Ureido Functionalization through Amine-Urea Transamidation under Mild Reaction Conditions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
2.3. Characterization
2.4. Kinetic Modeling
3. Results and Discussion
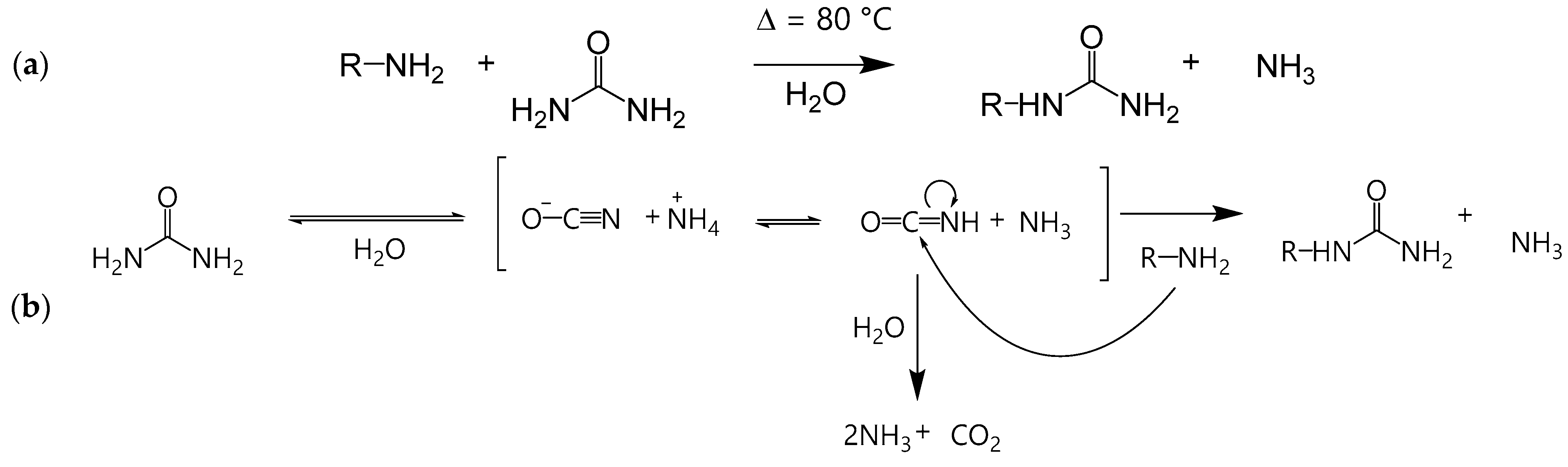
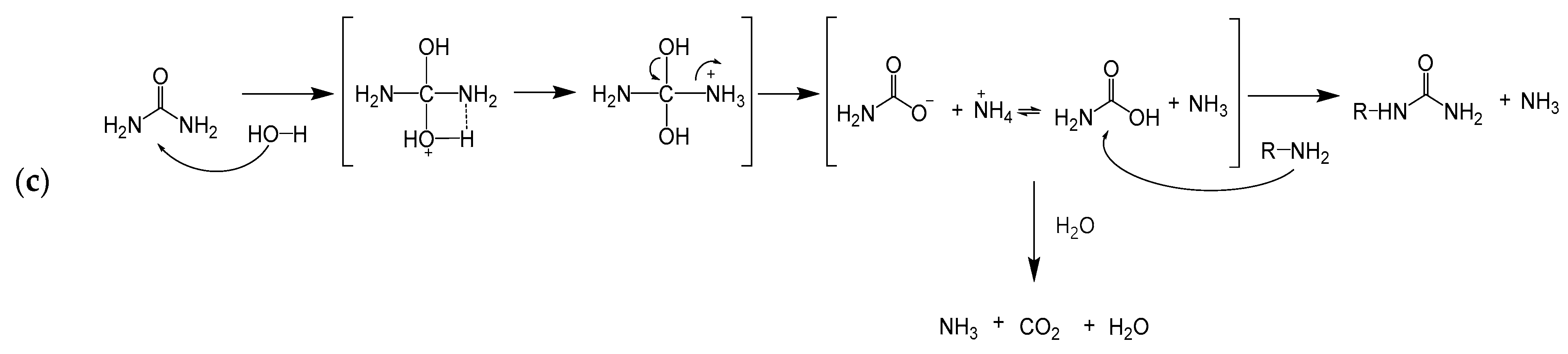
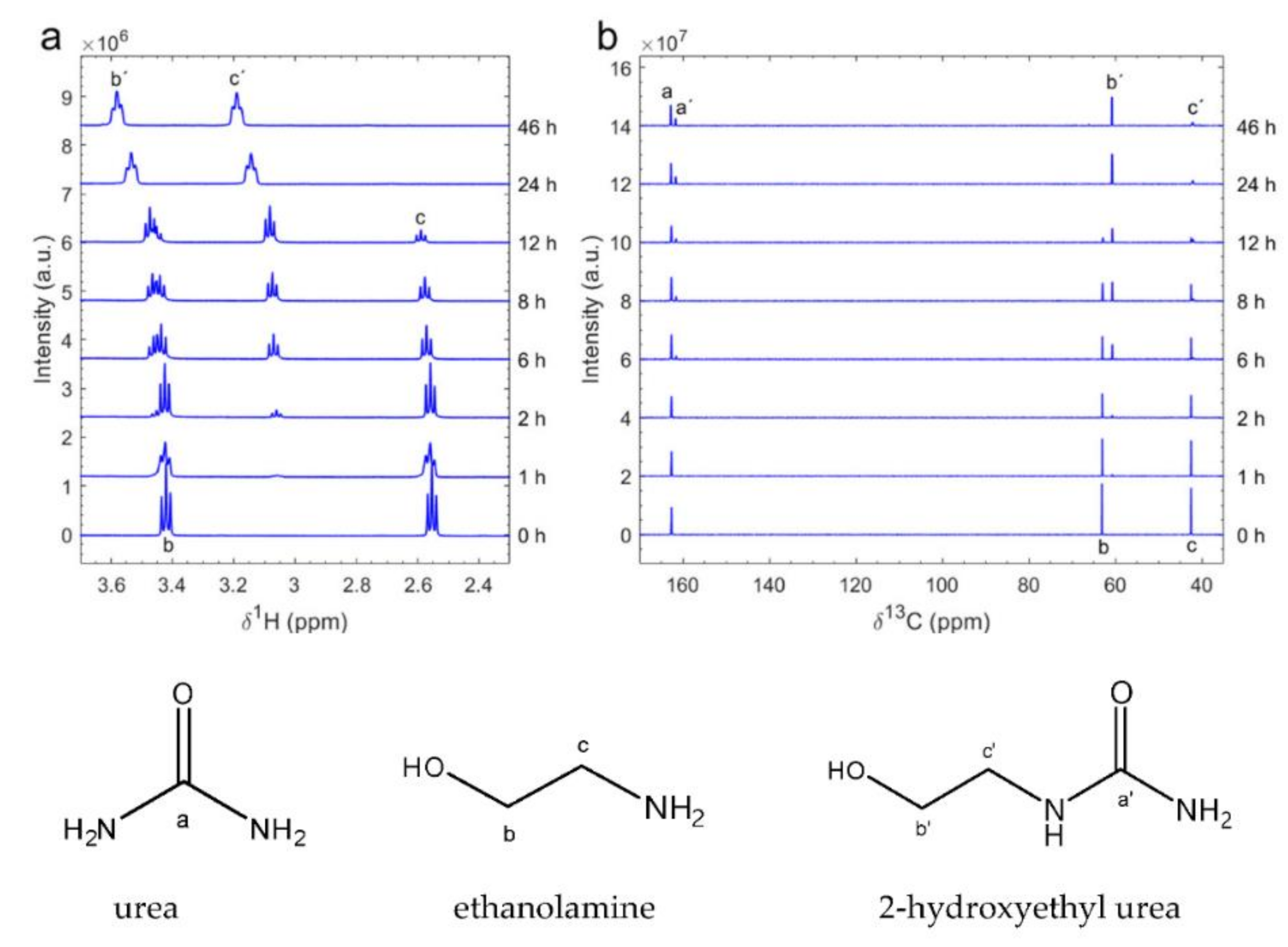
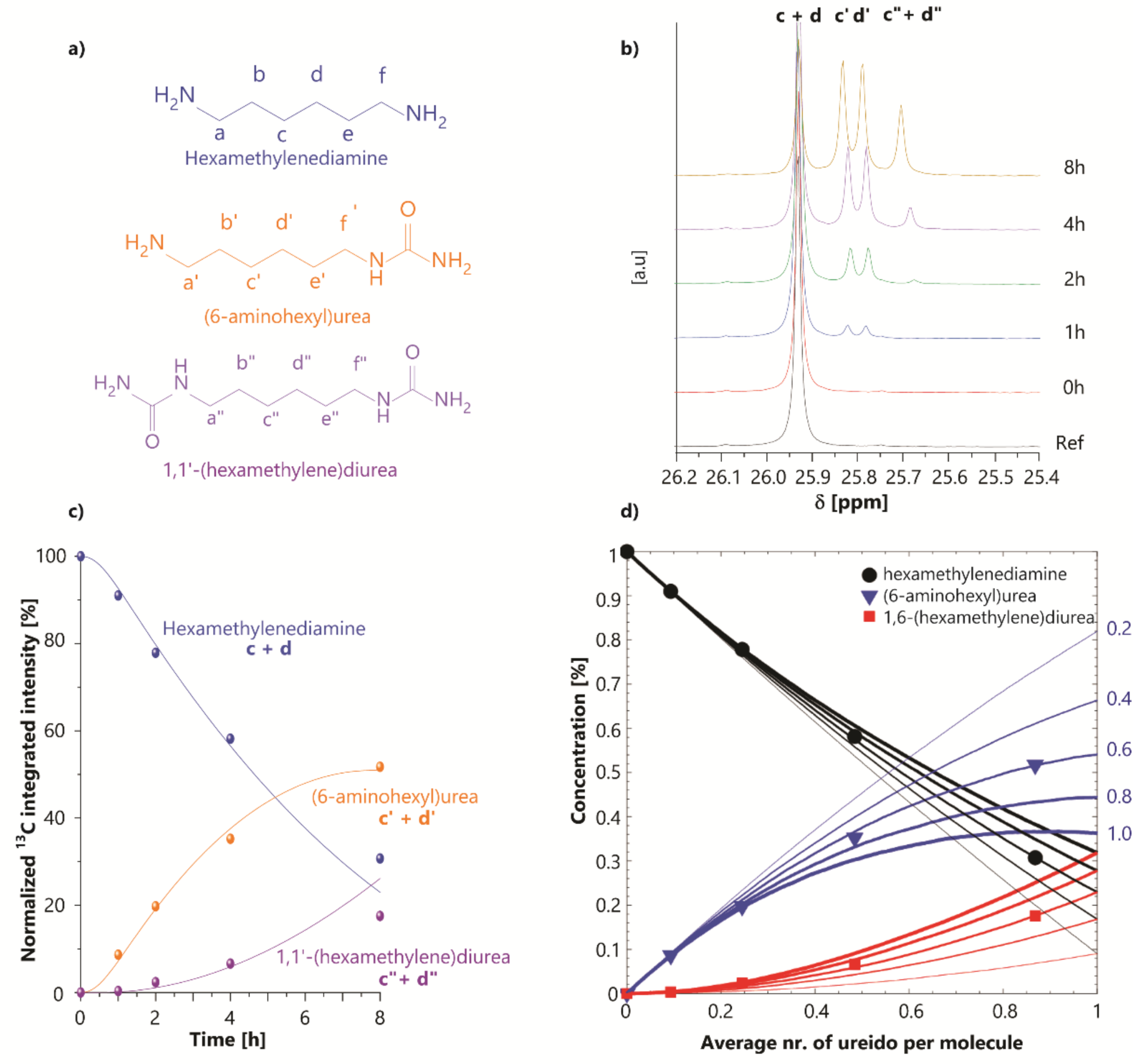
3.1. Ureido Formation
3.1.1. Effect of the Substrate
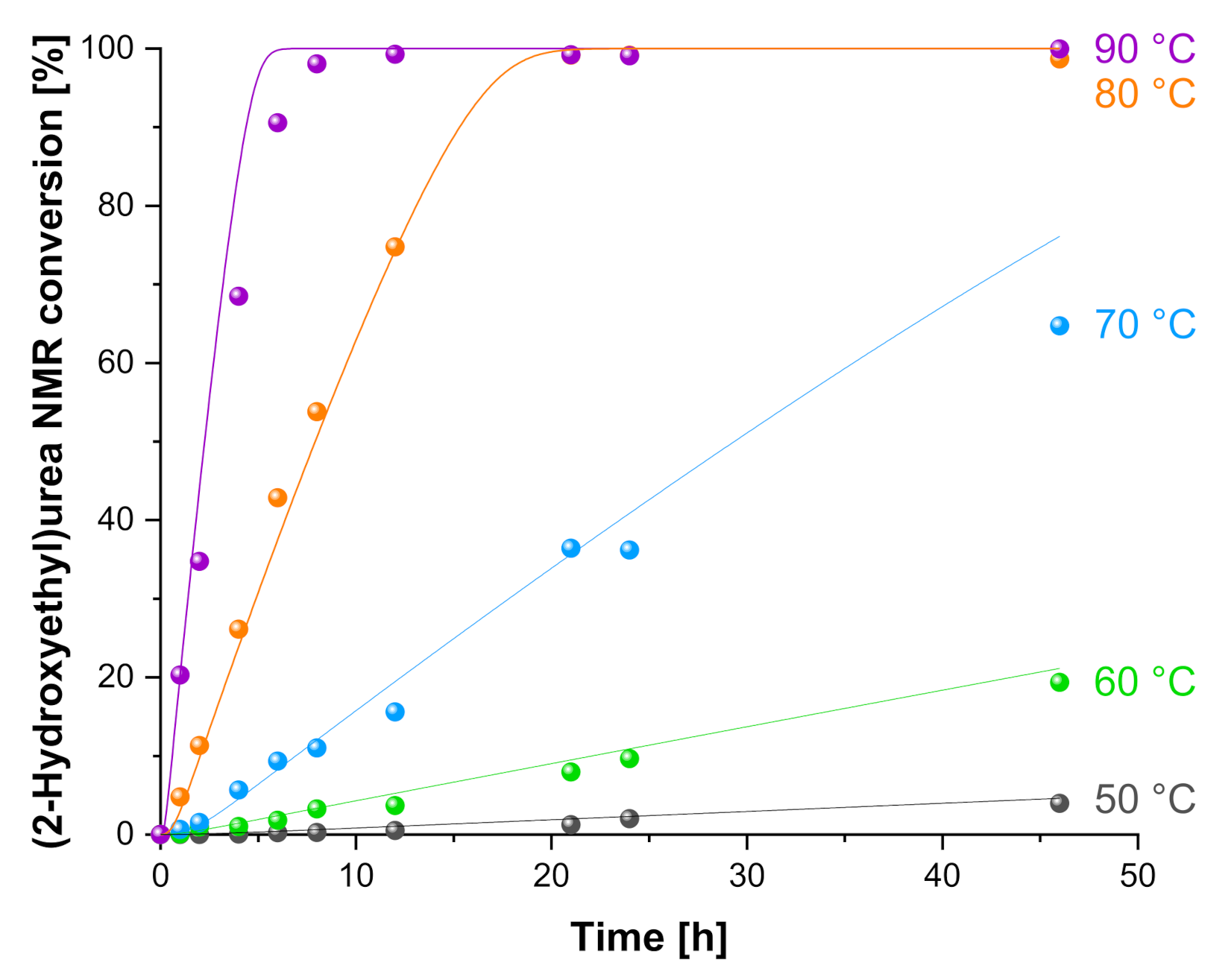
3.1.2. Effect of the Temperature
3.1.3. Effect of Urea: Ethanolamine Ratio and pH
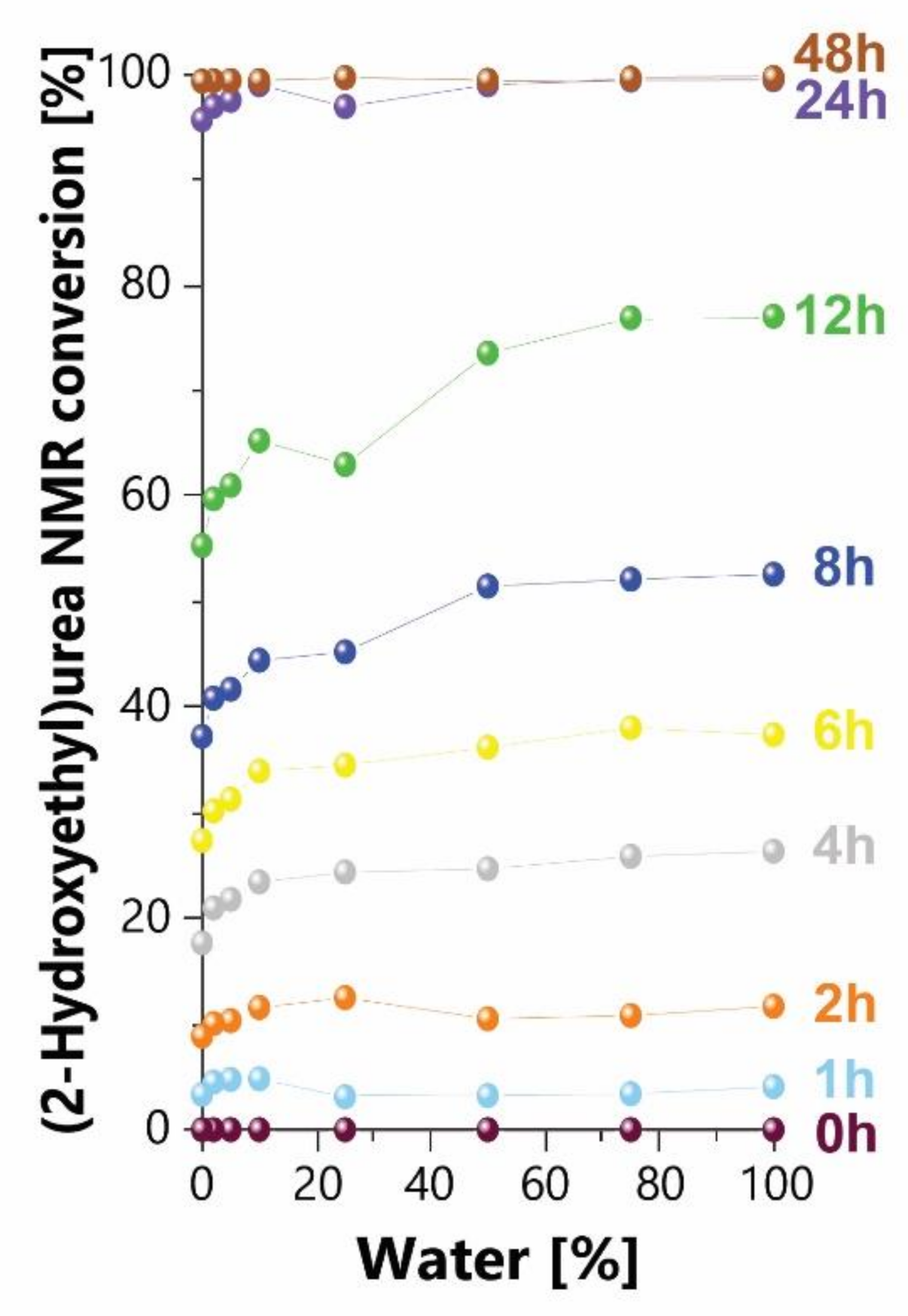
3.1.4. Effect of Ethanol as Solvent and Co-Solvent
3.2. Ureylene Formation
3.3. Effect of Catalysts
3.4. Advantages and Disadvantages of Urea Compared to KOCN for Ureido Formation
3.5. Kinetic Modeling of Uncatalyzed Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urech, F. XXI. Ueber Lacturaminsäure und Lactylharnstoff. Justus Liebig’s Ann. Chem. Pharm. 1873, 165, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent Advances in the Synthesis of Hydantoins: The State of the Art of a Valuable Scaffold. Chem. Rev. 2017, 117, 13757–13809. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.L.; Blanchard, K.C. PHENYLUREA and sym.-DIPHENYLUREA. Org. Synth. 1923, 3, 95. [Google Scholar]
- Davis, T.L. Di-Alkyl Urea. U.S. Patent 1,785,730, 23 December 1930. pp. 3–4. [Google Scholar]
- Erickson, J.G. Reactions of Long-chain Amines. II. Reactions with Urea1. J. Am. Chem. Soc. 1954, 76, 3977–3978. [Google Scholar] [CrossRef]
- Theis, S.; Hartrodt, B.; Kottra, G.; Neubert, K.; Daniel, H. Defining minimal structural features in substrates of the H(+)/peptide cotransporter PEPT2 using novel amino acid and dipeptide derivatives. Mol. Pharmacol. 2002, 61, 214–221. [Google Scholar] [CrossRef] [Green Version]
- McMeekin, T.L.; Cohn, E.J.; Weare, J.H. Studies in the Physical Chemistry of Amino Acids, Peptides and Related Substances. III. The Solubility of Derivatives of the Amino Acids in Alcohol—Water Mixtures. J. Am. Chem. Soc. 1935, 57, 626–633. [Google Scholar] [CrossRef]
- Stella, V.; Higuchi, T. Kinetics of the acid-catalyzed closure of hydantoic acids. Effect of 2-aryl and 2-alkyl substituents. J. Org. Chem. 1973, 38, 1527–1534. [Google Scholar] [CrossRef]
- Crosby, O. Urech hydantoin synthesis. In Catalysis from A to Z; Wiley-VCH Verlag GmbH & Co. KGaA: Weenheim, Germany, 2020; pp. 2856–2859. [Google Scholar]
- Löscher, W.; Reissmüller, E.; Ebert, U. Anticonvulsant effect of fosphenytoin in amygdala-kindled rats: Comparison with phenytoin. Epilepsy Res. 1998, 30, 69–76. [Google Scholar] [CrossRef]
- Scimeca, J.; Fast, D.; Zimmerman, A. Use of Allantoin as a Pro-Collagen Synthesis agent in Cosmetic Compositions. U.S. Patent Application No. 11/977,926, 28 May 2008. [Google Scholar]
- Cativiela, C.; Fraile, J.M.; Garcia, J.I.; Lázaro, B.; Mayoral, J.A.; Pallares, A. Heterogeneous catalysis in the synthesis and reactivity of allantoin. Green Chem. 2003, 5, 275–277. [Google Scholar] [CrossRef]
- Wermuth, C.G.; Bourguignon, J.J.; Schlewer, G.; Gies, J.P.; Schoenfelder, A.; Melikian, A.; Bouchet, M.J.; Chantreux, D.; Molimard, J.C. Synthesis and structure-activity relationships of a series of aminopyridazine derivatives of.gamma.-aminobutyric acid acting as selective GABA-A antagonists. J. Med. Chem. 1987, 30, 239–249. [Google Scholar] [CrossRef]
- Busse, W.D.; Carpenter, F.H. Synthesis and properties of carbonylbis(methionyl)insulin, a proinsulin analog which is convertible to insulin by cyanogen bromide cleavage. Biochemistry 1976, 15, 1649–1657. [Google Scholar] [CrossRef]
- Levit, G.L.; Radina, L.B.; Krasnov, V.P.; Gopko, V.F.; Nikiforova, N.V.; Peretolchina, N.M. Nω-Alkylnitrosocarbamoyl-α,ω-diaminocarboxylic acids. 3. Synthesis and antitumor activity of Nε-nitroso-Nε-[N′-(2-chloroethyl)carbamoyl]-L-lysine and Nε-[N′-(2-chloroethyl)-N′-nitroso-carbamoyl]-L-lysine. Pharm. Chem. J. 1996, 30, 306–309. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamazaki, T.; Ohta, H.; Shima, K.; Ohi, K.; Nishiyama, S.; Sugai, T. N-Carbamylamino Alcohols as the Precursors of Oxazolidinones via Nitrosation-Deamination Reaction. Synlett 2000, 2000, 189–192. [Google Scholar] [CrossRef]
- Lagrille, O.; Taillades, J.; Boiteau, L.; Commeyras, A. N-Carbamoyl Derivatives and Their Nitrosation by Gaseous NOx—A New, Promising Tool in Stepwise Peptide Synthesis. Eur. J. Org. Chem. 2002, 2002, 1026–1032. [Google Scholar] [CrossRef]
- Shimada, N.; Ino, H.; Maie, K.; Nakayama, M.; Kano, A.; Maruyama, A. Ureido-Derivatized Polymers Based on Both Poly(allylurea) and Poly(l-citrulline) Exhibit UCST-Type Phase Transition Behavior under Physiologically Relevant Conditions. Biomacromolecules 2011, 12, 3418–3422. [Google Scholar] [CrossRef]
- Delgado, G.E.; Seijas, L.E.; Mora, A.J.; González, T.; Briceño, A. Synthesis, Crystal Structure and Hydrogen-Bonding Patterns in (RS)-1-Carbamoyl Pyrrolidine-2-Carboxylic Acid. J. Chem. Crystallogr. 2012, 42, 388–393. [Google Scholar] [CrossRef]
- Khan, Z.; Rafiquee, M.Z.A.; Kabir-ud-din; Niaz, M.A.; Khan, A.A. Kinetics and mechanism of alkaline hydrolysis of urea and sodium cyanate. Indian J. Chem. Sect. A Inorg. Phys. Theor. Anal. Chem. 1996, 35, 1116–1119. [Google Scholar]
- Kravchenko, A.N.; Chikunov, I.E. Chemistry of ureido carboxylic and ureylene dicarboxylic acids. Russ. Chem. Rev. 2006, 75, 191–206. [Google Scholar] [CrossRef]
- Berlinerblau, J. Ueber die Einwirkung von Chlorcyan auf Ortho- und auf Para-Amidophenetol. J. Prakt. Chem. 1884, 30, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Dennis, J.M.; Steinberg, L.I.; Pekkanen, A.M.; Maiz, J.; Hegde, M.; Müller, A.J.; Long, T.E. Synthesis and characterization of isocyanate-free polyureas. Green Chem. 2017, 20, 243–249. [Google Scholar] [CrossRef]
- Shang, J.; Liu, S.; Ma, X.; Lu, L.; Deng, Y. A new route of CO2 catalytic activation: Syntheses of N-substituted carbamates from dialkyl carbonates and polyureas. Green Chem. 2012, 14, 2899–2906. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M.M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Kébir, N.; Benoit, M.; Legrand, C.; Burel, F. Non-isocyanate thermoplastic polyureas (NIPUreas) through a methyl carbamate metathesis polymerization. Eur. Polym. J. 2017, 96, 87–96. [Google Scholar] [CrossRef]
- Bizet, B.; Grau, E.; Cramail, H.; Asua, J.M. Water-based non-isocyanate polyurethane-ureas (NIPUUs). Polym. Chem. 2020, 11, 3786–3799. [Google Scholar] [CrossRef]
- Zareanshahraki, F.; Asemani, H.; Skuza, J.; Mannari, V. Synthesis of non-isocyanate polyurethanes and their application in radiation-curable aerospace coatings. Prog. Org. Coat. 2020, 138, 105394. [Google Scholar] [CrossRef]
- Vessally, E.; Soleimani-Amiri, S.; Hosseinian, A.; Edjlali, L.; Babazadeh, M. Chemical fixation of CO2 to 2-aminobenzonitriles: A straightforward route to quinazoline-2,4(1H,3H)-diones with green and sustainable chemistry perspectives. J. CO2 Util. 2017, 21, 342–352. [Google Scholar] [CrossRef]
- Long, Y.; Zheng, L.; Gu, Y.; Lin, H.; Xie, X. Carbon dioxide adduct from polypropylene glycol grafted polyethyleneimine as a climate-friendly blowing agent for polyurethane foams. Polymer 2014, 55, 6494–6503. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Whelan, J.M.; Hill, M.; Cotter, R.J. Multiple Cyclic Carbonate Polymers. U.S. Patent 3,072,613, 8 January 1963. [Google Scholar]
- Asemani, H.; Mannari, V. Synthesis and evaluation of non-isocyanate polyurethane polyols for heat-cured thermoset coatings. Prog. Org. Coat. 2019, 131, 247–258. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Zohuriaan-Mehr, M.; Rostami, A. Bio-based thermo-healable non-isocyanate polyurethane DA network in comparison with its epoxy counterpart. J. CO2 Util. 2017, 18, 294–302. [Google Scholar] [CrossRef]
- Dechent, S.-E.; Kleij, A.W.; Luinstra, G.A. Fully bio-derived CO2 polymers for non-isocyanate based polyurethane synthesis. Green Chem. 2020, 22, 969–978. [Google Scholar] [CrossRef]
- Schmidt, S.; Göppert, N.E.; Bruchmann, B.; Mülhaupt, R. Liquid sorbitol ether carbonate as intermediate for rigid and segmented non-isocyanate polyhydroxyurethane thermosets. Eur. Polym. J. 2017, 94, 136–142. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Esmaeili, N.; Zohuriaan-Mehr, M.; Salimi, A.; Vafayan, M.; Meyer, W. Tannic acid derived non-isocyanate polyurethane networks: Synthesis, curing kinetics, antioxidizing activity and cell viability. Thermochim. Acta 2018, 664, 64–72. [Google Scholar] [CrossRef]
- Maisonneuve, L.; More, A.S.; Foltran, S.; Alfos, C.; Robert, F.; Landais, Y.; Tassaing, T.; Grau, E.; Cramail, H. Novel green fatty acid-based bis-cyclic carbonates for the synthesis of isocyanate-free poly(hydroxyurethane amide)s. RSC Adv. 2014, 4, 25795–25803. [Google Scholar] [CrossRef] [Green Version]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Samanta, S.; Selvakumar, S.; Bahr, J.; Wickramaratne, D.S.; Sibi, M.; Chisholm, B.J. Synthesis and Characterization of Polyurethane Networks Derived from Soybean-Oil-Based Cyclic Carbonates and Bioderivable Diamines. ACS Sustain. Chem. Eng. 2016, 4, 6551–6561. [Google Scholar] [CrossRef]
- Loulergue, P.; Amela-Cortes, M.; Cordier, S.; Molard, Y.; Lemiègre, L.; Audic, J.-L. Polyurethanes prepared from cyclocarbonated broccoli seed oil (PUcc): New biobased organic matrices for incorporation of phosphorescent metal nanocluster. J. Appl. Polym. Sci. 2017, 134, 45339. [Google Scholar] [CrossRef]
- Bähr, M.; Mülhaupt, R. Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Mahendran, A.R.; Aust, N.; Wuzella, G.; Müller, U.; Kandelbauer, A. Bio-Based Non-Isocyanate Urethane Derived from Plant Oil. J. Polym. Environ. 2012, 20, 926–931. [Google Scholar] [CrossRef]
- Alburquerque, N.G.; Zhao, S.; Adilien, N.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Strong, Machinable, and Insulating Chitosan–Urea Aerogels: Toward Ambient Pressure Drying of Biopolymer Aerogel Monoliths. ACS Appl. Mater. Interfaces 2020, 12, 22037–22049. [Google Scholar] [CrossRef]
- Van D Kerk, G.J.M. Linear Polyureas Prepared from Half-Polymers. U.S. Patent 3156672A, 10 November 1964. pp. 3–5. [Google Scholar]
- Nitschke, C.; Scherr, G. Urea Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weenheim, Germany, 2010; Volume 11, pp. 1–9. [Google Scholar]
- Gabler, R.; Müller, H. Preparation of Linear Polyureas Utilizing a Cyclic Amide Solvent. U.S. Patent 3185656A, 25 May 1965. pp. 1–2. [Google Scholar]
- Markiewitz, K. US3763106A-Polymers Preapred by Reacting Urea with an Amino Alcohol or Diamine Followed by Methylolation with Formaldehyde. U.S. Patent 3,763,106, 1973. [Google Scholar]
- Arnold, H.W. Process of Making Aliphatic Polyureas. U.S. Patent 2145242 A, 31 January 1939. p. 3. [Google Scholar]
- Fleischer, A. Ueber Bildung von Azoverbindungen. Eur. J. Inorg. Chem. 1876, 9, 992–995. [Google Scholar] [CrossRef] [Green Version]
- Baeyer, A. Mittheilungen aus dem organischen Laboratorium des Gewerbe-Institutes in Berlin: III. Notiz über die Einwirkung von Phenylsäure und Anilin auf Harnstoff. Eur. J. Org. Chem. 1864, 131, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Olin, J.F. Manufacture and Purification of Urea Derivatives. U.S. Patent 2,257,717, 30 September 1941. [Google Scholar]
- Babu, S.S.; Shahid, M.; Gopinath, P. Dual palladium–photoredox catalyzed chemoselective C–H arylation of phenylureas. Chem. Commun. 2020, 56, 5985–5988. [Google Scholar] [CrossRef]
- Chamni, S.; Zhang, J.; Zou, H. Benign synthesis of unsymmetrical arylurea derivatives using 3-substituted dioxazolones as isocyanate surrogates. Green Chem. Lett. Rev. 2020, 13, 246–257. [Google Scholar] [CrossRef]
- Gezegen, H.; Gürdere, M.B.; Dinçer, A.; Özbek, O.; Koçyiğit, Ü.M.; Taslimi, P.; Tüzün, B.; Budak, Y.; Ceylan, M. Synthesis, molecular docking, and biological activities of new cyanopyridine derivatives containing phenylurea. Arch. Pharm. 2020, 354, e2000334. [Google Scholar] [CrossRef]
- Ayyangar, N.R.; Chwodhary, A.R.; Kalkote, U.R.; Natu, A.A. A Non-phosgene Route for the Synthesis of Diethyldiphenylurea. Chem. Ind. 1988, 11, 599–600. [Google Scholar]
- Arylureas II. Urea Method p-Ethoxyphenylurea. Org. Synth. 1951, 31, 11.
- Jang, J.; Khanom, S.; Moon, Y.; Shin, S.; Lee, O.R. PgCYP76B93 docks on phenylurea herbicides and its expression enhances chlorotoluron tolerance in Arabidopsis. Appl. Biol. Chem. 2020, 63, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, P.; Guo, Y.; Ji, H.; Liang, F. Phenylurea herbicide degradation and N-nitrosodimethylamine formation under various oxidation conditions: Relationships and transformation pathways. Environ. Pollut. 2021, 269, 116122. [Google Scholar] [CrossRef]
- Sharma, P.; Suri, C.R. Biotransformation and biomonitoring of phenylurea herbicide diuron. Bioresour. Technol. 2011, 102, 3119–3125. [Google Scholar] [CrossRef]
- Hois, P.; Reichert, J. Bridged and Possibly Methylolized 4,5-bis-di-hydroxy-imidazolidin-2-ones, Process for Their Production and Use Thereof. WO1998005650A1, 12 February 1998. [Google Scholar]
- Fischer, K.; Steimmig, A.; Bille, H.; Petersen, H.; Tulo, H. Manufacture of Easy-Care Finishing Agents for Cellulosic Textiles. U.S. Patent 3,996,178, 7 December 1976. [Google Scholar]
- Ganesan, K.; Heyer, M.; Ratke, L.; Milow, B. Facile Preparation of Nanofibrillar Networks of “Ureido-Chitin” Containing Ureido and Amine as Chelating Functional Groups. Chem. Eur. J. 2018, 24, 19332–19340. [Google Scholar] [CrossRef]
- Polino, D.; Parrinello, M. Kinetics of Aqueous Media Reactions via Ab Initio Enhanced Molecular Dynamics: The Case of Urea Decomposition. J. Phys. Chem. B 2019, 123, 6851–6856. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Jorgensen, W.L. Why Urea Eliminates Ammonia Rather than Hydrolyzes in Aqueous Solution. J. Phys. Chem. B 2007, 111, 720–730. [Google Scholar] [CrossRef] [Green Version]
- Ware, E. The Chemistry of the Hydantoins. Chem. Rev. 1950, 46, 403–470. [Google Scholar] [CrossRef]
- Zarzyka-Niemiec, I. Products of reaction between N,N′-Bis(2-hydroxyethyl)urea and ethylene carbonate and their application to obtain polyurethane foams. J. Appl. Polym. Sci. 2009, 114, 1141–1149. [Google Scholar] [CrossRef]
- Schuster, F.; Ngamgoue, F.N.; Goetz, T.; Hirth, T.; Weber, A.; Bach, M. Investigations of a catalyst system regarding the foamability of polyurethanes for reactive inkjet printing. J. Mater. Chem. C 2017, 5, 6738–6744. [Google Scholar] [CrossRef]
- Bernardini, J.; Licursi, D.; Anguillesi, I.; Cinelli, P.; Coltelli, M.-B.; Antonetti, C.; Galletti, A.M.R.; Lazzeri, A. Exploitation of Arundo donax L. Hydrolysis Residue for the Green Synthesis of Flexible Polyurethane Foams. BioResources 2017, 12, 3630–3655. [Google Scholar] [CrossRef] [Green Version]
- Pilch-Pitera, B.; Kędzierski, M.; Olejnik, E.; Zapotoczny, S. Structure and properties of polyurethane-based powder clear coatings systems modified with hydrotalcites. Prog. Org. Coat. 2016, 95, 120–126. [Google Scholar] [CrossRef]
- Simpson, S.S. Process for Manufacturing Poylurethane Using a Metal Acetyl Acetonate/Acetyl Acetone Catalyst System and the Product Made Therefrom. U.S. Patent 5,733,945, 31 March 1998. [Google Scholar]
- Banihashemi, A.; Hazarkhani, H.; Abdolmaleki, A. Efficient and rapid synthesis of polyureas and polythioureas from the reaction of urea and thiourea with diamines under microwave irradiation. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2106–2111. [Google Scholar] [CrossRef]
- Sumner, J.B.; Hand, D.B.; Holloway, R.G. Studies of the intermediate products formed during the hydrolysis of urea by urease. J. Biol. Chem. 1931, 91, 333–341. [Google Scholar] [CrossRef]
- Jespersen, N.D. Thermochemical study of the hydrolysis of urea by urease. J. Am. Chem. Soc. 1975, 97, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects, catalytic, and non-catalytic properties—A review. J. Adv. Res. 2018, 13, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Wlodarczyk, D.; Méricq, J.-P.; Soussan, L.; Bouyer, D.; Faur, C. Enzymatic gelation to prepare chitosan gels: Study of gelation kinetics and identification of limiting parameters for controlled gel morphology. Int. J. Biol. Macromol. 2018, 107, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Chenite, A.; Gori, S.; Shive, M.; Desrosiers, E.; Buschmann, M. Monolithic gelation of chitosan solutions via enzymatic hydrolysis of urea. Carbohydr. Polym. 2006, 64, 419–424. [Google Scholar] [CrossRef]
- Krajewska, B.; Chudy, M.; Drozdek, M.; Brzózka, Z. Potentiometric Study of Urease Kinetics over pH 5.36–8.21. Electroanalysis 2003, 15, 460–466. [Google Scholar] [CrossRef]
- Takeshita, S.; Zhao, S.; Malfait, W.J. Transparent, Aldehyde-Free Chitosan Aerogel. Carbohydr. Polym. 2021, 251, 117089. [Google Scholar] [CrossRef]
- Tiwari, L.; Kumar, V.; Kumar, B.; Mahajan, D. A practically simple, catalyst free and scalable synthesis of N-substituted ureas in water. RSC Adv. 2018, 8, 21585–21595. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, A.; Shimada, N.; Maruyama, A.; Ishihara, K.; Nakai, K.; Yusa, S.-I. Preparation of upper critical solution temperature (UCST) responsive diblock copolymers bearing pendant ureido groups and their micelle formation behavior in water. Soft Matter 2015, 11, 5204–5213. [Google Scholar] [CrossRef]
- Sharafi-Kolkeshvandi, M.; Nikpour, F. A facile and convenient approach for the one-pot synthesis of 2,4(1H,3H)-quinazolinediones. Chin. Chem. Lett. 2012, 23, 431–433. [Google Scholar] [CrossRef]
- Welles, H.L.; Giaquinto, A.R.; Lindstrom, R.E. Degradation of Urea in Concentrated Aqueous Solution. J. Pharm. Sci. 1971, 60, 1212–1216. [Google Scholar] [CrossRef]



| Reactant | Product/s | Amine-to-Ureido Conversion (NMR) [%] |
|---|---|---|
 N,N′-dimethyl-1,3-propanediamine | 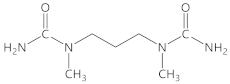 1,1′-(propane-1,3-diyl)bis(1-methylurea)  1-methyl-1-(3-(methylamino)propyl)urea | 97 |
| 3 | ||
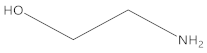 Ethanolamine | 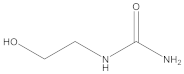 2-hydroxyethyl urea | 99 |
 5-amino-1-pentanol |  1-(5-hydroxypentyl)urea | 99 |
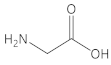 Glycine | 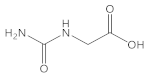 Hydantoic acid | 95 |
 Hexamethylenediamine | 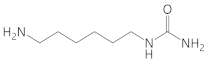 (6-aminohexyl)urea 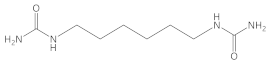 1,1′-(hexamethylene)diurea | * |
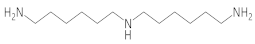 Bis-(hexamethylene)triamine | 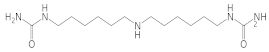 1,1′-(azanediylbis(hexane-6,1-diyl))diurea  1,1-bis(6-ureidohexyl)urea | 12 ** |
| 88 ** | ||
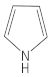 Pyrrole | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Alburquerque, N.; Zhao, S.; Rentsch, D.; Koebel, M.M.; Lattuada, M.; Malfait, W.J. Ureido Functionalization through Amine-Urea Transamidation under Mild Reaction Conditions. Polymers 2021, 13, 1583. https://doi.org/10.3390/polym13101583
Guerrero-Alburquerque N, Zhao S, Rentsch D, Koebel MM, Lattuada M, Malfait WJ. Ureido Functionalization through Amine-Urea Transamidation under Mild Reaction Conditions. Polymers. 2021; 13(10):1583. https://doi.org/10.3390/polym13101583
Chicago/Turabian StyleGuerrero-Alburquerque, Natalia, Shanyu Zhao, Daniel Rentsch, Matthias M. Koebel, Marco Lattuada, and Wim J. Malfait. 2021. "Ureido Functionalization through Amine-Urea Transamidation under Mild Reaction Conditions" Polymers 13, no. 10: 1583. https://doi.org/10.3390/polym13101583
APA StyleGuerrero-Alburquerque, N., Zhao, S., Rentsch, D., Koebel, M. M., Lattuada, M., & Malfait, W. J. (2021). Ureido Functionalization through Amine-Urea Transamidation under Mild Reaction Conditions. Polymers, 13(10), 1583. https://doi.org/10.3390/polym13101583








