Fabrication of Carbon Fiber Reinforced Aromatic Polyamide Composites and Their Thermal Conductivities with a h-BN Filler
Abstract
:1. Introduction
2. Experimentals
2.1. Materials
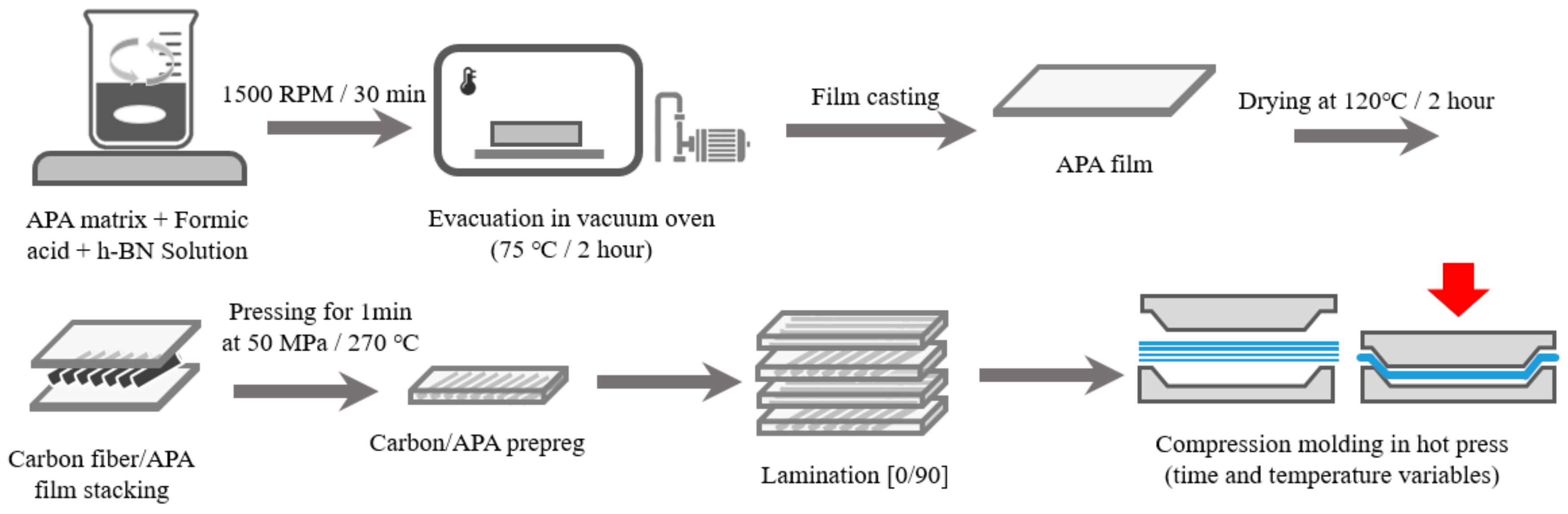
2.2. Composite Fabrication
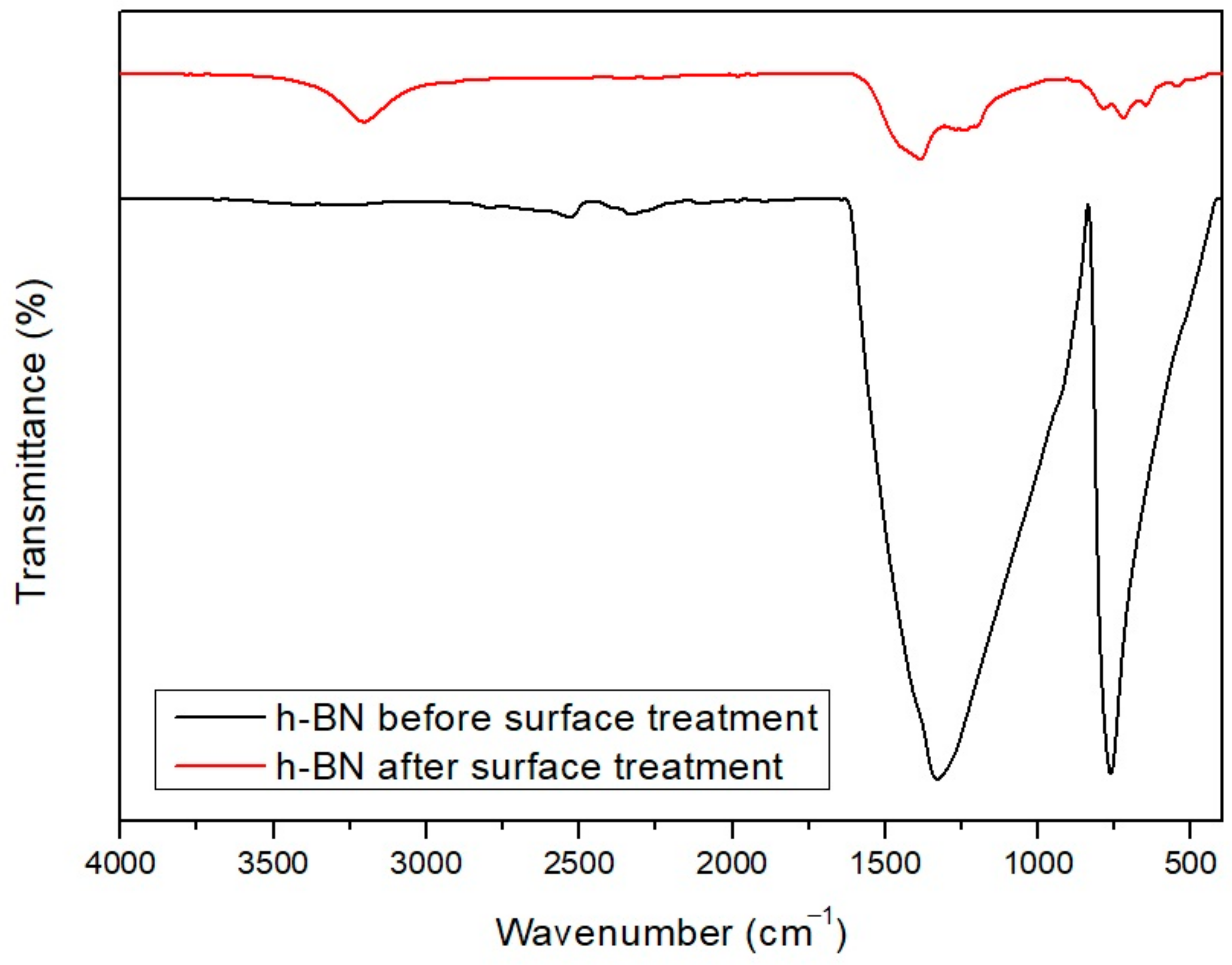
2.3. h-BN Surface Modification and Composite Fabrication
2.4. Characterization
3. Results and Discussions
3.1. Crystallization Behaviors
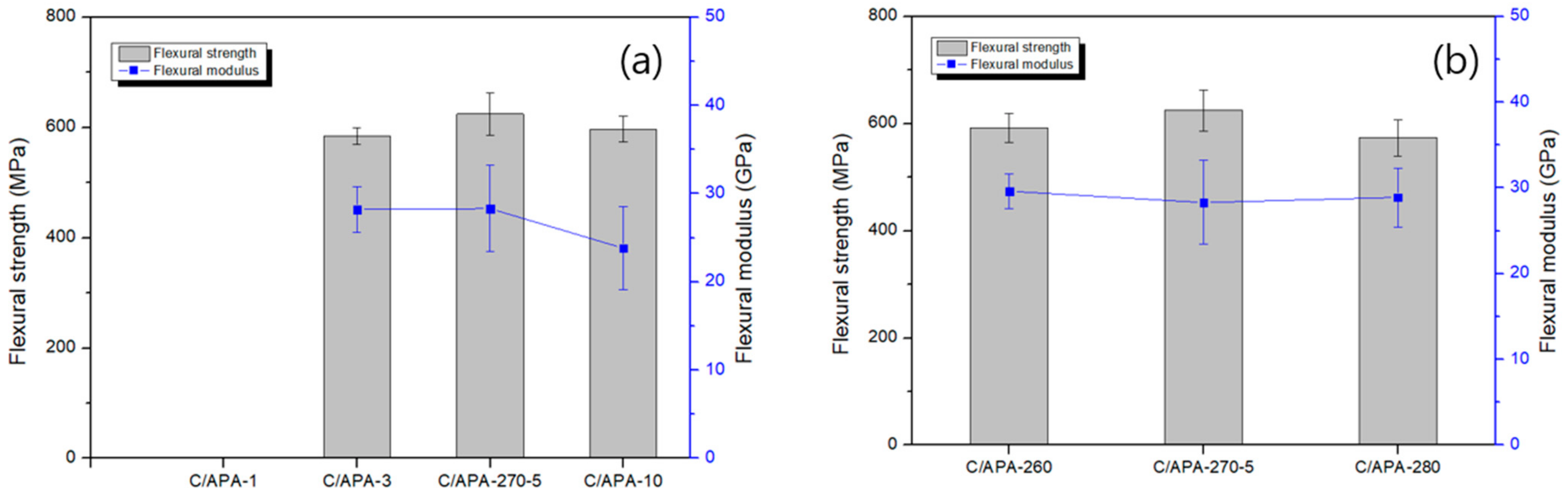
3.2. Mechanical Properties of Composites with Moding Conditions
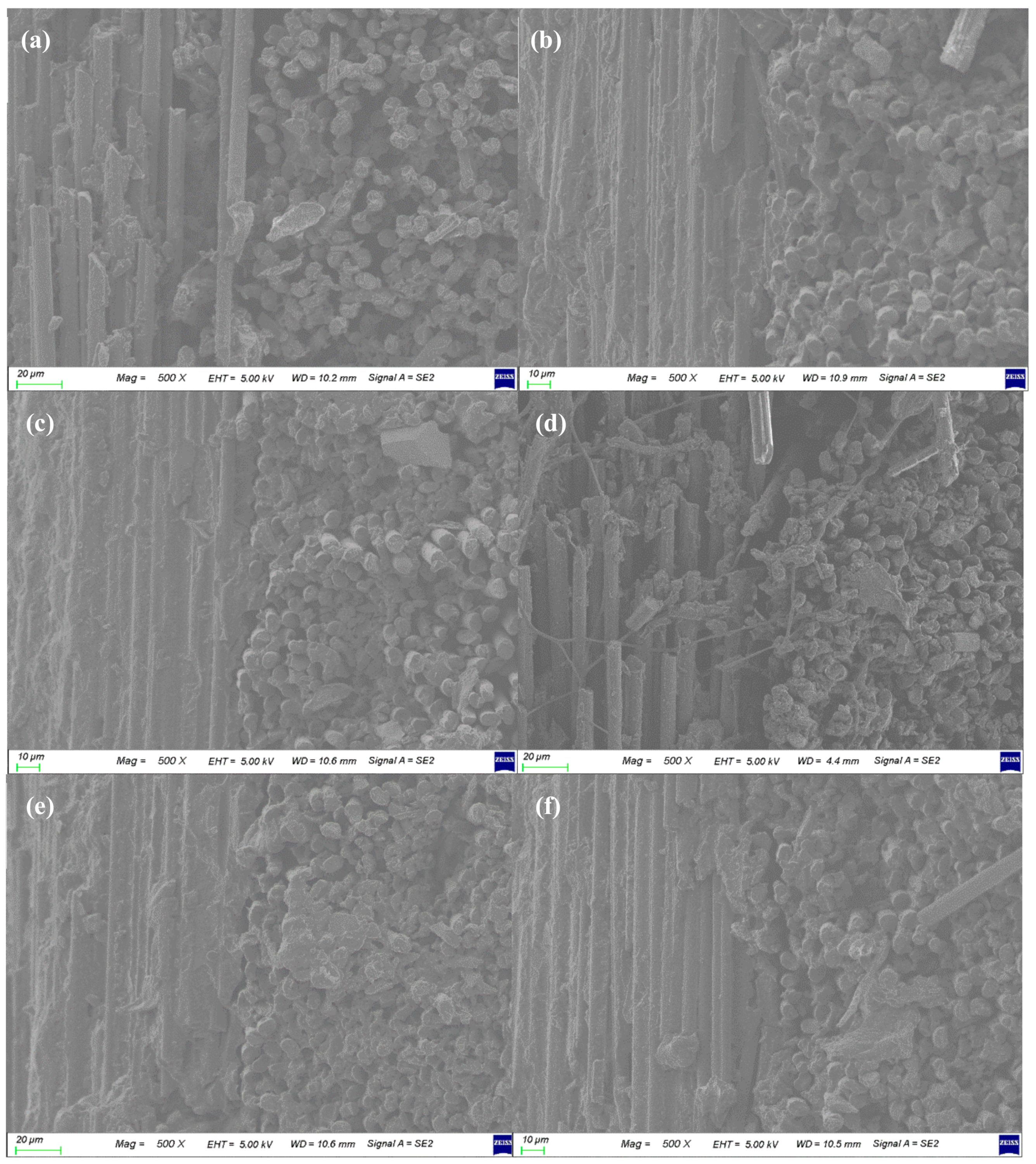
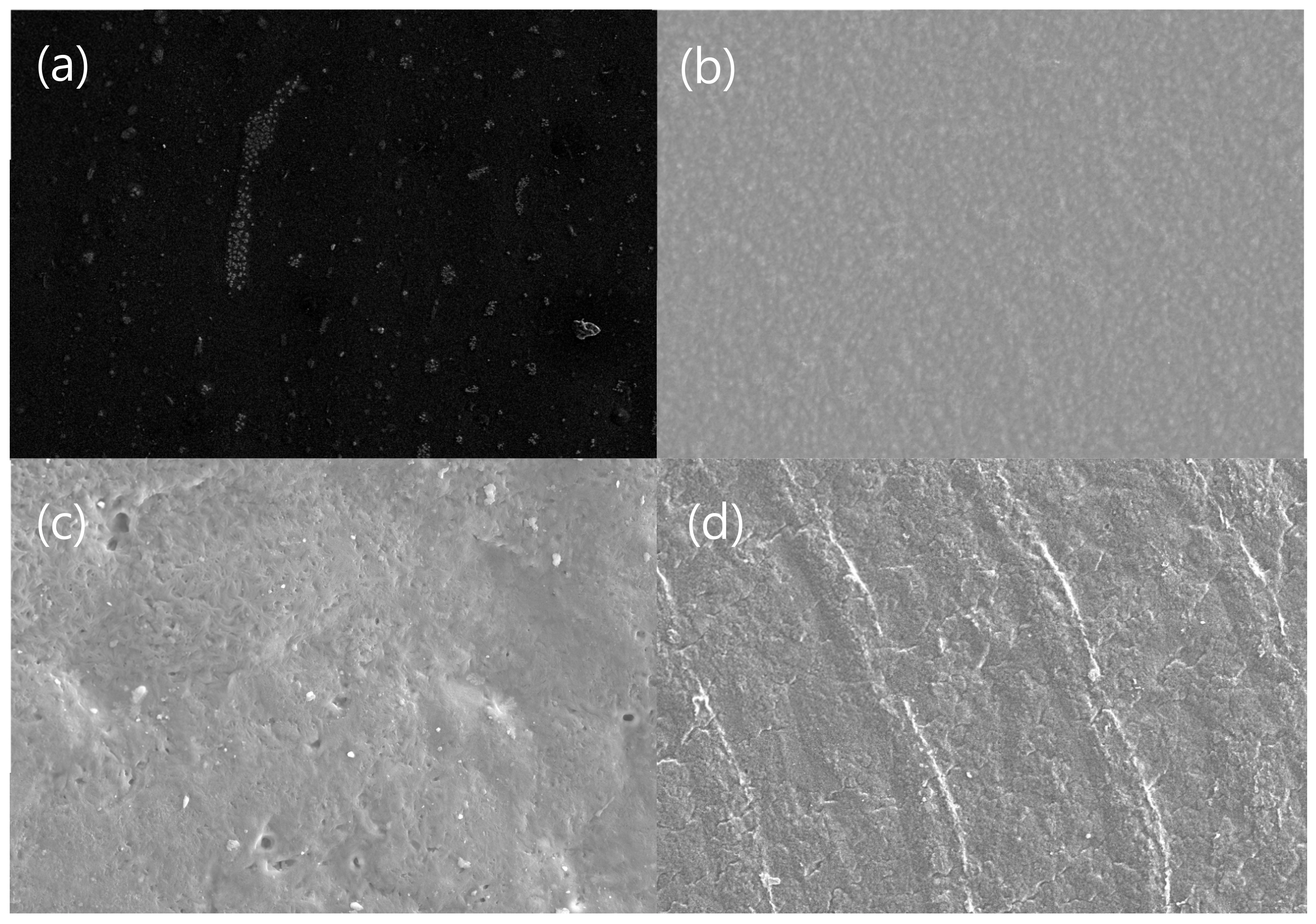
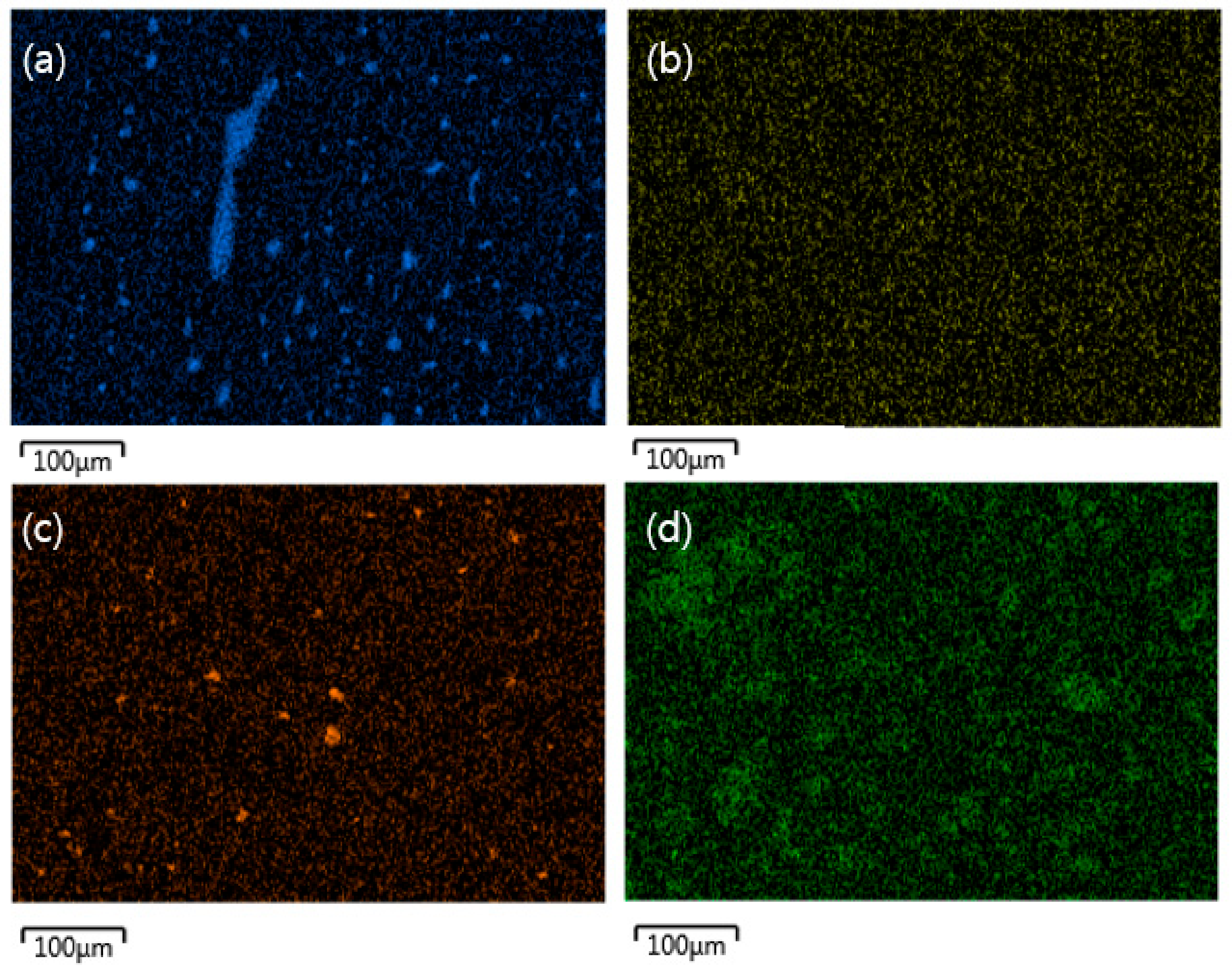
3.3. Morphologies of Carbon/APA Composites
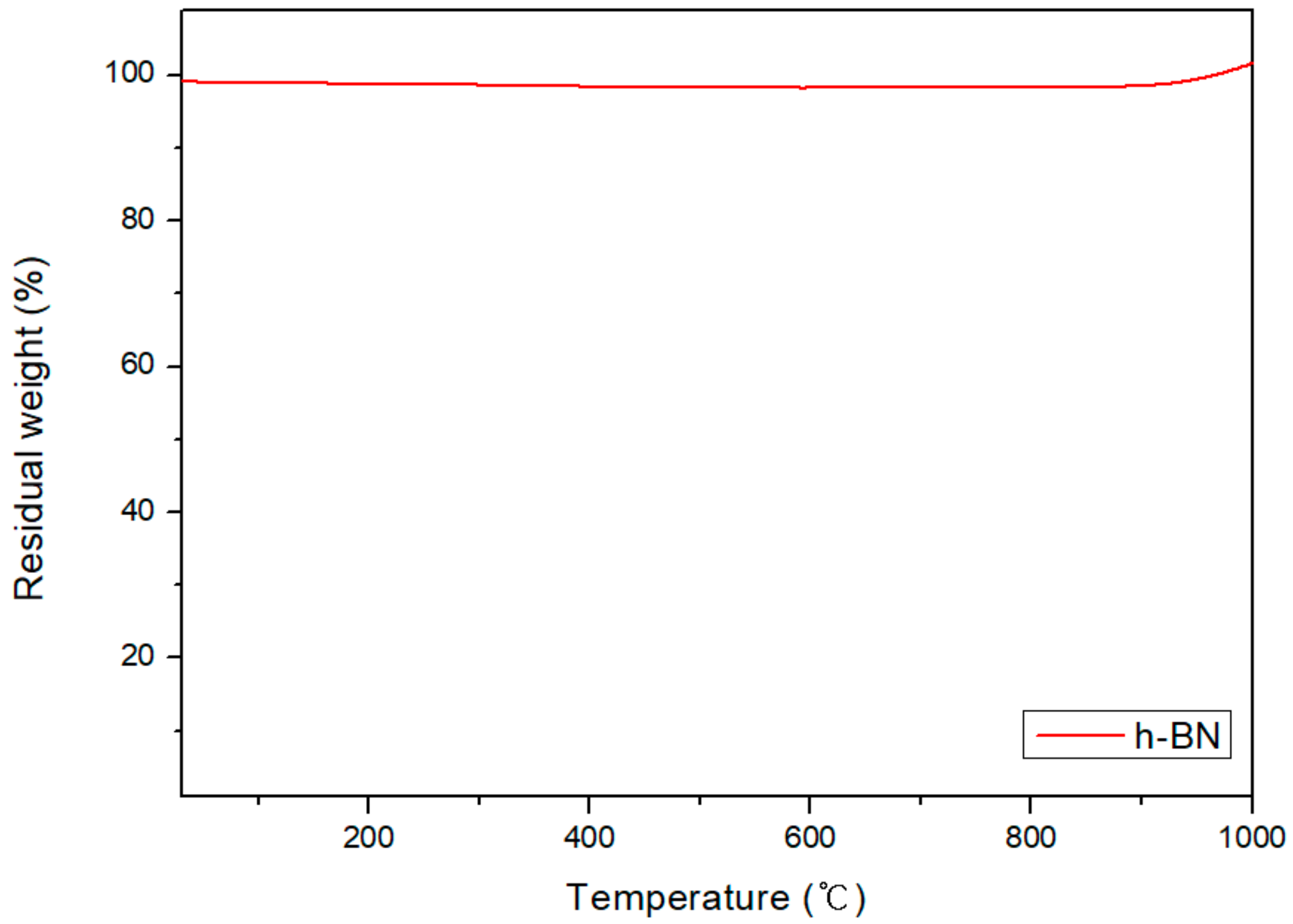
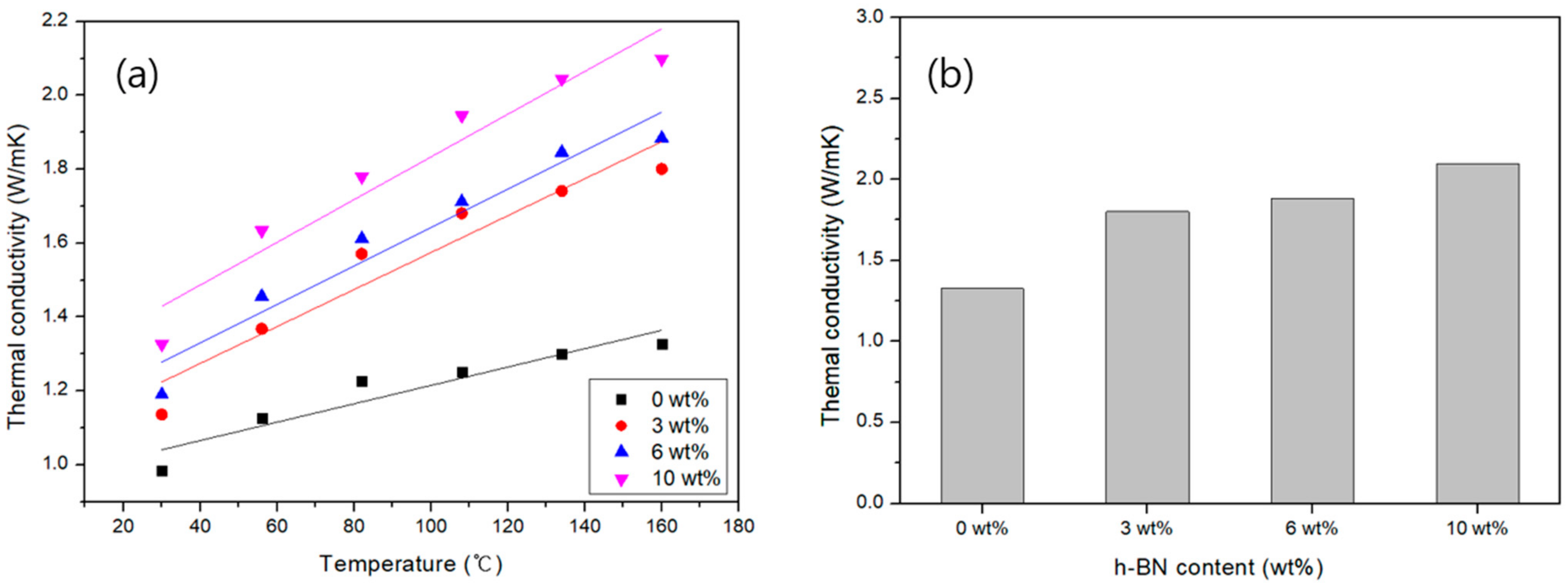
3.4. Theaml Conductivities of Carbon/APA(h-BN)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moon, C.K.; Ann, C.H.; Lee, J.O.; Cho, H.H.; Park, J.M.; Park, T.W. Improvement of Mechanical Properties in Carbon Fiber Reinforced Thermoplastic Composites by Surface Modification. Polymers 1993, 17, 644–653. [Google Scholar]
- Lee, Y.S.; Song, S.A.; Kim, W.J.; Kim, S.S.; Jung, Y.S. Fabrication and Characterization of the Carbon Fiber Composite Sheets. Compos. Res. 2015, 28, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A. Carbon Fiber Composite. Phys. High. Technol. 2003, 12, 31–35. [Google Scholar]
- Zushi, H.; Tamura, M.; Ohsawa, I.; Uzawa, K.; Takahashi, J.; Yasuda, H. Evaluation on mechanical properties of carbon fiber reinforced polypropylene. J. Jpn. Compos. Mater. 2006, 32, 153–162. [Google Scholar] [CrossRef]
- Offringa, A.R. Thermoplastic composites-rapid processing application. Compos. Part A Appl. Sci. Manuf. 1996, 27, 329–336. [Google Scholar] [CrossRef]
- Yan, X.; Imai, Y.; Nagata, K.; Sto, K.; Hotta, Y. Relationship study between crystal structure and thermal/mechanical properites of polyamide 6 reinforced and unreinforced by carbon fiber from macro and local view. Polymers 2014, 55, 6186–12194. [Google Scholar] [CrossRef]
- Renzai, F.; Yunus, R.; Ibrahim, N.A.; Mahdi, E.S. Development of short-carbon-fiber-reinforced polypropylene composite for car bonnet. Polym. Plast. Technol. Eng. 2008, 47, 351–357. [Google Scholar]
- Tanaka, K.; Kashihara, H.; Katayama, T. Vacuum assisted high speed compression molding and evaluation of mechanical properties of continuous carbon fiber reinforced polycarbonate composite. J. Soc. Mater. Sci. Jpn. 2011, 60, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Irisawa, T.; Hashimoto, R.; Arai, M.; Tanabe, Y. The suitability evaluation of aromatic amorphous thermoplastics as matrix resin for CFRTP having high thermal stability. J. Fiber Sci. Technol. 2017, 73, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Karsli, N.G.; Aytac, A. Effects of maleated polypropylene on the morphology, thermal and mechanical properties of short carbon fiber reinforced polypropylene composites. Mater. Des. 2011, 32, 4069–4073. [Google Scholar] [CrossRef]
- Lee, H.; Ohsawa, I.J. Takahashi, Effect of plasma surface treatment of recycled carbon fiber on carbon fiber-reinforced plastics (CFRP) interfacial properties. Appl. Surf. Sci. 2015, 328, 241–246. [Google Scholar] [CrossRef]
- Li, J. Interfacial studies on the O3 modified carbon fiber-reinforced polyamide 6 composites. Appl. Surf. Sci. 2008, 255, 2822–2824. [Google Scholar] [CrossRef]
- Nishikawa, M.; Fukuzo, A.; Matsuda, N.; Hojo, M. Evaluation of elastic-plastic response of discontinuous carbon fiber-reinforced thermoplastics: Experiments and considerations based on load-transfer-based micromechanical simulation. Research of fiber length and fiber-matrix adhesion in carbon fiber reinforced polypropylene. Compos. Sci. Technol. 2018, 155, 117–125. [Google Scholar]
- Fujita, R.; Nagano, H. Novel fiber orientation evaluation method for CFRP/CFRTP based on measurement of anisotropic in-plane thermal diffusivity distribution. Compos. Sci. Technol. 2017, 140, 116–122. [Google Scholar] [CrossRef]
- Ishikawa, T. Overview of carbon fiber reinforced composites (CFRP) applications to automotive structural parts, -focused on thermoplastic CFRP-. J. Jpn. Soc. Prec. Eng. 2015, 81, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.R.; Drzal, L.T. Manufacture of powder-impregnated thermoplastic composites. J. Therm. Compos. Mater. 1990, 3, 325–355. [Google Scholar] [CrossRef]
- L-Dessouky, H.M.E.; Lawrence, C.A. Ultra-lightweight carbon fiber/thermoplastic composite material using spread tow technology. Compos. B. Eng. 2013, 50, 91–97. [Google Scholar] [CrossRef]
- Lebrun, G.; Bureau, M.N.; Denault, J. Evaluation of bias-extension and picture-frame test methods for the measurement of intraply shear properties of PP/glass commingled fabrics. Compos. Struct. 2003, 61, 341–352. [Google Scholar] [CrossRef]
- Ben, G.; Ozeki, H.; Nakamura, K.; Hirayama, N.; Namaizawa, M.; Kobayashi, M.; Azuma, H. Mechanical properties and molding conditions of CFRTP composed of carbon fabrics and In-situ polymerizable thermoplastic resin. J. Jpn. Compos. Mater. 2013, 39, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Ben, G.; Sakata, K. Fast fabrication method and evaluation of performance of hybrid FRTPs for applying them to automotive structural members. Compos. Struct. 2015, 133, 1160–1167. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C. Sequence of Enthalpy Relaxation, Homogeneous Crystal Nucleation and Crystal Growth in Glassy Polyamide 6. Eur. Polym. 2014, 53, 100–108. [Google Scholar] [CrossRef]
- Kiziltas, A.; Nazari, B.; Gardner, D.J. Polyamide 6–Cellulose Composites: Effect of Cellulose Composition on Melt Rheology and Crystallization Behavior. Polym. Eng. Sci. 2014, 54, 739–746. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, S.; Yokozeki, T.; Ueda, M.; Yang, Y.; Elbadry, E.A.; Hamada, H.; Sugahara, Y. Effect of hot water on the mechanical performance of unidirectional carbon fiber-reinforced nylon 6 composites. Compos. Sci. Technol. 2020, 200, 108426. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Ge, Q.S.; Guo, Z.X.; Yu, J. Effects of electrically inert fillers on the properties of poly(m-xylene adipamide)/multiwalled carbon nanotube composites. Chin. J. Polym. Sci. 2016, 34, 1032–1038. [Google Scholar] [CrossRef]
- Guo, Y.l.; Zhang, R.Z.; Wu, K.; Chen, F.; Fu, Q. Preparation of nylon MXD6/EG/CNTs ternary composites with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Chin. J. Polym. Sci. 2017, 35, 1497–1507. [Google Scholar] [CrossRef]
- Chen, L.; Tuo, X.L.; Fan, X.C.; Xie, C.J.; Guo, B.H.; Yu, J.; Hu, P.; Guo, Z.-X. Enhanced mechanical properties of poly(arylene sulfide sulfone) membrane by Co-electrospinning with poly(m-xylene adipamide). Chin. J. Polym. Sci. 2019, 37. [Google Scholar] [CrossRef]
- Fereydoon, M.; Tabatabaei, S.H.; Ajji, A. Rehological crystal structure, barrier, and mechanical properties of PA6 and MXD6 nanocomposites films. Polym. Eng. Sci. 2014, 54, 2617–2631. [Google Scholar] [CrossRef]
- Tanaka, K.; Ogata, S.; Kobayashi, R.; Tarura, T.; Kouno, T. A molecular dynamics study on thermal conductivity of thin epoxy polymer sandwiched between alumina fillers in heat-dissipation composite material. Int. J. Heat Mass Transf. 2015, 89, 714–723. [Google Scholar] [CrossRef]
- Zheng, Z.; Cox, M.; Li, B. Surface modification of hexagonal boron nitride nanomaterials. J. Matter. Sci. 2018, 53, 66–99. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.P. Crystallization Behavior of Nylon 6 Nano composite. Polymers 2003, 44, 3945–3961. [Google Scholar] [CrossRef]
- Feng, N.; Wang, X.; Wu, D. Surface modification of recycled carbon fiber and its reinforcement effect on nylon 6 composites: Mechanical properties, morphology and crystallization behaviors. Curr. Appl. Phys. 2013, 13, 2038–2050. [Google Scholar] [CrossRef]
- Jiasheng, Q.; Pingsheng, H. Non-isothermal Crystallization of HDPE/nano-SiO2 Composite. J. Mater. Sci. 2003, 38, 2299–2304. [Google Scholar] [CrossRef]
- Jung, J.W.; Cho, W.G.; Lee, H.R.; Won, J.S.; Bae, I.J.; Lee, S.G. Non-isothermal Crystallization Behavior and Property Characterization with the Cooling Rate of Carbon Fiber/Polyamide 6 Composite. J. Kor. Soc. Text. Eng. Chem. 2018, 55, 450–456. [Google Scholar]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured Boron Nitride With High Water Dispersibility For Boron Neutron Capture Therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar] [CrossRef] [Green Version]







| Properties | Units | APA(MXD6) | PA6 | PA66 |
|---|---|---|---|---|
| Water absorption | % | 5.8 | 11.5 | 9.9 |
| Deflection temperature under load | °C | 96 | 57 | 60 |
| (Melting point) Tm | °C | 243 | 225 | 268 |
| Tensile strength | MPa | 99.0 | 61.8 | 76.5 |
| Tensile modulus | GPa | 4.7 | 2.5 | 3.1 |
| Flexural strength | MPa | 157 | 123 | 127 |
| Flexural modulus | GPa | 4.4 | 2.4 | 2.9 |
| Sample Code | Molding Temperature/Time | Sample Code | Molding Temperature/Time |
|---|---|---|---|
| C/APA-260 | 260/5 | C/APA-1 | 270/1 |
| C/APA-270-5 | 270/5 | C/APA-3 | 270/3 |
| C/APA-280 | 280/5 | C/APA-270-5 | 270/5 |
| - | - | C/APA-10 | 270/10 |
| C/APA-270-5 | C/APA(h-BN)-270-5 | |
|---|---|---|
| Tensile strength (MPa) | 690.6 | 681.2 |
| Tensile modulus (GPa) | 10.8 | 12.7 |
| Flexural strength (MPa) | 624.3 | 590.4 |
| Flexural modulus (GPa) | 28.3 | 33.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Lee, P.G.; Bae, I.-J.; Won, J.S.; Jeon, M.H.; Lee, S.G. Fabrication of Carbon Fiber Reinforced Aromatic Polyamide Composites and Their Thermal Conductivities with a h-BN Filler. Polymers 2021, 13, 21. https://doi.org/10.3390/polym13010021
Lee MJ, Lee PG, Bae I-J, Won JS, Jeon MH, Lee SG. Fabrication of Carbon Fiber Reinforced Aromatic Polyamide Composites and Their Thermal Conductivities with a h-BN Filler. Polymers. 2021; 13(1):21. https://doi.org/10.3390/polym13010021
Chicago/Turabian StyleLee, Min Jun, Pil Gyu Lee, Il-Joon Bae, Jong Sung Won, Min Hong Jeon, and Seung Goo Lee. 2021. "Fabrication of Carbon Fiber Reinforced Aromatic Polyamide Composites and Their Thermal Conductivities with a h-BN Filler" Polymers 13, no. 1: 21. https://doi.org/10.3390/polym13010021
APA StyleLee, M. J., Lee, P. G., Bae, I.-J., Won, J. S., Jeon, M. H., & Lee, S. G. (2021). Fabrication of Carbon Fiber Reinforced Aromatic Polyamide Composites and Their Thermal Conductivities with a h-BN Filler. Polymers, 13(1), 21. https://doi.org/10.3390/polym13010021






