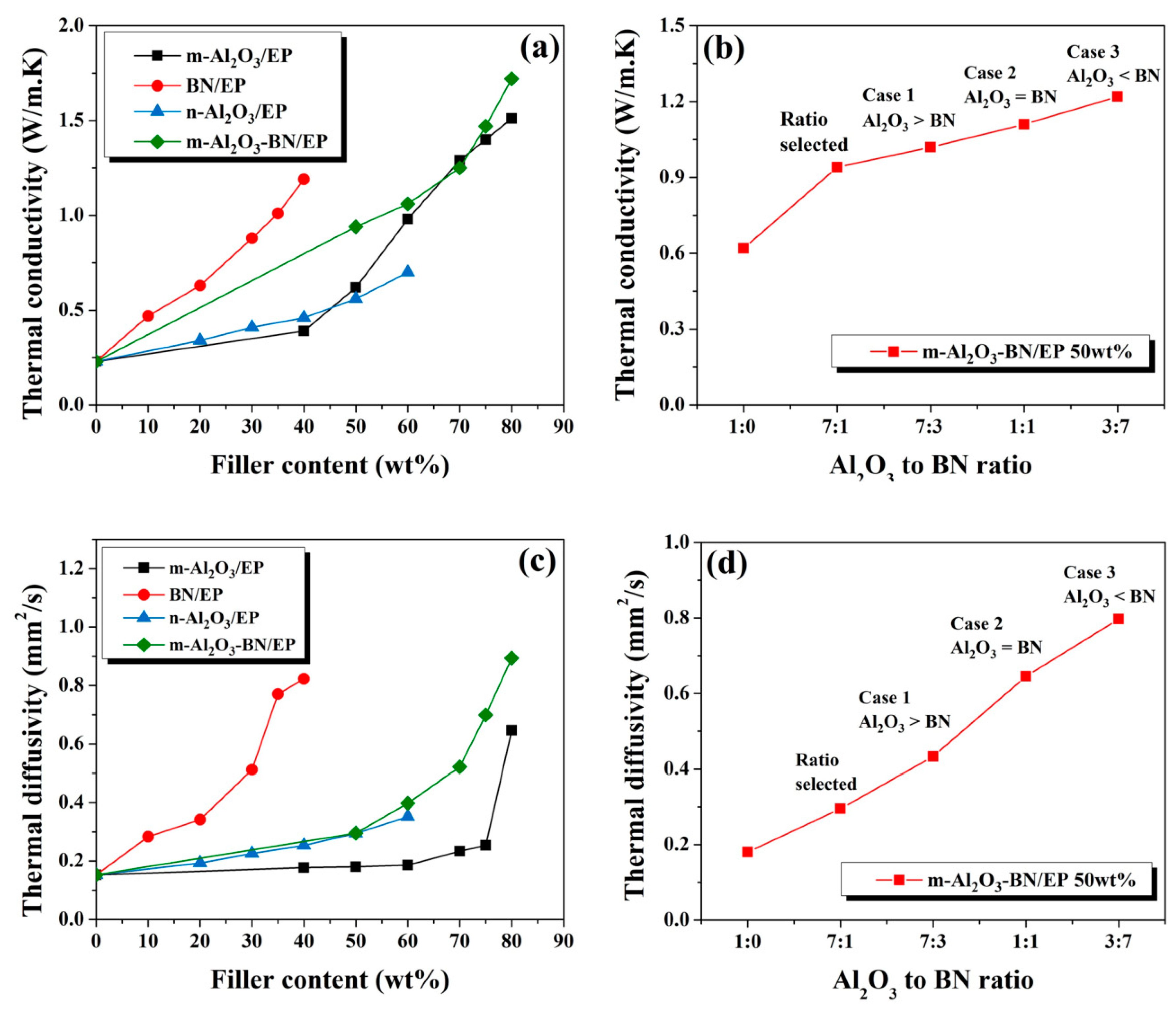
3.4. Thermal Conductivity of EP Composites
3.5. Composites Thermal Management Capacity
3.6. Glass Transition Temperature and Coefficient of Thermal Expansion of EP Composites
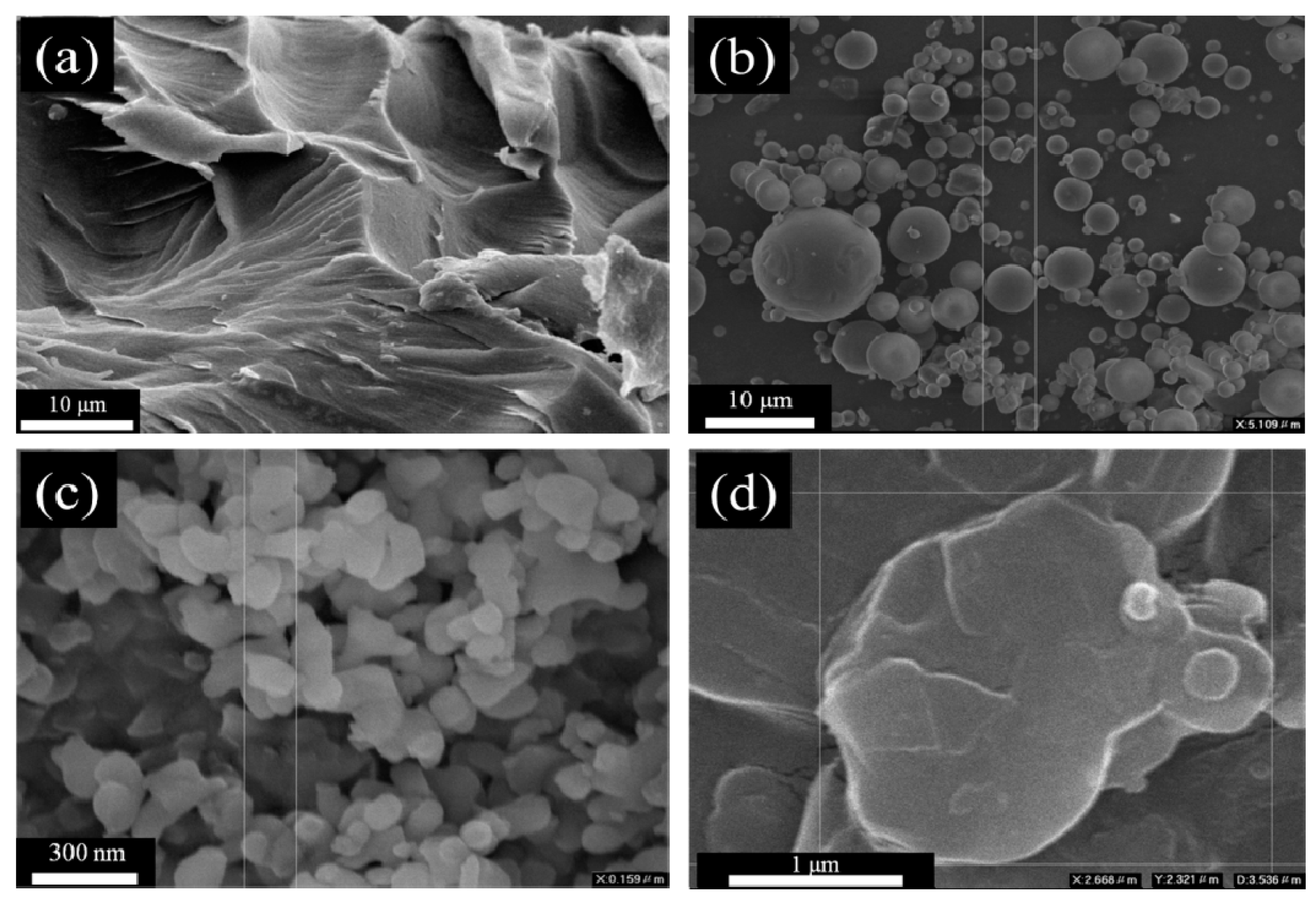
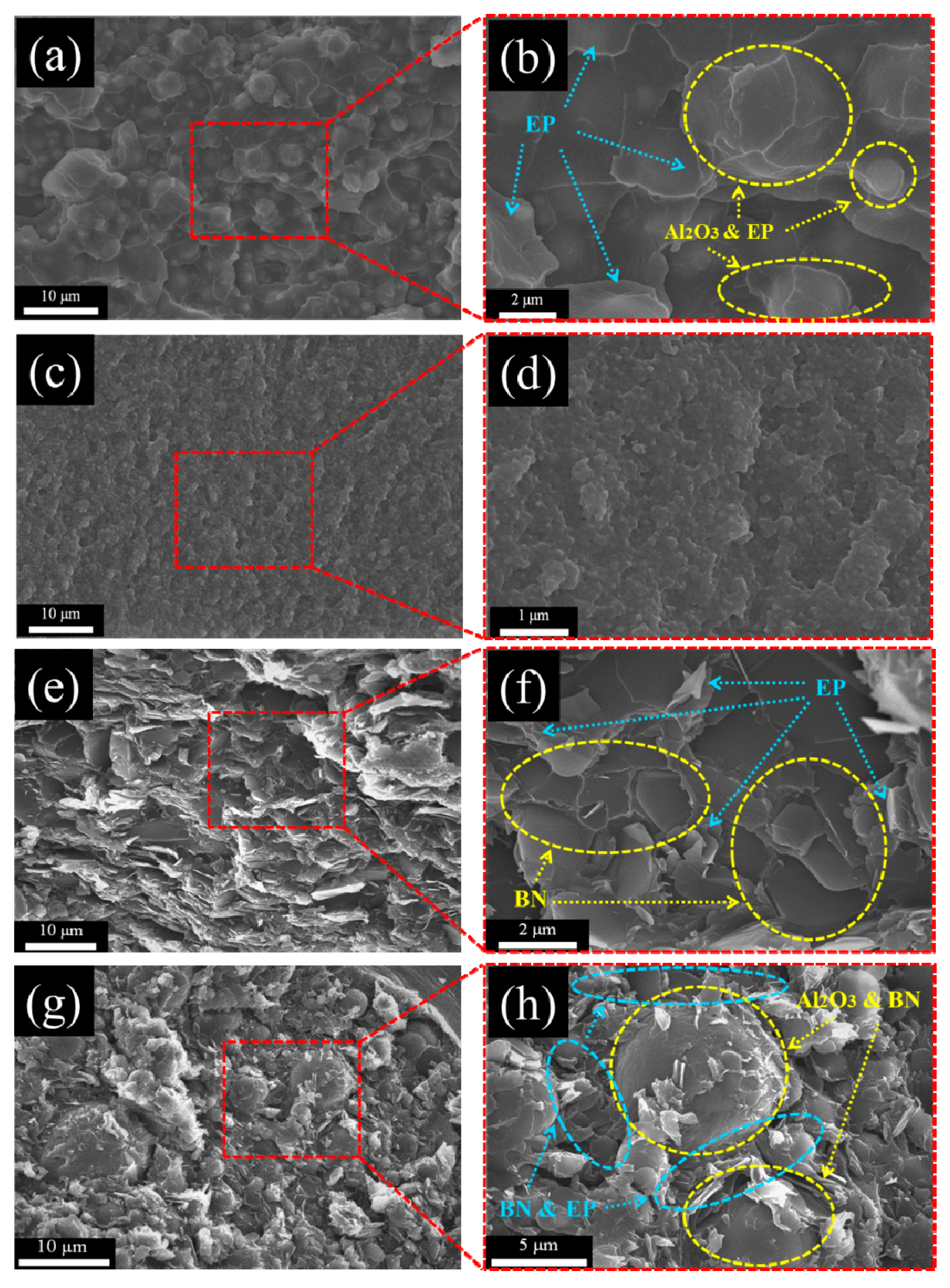
3.7. Morphological Observation of m-Al2O3-BN/EP Composites
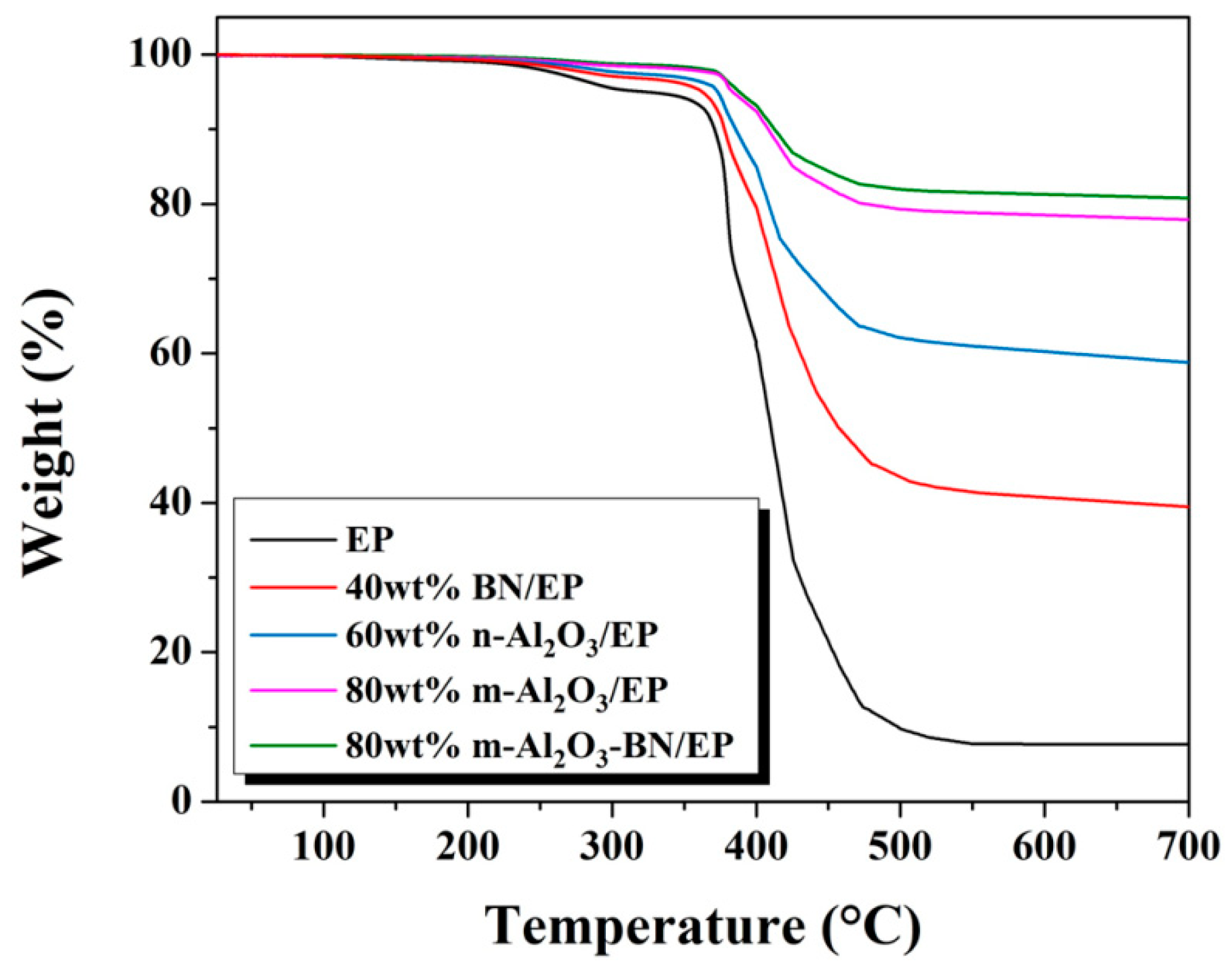
3.8. Thermal Stability
3.9. Electrical and Dielectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wie, J.; Kim, J. Thermal properties of binary filler hybrid composite with graphene oxide and pyrolyzed silicon-coated boron nitride. Polymers 2020, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, R.; Li, Y.; Li, H.; Wu, Z.; Huang, J.; Yu, B.; Gao, X.; Li, J.; Li, L. Optimization of boron nitride sphere loading in epoxy: Enhanced thermal conductivity and excellent electrical insulation. Polymers 2019, 11, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, F.; Zhao, X.; Zhang, W.; Li, S.; Lu, Y.; Zhang, L. Jelly-inspired construction of the three-dimensional interconnected BN network for lightweight, thermally conductive, and electrically insulating rubber composites. ACS Appl. Electron. Mater. 2020, 2, 1661–1669. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.; Liu, J. From lithographically patternable to genetically patternable electronic materials for miniaturized, scalable, and soft implantable bioelectronics to interface with nervous and cardiac systems. ACS Appl. Electron. Mater. 2020. In Press. [Google Scholar] [CrossRef]
- Pan, C.T.; Wang, S.Y.; Yen, C.K.; Ho, C.K.; Yen, J.F.; Chen, S.W.; Fu, F.R.; Lin, Y.T.; Lin, C.H.; Kumar, A.; et al. Study on delamination between polymer materials and metals in IC packaging process. Polymers 2019, 11, 940. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, J.; Zhou, J.; Li, B. Thermal conductivity of polymers and their nanocomposites. Adv. Mater. 2018, 30, 1705544. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Lv, X.; Tang, Y.; Ge, G.; Zhang, P.; Li, Y. Effect of alumina nanowires on the thermal conductivity and electrical performance of epoxy composites. Polymers 2020, 12, 2126. [Google Scholar] [CrossRef]
- Wang, J.; Suzuki, R.; Ogata, K.; Nakamura, T.; Dong, A.; Weng, W. Near-linear responsive and wide-range pressure and stretch sensor based on hierarchical graphene-based structures via solvent-free preparation. Polymers 2020, 12, 1814. [Google Scholar] [CrossRef]
- Cataldi, P.; Steiner, P.; Raine, T.; Lin, K.; Kocabas, C.; Young, R.J.; Bissett, M.; Kinloch, I.A.; Papageorgiou, D.G. Multifunctional biocomposites based on polyhydroxyalkanoate and graphene/carbon nanofiber hybrids for electrical and thermal applications. ACS Appl. Polym. Mater. 2020, 2, 3525–3534. [Google Scholar] [CrossRef]
- Song, S.H.; Park, K.H.; Kim, B.H.; Choi, Y.W.; Jun, G.H.; Lee, D.J.; Kong, B.S.; Paik, K.W.; Jeon, S. Enhanced thermal conductivity of epoxy–graphene composites by using non-oxidized graphene flakes with non-covalent functionalization. Adv. Mater. 2013, 25, 732–737. [Google Scholar] [CrossRef]
- Ruhkopf, J.; Sawallich, S.; Nagel, M.; Otto, M.; Plachetka, U.; Kremers, T.; Schnakenberg, U.; Kataria, S.; Lemme, M.C. Role of substrate surface morphology on the performance of graphene inks for flexible electronics. ACS Appl. Electron. Mater. 2019, 1, 1909–1916. [Google Scholar] [CrossRef]
- Ren, L.; Pashayi, K.; Fard, H.R.; Kotha, S.P.; Borca-Tasciuc, T.; Ozisik, R. Engineering the coefficient of thermal expansion and thermal conductivity of polymers filled with high aspect ratio silica nanofibers. Compos. B Eng. 2014, 58, 228–234. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Y.; Raghavan, S.; Moon, K.S.; Sitaraman, S.K.; Wong, C.P. Magnetic alignment of hexagonal boron nitride platelets in polymer matrix: Toward high performance anisotropic polymer composites for electronic encapsulation. ACS Appl. Mater. Interfaces 2013, 5, 7633–7640. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, M.; Nitzsche, F.; Laliberte, J.; Hind, S.; Robitaille, F.; Labrosse, M.R. Thermal properties of doubly reinforced fiberglass/epoxy composites with graphene nanoplatelets, graphene oxide and reduced-graphene oxide. Compos. B Eng. 2019, 164, 1–9. [Google Scholar] [CrossRef]
- Lee, W.S.; Yu, J. Comparative study of thermally conductive fillers in underfill for the electronic components. Diam. Relat. Mater. 2005, 14, 1647–1653. [Google Scholar]
- Wang, Y.; Wu, W.; Drummer, D.; Liu, C.; Tomiak, F.; Schneider, K.; Huang, Z. Achieving a 3D thermally conductive while electrically insulating network in polybenzoxazine with a novel hybrid filler composed of boron nitride and carbon nanotubes. Polymers 2020, 12, 2331. [Google Scholar] [CrossRef]
- Akhtar, M.W.; Lee, Y.S.; Yoo, D.J.; Kim, J.S. Alumina-graphene hybrid filled epoxy composite: Quantitative validation and enhanced thermal conductivity. Compos. B Eng. 2017, 131, 184–195. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Lindsay, L.; Cherns, D.; Pomeroy, J.W.; Liu, S.; Kuball, M. Modulating the thermal conductivity in hexagonal boron nitride via controlled boron isotope concentration. Commun. Phys. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving high thermal conductivity in epoxy composites: Effect of boron nitride particle size and matrix-filler interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.C.; Lima, A.M.; Demosthenes, L.C.C.; Oliveira, M.S.; Costa, U.O.; Bezerra, W.B.A.; Monteiro, S.N.; Rodriguez, R.J.S.; Deus, J.F.; Anacleto Pinheiro, W. Ballistic performance of ramie fabric reinforcing graphene oxide-incorporated epoxy matrix composite. Polymers 2020, 12, 2711. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J. Thermal conductivity of epoxy composites with a binary-particle system of aluminum oxide and aluminum nitride fillers. Compos. B Eng. 2013, 51, 140–147. [Google Scholar] [CrossRef]
- Fang, L.; Wu, C.; Qian, R.; Xie, L.; Yang, K.; Jiang, P. Nano–micro structure of functionalized boron nitride and aluminum oxide for epoxy composites with enhanced thermal conductivity and breakdown strength. RSC Adv. 2014, 4, 21010–21017. [Google Scholar] [CrossRef]
- Zhang, Y.; Choi, J.R.; Park, S.J. Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf. 2017, 101, 227–236. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Zhang, Y.; Li, Z.; Wu, G. Enhanced through-plane thermal conductivity of PTFE composites with hybrid fillers of hexagonal boron nitride platelets and aluminum nitride particles. Compos. B Eng. 2018, 153, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Zhan, Y.; Zhang, L.; Shi, H.; Xu, Z. Poly (dopamine) assisted epoxy functionalization of hexagonal boron nitride for enhancement of epoxy resin anticorrosion performance. Polym. Adv. Technol. 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Bian, W.; Yao, T.; Chen, M.; Zhang, C.; Shao, T.; Yang, Y. The synergistic effects of the micro-BN and nano-Al2O3 in micro-nano composites on enhancing the thermal conductivity for insulating epoxy resin. Compos. Sci. Technol. 2018, 168, 420–428. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, P.; Li, G.; Zhou, F.; Lu, D.; Sun, R.; Wong, C.P. Spherical and flake-like BN filled epoxy composites: Morphological effect on the thermal conductivity, thermo-mechanical and dielectric properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3564–3572. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Li, Y.; Zhao, D.; Yin, H. Hybrid fillers of hexagonal and cubic boron nitride in epoxy composites for thermal management applications. RSC Adv. 2019, 9, 7388–7399. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Xu, C.; Hou, J.; Yao, Y.; Zhang, Q.; Grami, M.E.; He, L.; Wang, N.; Qu, X. Preparation and properties of thermally conductive polyimide/boron nitride composites. RSC Adv. 2016, 6, 18279–18287. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Z.; Drummer, D. Thermal conductivity of aluminosilicate- and aluminum oxide-filled thermosets for injection molding: Effect of filler content, filler size and filler geometry. Polymers 2018, 10, 457. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.S.; Li, J.; Deng, B.J.; Zhao, X.S. Preparation and characterization of long-chain alkyl silane-functionalized graphene film. J. Mater. Sci. 2013, 48, 156–161. [Google Scholar] [CrossRef]
- Wan, Y.J.; Gong, L.X.; Tang, L.C.; Wu, L.B.; Jiang, J.X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Adamczyk, A.; Długoń, E. The FTIR studies of gels and thin films of Al2O3–TiO2 and Al2O3–TiO2–SiO2 systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 89, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Xie, F.; Chen, J.; Cao, H.; Xu, J.B. Facile and environmentally friendly solution-processed aluminum oxide dielectric for low-temperature, high-performance oxide thin-film transistors. ACS Appl. Mater. Interfaces 2015, 7, 5803–5810. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of mimusops elengi, linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Lin, Z.; Mcnamara, A.; Liu, Y.; Moon, K.S.; Wong, C.P. Exfoliated hexagonal boron nitride-based polymer nanocomposite with enhanced thermal conductivity for electronic encapsulation. Compos. Sci. Technol. 2014, 90, 123–128. [Google Scholar] [CrossRef]
- Liang, D.; Ren, P.; Ren, F.; Jin, Y.; Wang, J.; Feng, C.; Duan, Q. Synergetic enhancement of thermal conductivity by constructing BN and AlN hybrid network in epoxy matrix. J. Polym. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Li, G.; He, Y.; Zhu, P.; Zhao, T.; Sun, R.; Lu, D.; Wong, C.P. Tailored surface chemistry of SiO2 particles with improved rheological, thermal-mechanical and adhesive properties of epoxy based composites for underfill applications. Polymer 2018, 156, 111–120. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Ye, Y.S.; Xue, Z.; Xue, Y.; Xie, X.; Mai, Y.W. High-performance epoxy/silica coated silver nanowire composites as underfill material for electronic packaging. Compos. Sci. Technol. 2014, 105, 80–85. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ma, J.; Wu, J.; Yung, K.C.; Xie, C.S. Study on the properties of the epoxy-matrix composites filled with thermally conductive AlN and BN ceramic particles. J. Appl. Polym. Sci. 2010, 118, 2754–2764. [Google Scholar] [CrossRef]
- Hong, J.P.; Yoon, S.W.; Hwang, T.; Oh, J.S.; Hong, S.C.; Lee, Y.; Nam, J.D. High Thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Chiu, C.W.; Lin, C.A.; Hong, P.D. Melt-spinning and thermal stability behavior of TiO2 nanoparticle/polypropylene nanocomposite fibers. J. Polym. Res. 2010, 18, 367–372. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Spray-assisted assembled spherical boron nitride as fillers for polymers with enhanced thermally conductivity. Chem. Eng. J. 2019, 370, 166–175. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Shi, X.; Yang, X.; Gu, J. Factors affecting thermal conductivities of the polymers and polymer composites: A review. Compos. Sci. Technol. 2020, 193, 108134. [Google Scholar] [CrossRef]
- Ren, L.; Li, Q.; Lu, J.; Zeng, X.; Sun, R.; Wu, J.; Xu, J.B.; Wong, C.P. Enhanced thermal conductivity for Ag-deposited alumina sphere/epoxy resin composites through manipulating interfacial thermal resistance. Compos. Part A Appl. Sci. Manuf. 2018, 107, 561–569. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Ren, L.; Yao, Y.; Wang, M.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Nacre-inspired polymer composites with high thermal conductivity and enhanced mechanical strength. Compos. Part A Appl. Sci. Manuf. 2019, 121, 92–99. [Google Scholar] [CrossRef]
- Owais, M.; Zhao, J.; Imani, A.; Wang, G.; Zhang, H.; Zhang, Z. Synergetic effect of hybrid fillers of boron nitride, graphene nanoplatelets, and short carbon fibers for enhanced thermal conductivity and electrical resistivity of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2019, 117, 11–22. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Wang, H.; Hu, Z.; Yu, Y. High-performance light-emitting diodes encapsulated with silica-filled epoxy materials. ACS Appl. Mater. Interfaces 2013, 5, 8968–8981. [Google Scholar] [CrossRef]
- Qu, J.; Wong, C.P. Effective elastic modulus of underfill material for flip-chip applications. IEEE Trans. Compon. Packaging Manuf. Technol. 2002, 25, 53–55. [Google Scholar]
- Yao, Y.; Lu, G.Q.; Boroyevich, D.; Ngo, K.D. Survey of high-temperature polymeric encapsulants for power electronics packaging. IEEE Trans. Compon. Packaging Manuf. Technol. 2015, 5, 168–181. [Google Scholar]
- Mao, D.; Chen, J.; Ren, L.; Zhang, K.; Yuen, M.M.; Zeng, X.; Sun, R.; Xu, J.B.; Wong, C.P. Spherical core-shell Al@Al2O3 filled epoxy resin composites as high-performance thermal interface materials. Compos. Part A Appl. Sci. Manuf. 2019, 123, 260–269. [Google Scholar] [CrossRef]
- Cui, W.; Du, F.; Zhao, J.; Zhang, W.; Yang, Y.; Xie, X.; Mai, Y.W. Improving thermal conductivity while retaining high electrical resistivity of epoxy composites by incorporating silica-coated multi-walled carbon nanotubes. Carbon 2011, 49, 495–500. [Google Scholar] [CrossRef]
- Cao, T.; Yuan, L.; Gu, A.; Liang, G. Fabrication and origin of new flame retarding bismaleimide resin system with low dielectric constant and loss based on microencapsulated hexaphenoxycyclotriphosphazene in low phosphorus content. Polym. Degrad. Stab. 2015, 121, 157–170. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, X.; Zhang, X.; Sun, R.; Tian, X.; Zeng, Y.; Xu, J.B.; Wong, C.P. Silver nanoparticle-modified alumina microsphere hybrid composites for enhanced energy density and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2019, 119, 299–309. [Google Scholar] [CrossRef]
- Huang, Y.; Schadler, L.S. Understanding the strain-dependent dielectric behavior of carbon black reinforced natural rubber–an interfacial or bulk phenomenon. Compos. Sci. Technol. 2017, 142, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, F.; Tian, H.; Wang, C.; Guo, Z.; Liu, P.; Wang, Q. Synergetic enhancement of mechanical and electrical strength in epoxy/silica nanocomposites via chemically-bonded interface. Compos. Sci. Technol. 2018, 167, 539–546. [Google Scholar] [CrossRef]
- Su, L.; Zeng, X.; He, H.; Tao, Q.; Komarneni, S. Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Appl. Clay Sci. 2017, 148, 103–108. [Google Scholar] [CrossRef]






| Material | Mean Particle Size (μm) | Thermal Conductivity (W m−1K−1) | Density (g/cm3) |
|---|---|---|---|
| BN | 2.5 | 220 | 2.1 |
| Micro Al2O3 | 5 | 29 | 3.89 |
| Nano Al2O3 | 0.19 | 27 | |
| Epoxy | 0.22 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee Sanchez, W.A.; Huang, C.-Y.; Chen, J.-X.; Soong, Y.-C.; Chan, Y.-N.; Chiou, K.-C.; Lee, T.-M.; Cheng, C.-C.; Chiu, C.-W. Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials. Polymers 2021, 13, 147. https://doi.org/10.3390/polym13010147
Lee Sanchez WA, Huang C-Y, Chen J-X, Soong Y-C, Chan Y-N, Chiou K-C, Lee T-M, Cheng C-C, Chiu C-W. Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials. Polymers. 2021; 13(1):147. https://doi.org/10.3390/polym13010147
Chicago/Turabian StyleLee Sanchez, William Anderson, Chen-Yang Huang, Jian-Xun Chen, Yu-Chian Soong, Ying-Nan Chan, Kuo-Chan Chiou, Tzong-Ming Lee, Chih-Chia Cheng, and Chih-Wei Chiu. 2021. "Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials" Polymers 13, no. 1: 147. https://doi.org/10.3390/polym13010147
APA StyleLee Sanchez, W. A., Huang, C.-Y., Chen, J.-X., Soong, Y.-C., Chan, Y.-N., Chiou, K.-C., Lee, T.-M., Cheng, C.-C., & Chiu, C.-W. (2021). Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials. Polymers, 13(1), 147. https://doi.org/10.3390/polym13010147








