Adsorption Characteristics of Polymer Solutions on Media Surfaces and Their Main Influencing Factors
Abstract
1. Introduction
2. Experiment
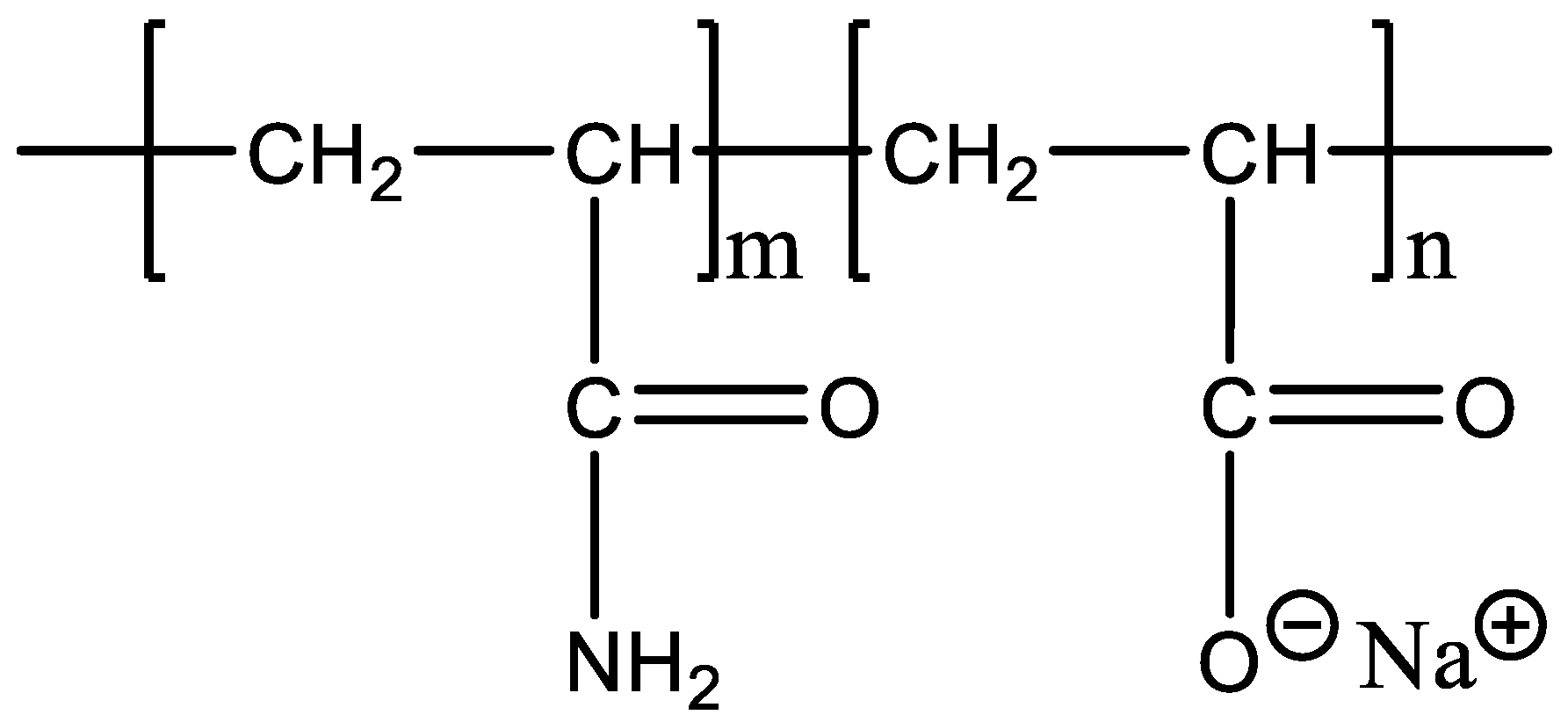
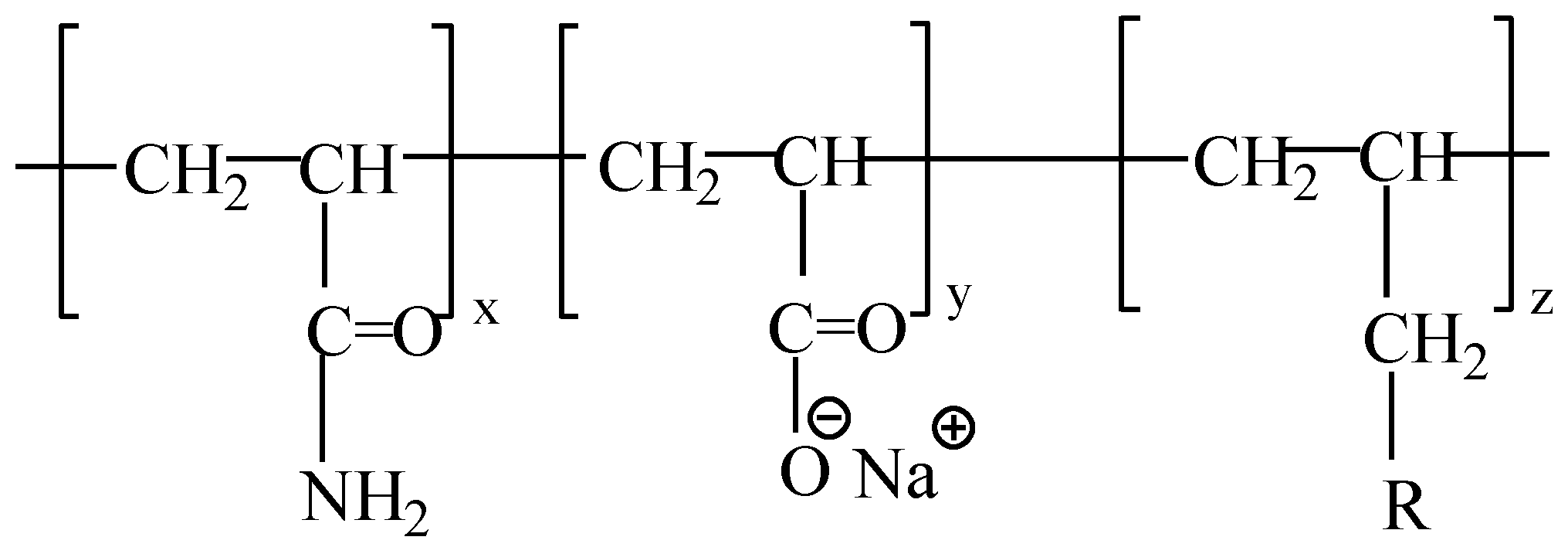
2.1. Experimental Materials and Equipments
2.2. Experimental Contents and Steps
2.2.1. Static Adsorption Capacity
2.2.2. Effect of Contact Time on Adsorption Capacity
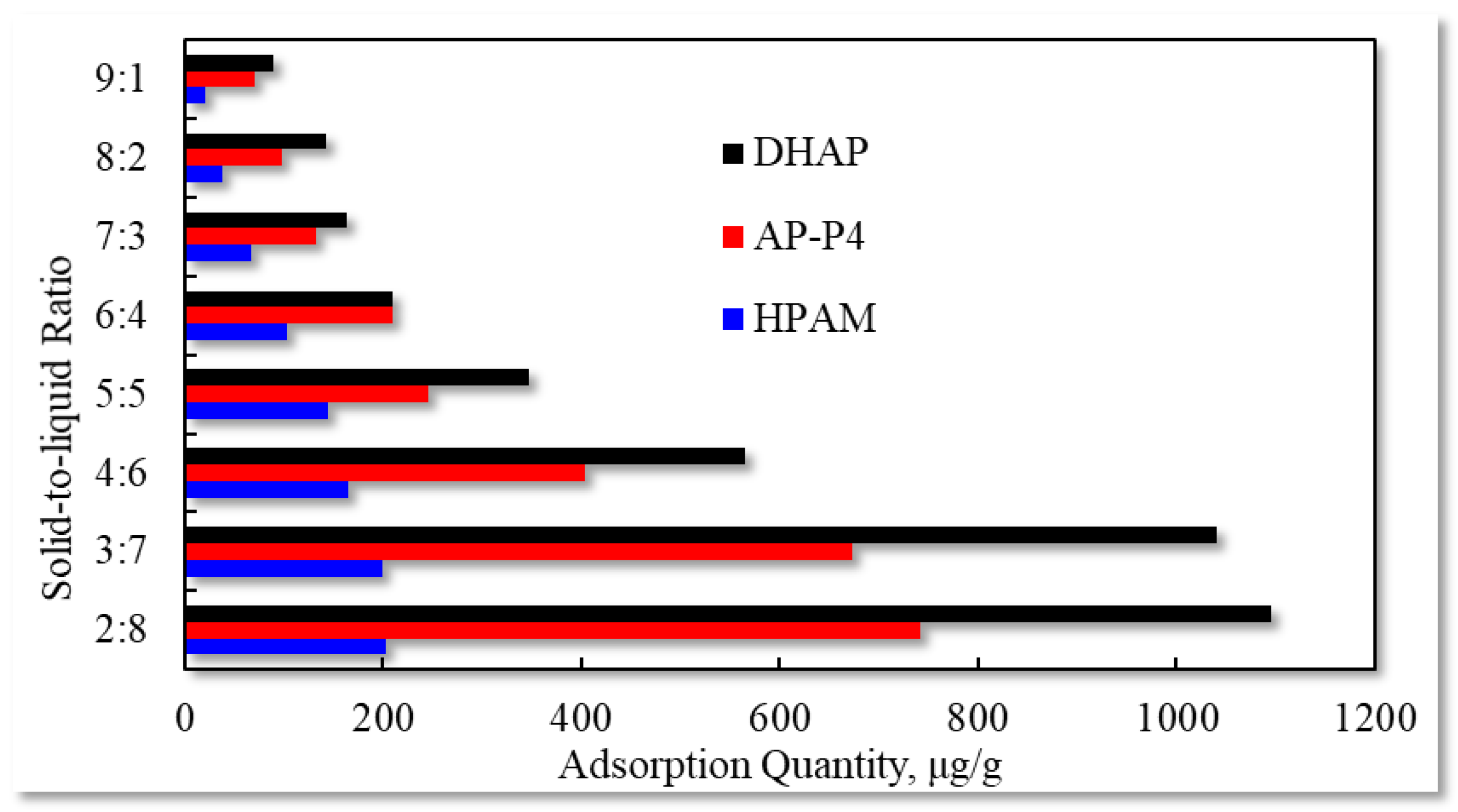
2.2.3. Influence of Solid to Liquid Ratio on Adsorption Capacity
2.2.4. Impact of Effective Contact Area on Adsorption Capacity
2.2.5. Influence of Fluid Movement on Adsorption Capacity
3. Results and Discussion
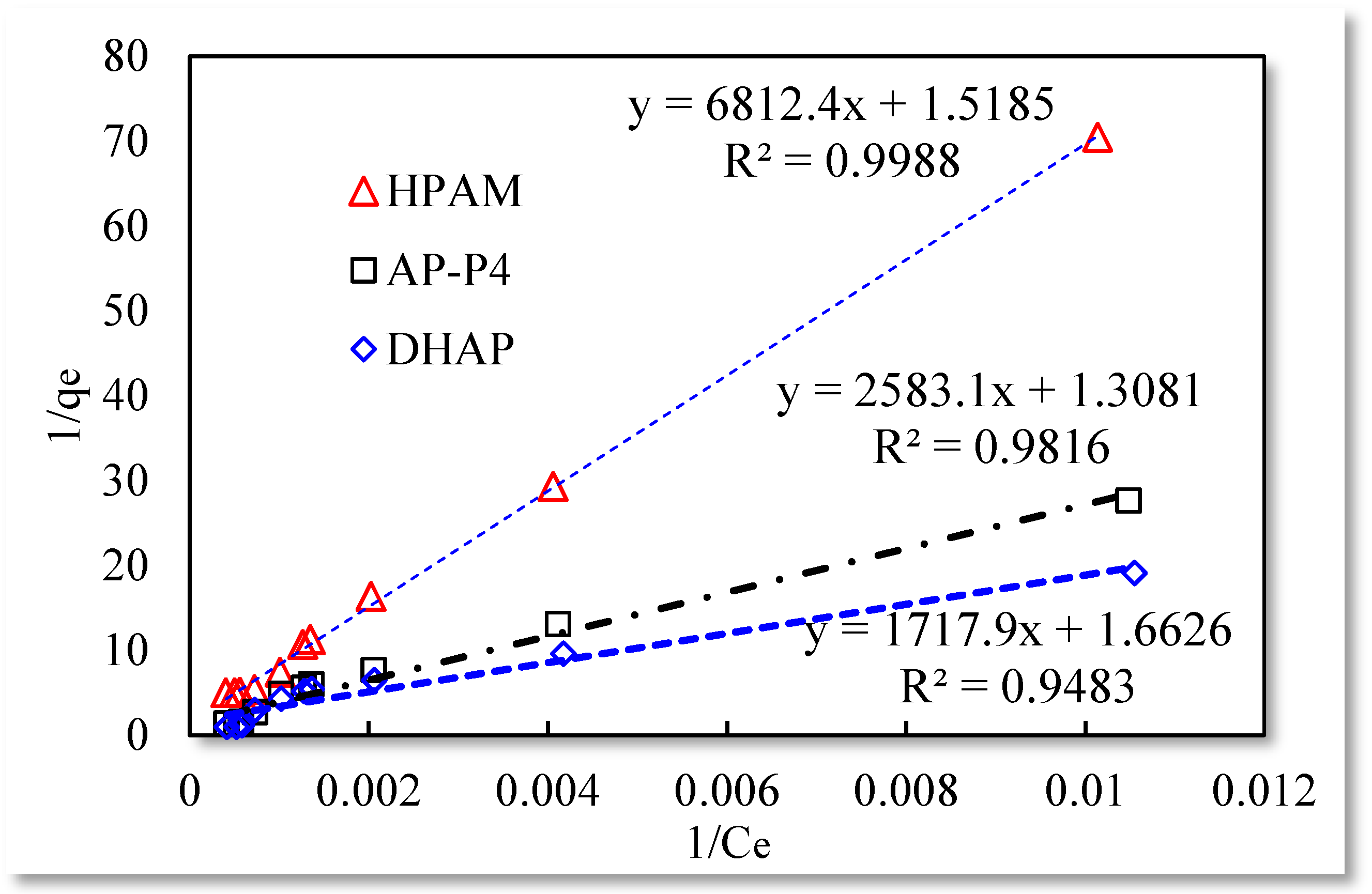
3.1. Static Adsorption Capacity
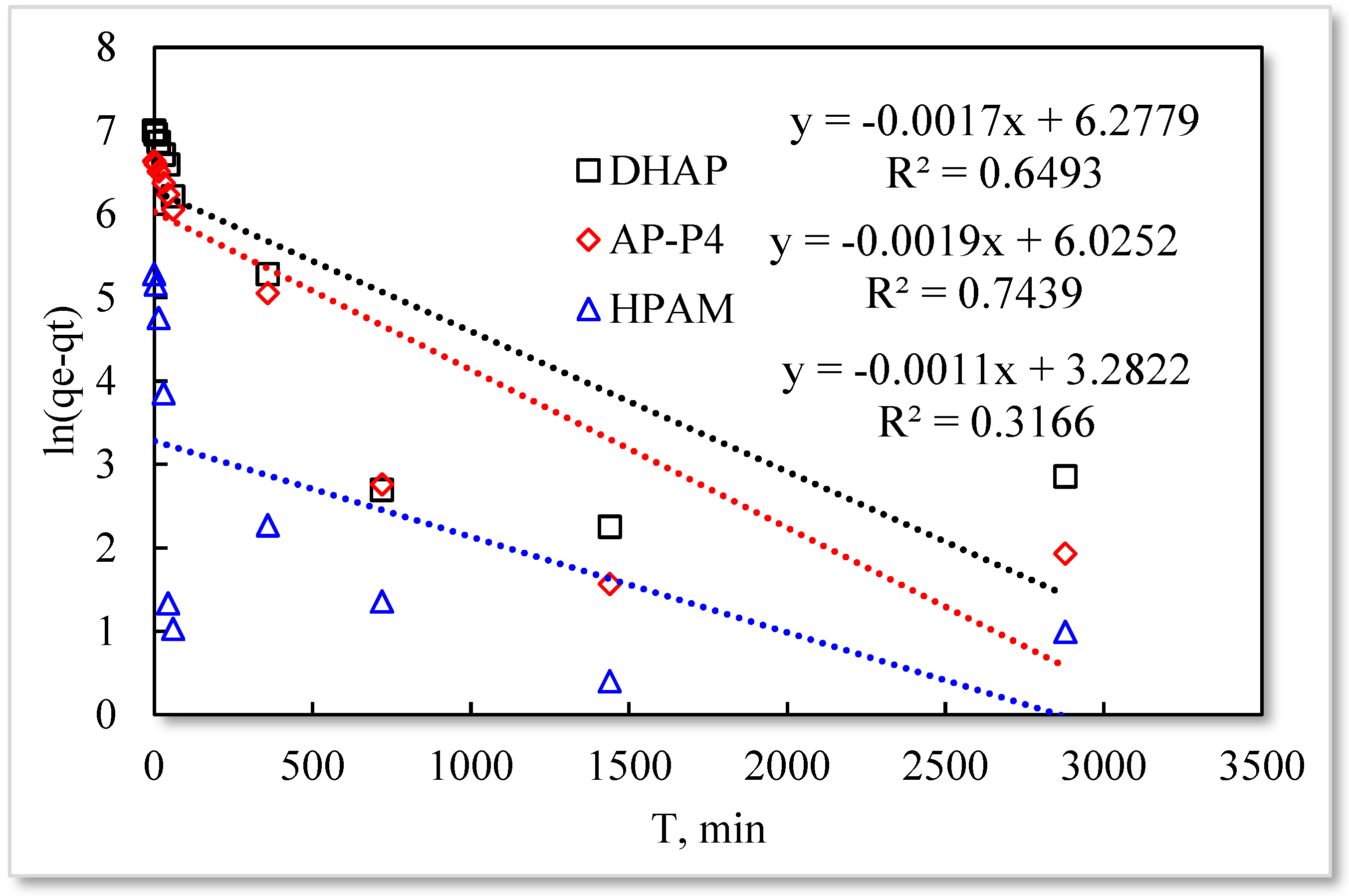
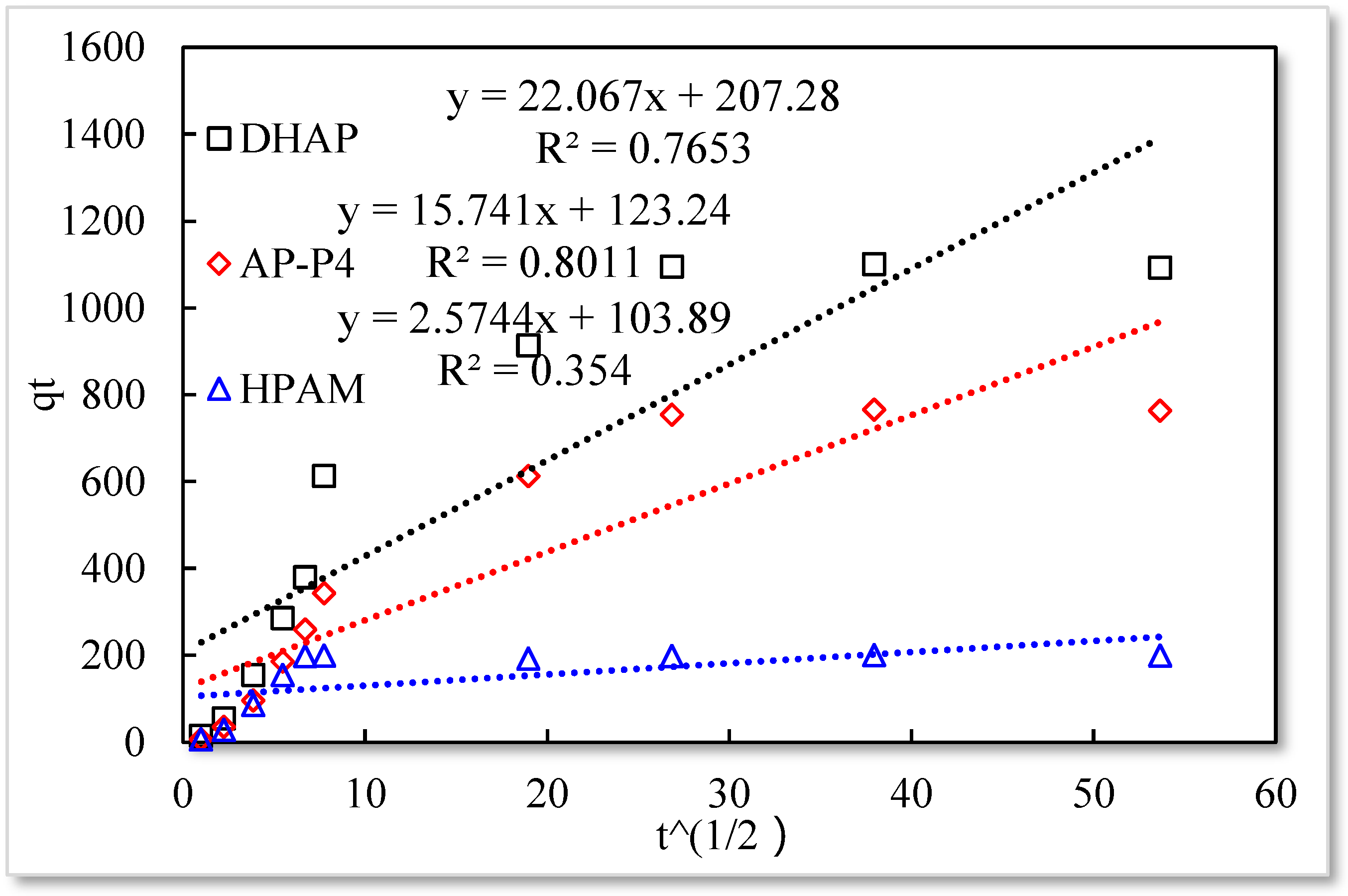
3.2. Effect of Contact Time on the Adsorption Capacity Polymers
3.3. Influence of Effective Concentration on Adsorption Capacity
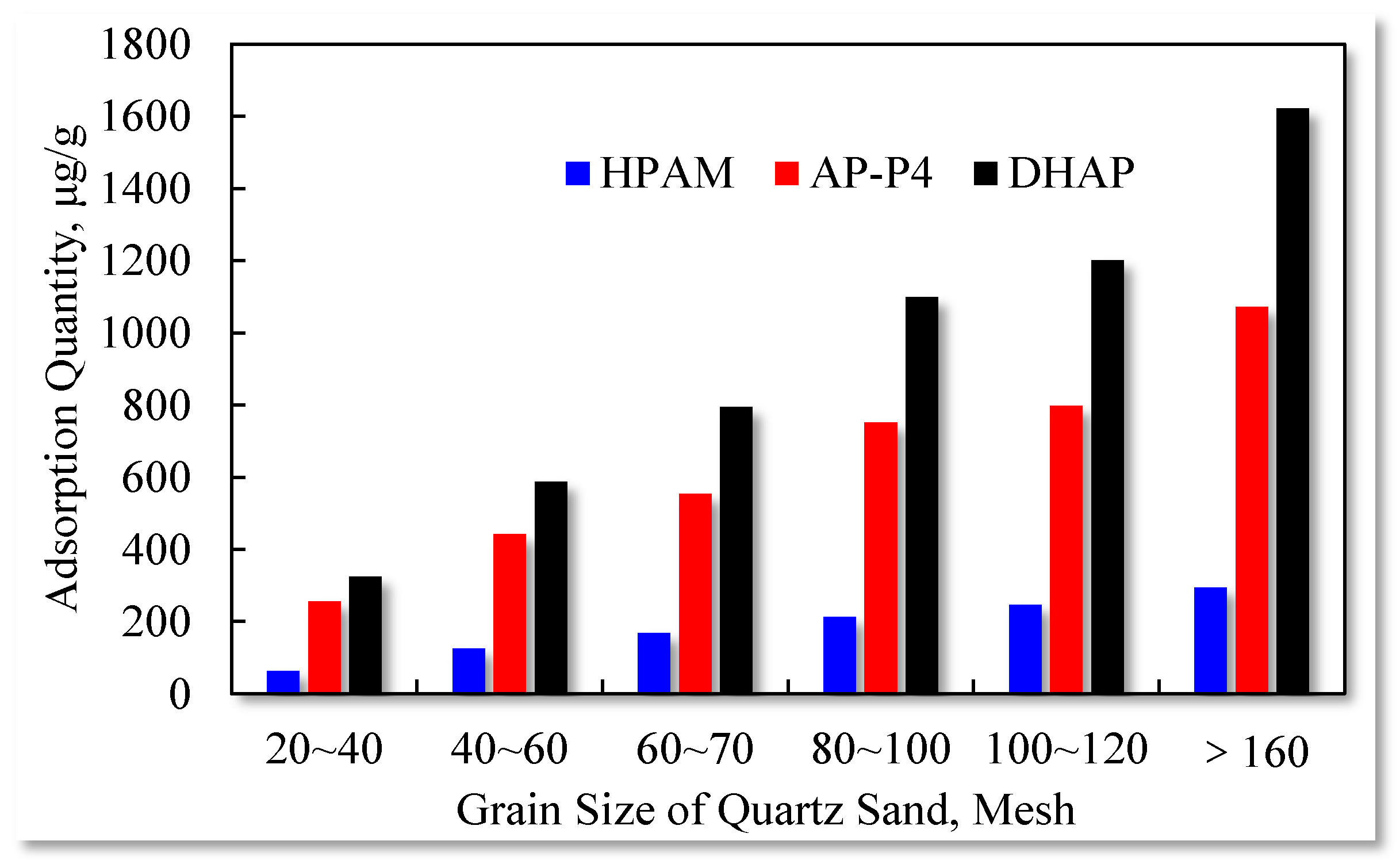
3.4. Impact of Effective Contact Area on Adsorption Capacity
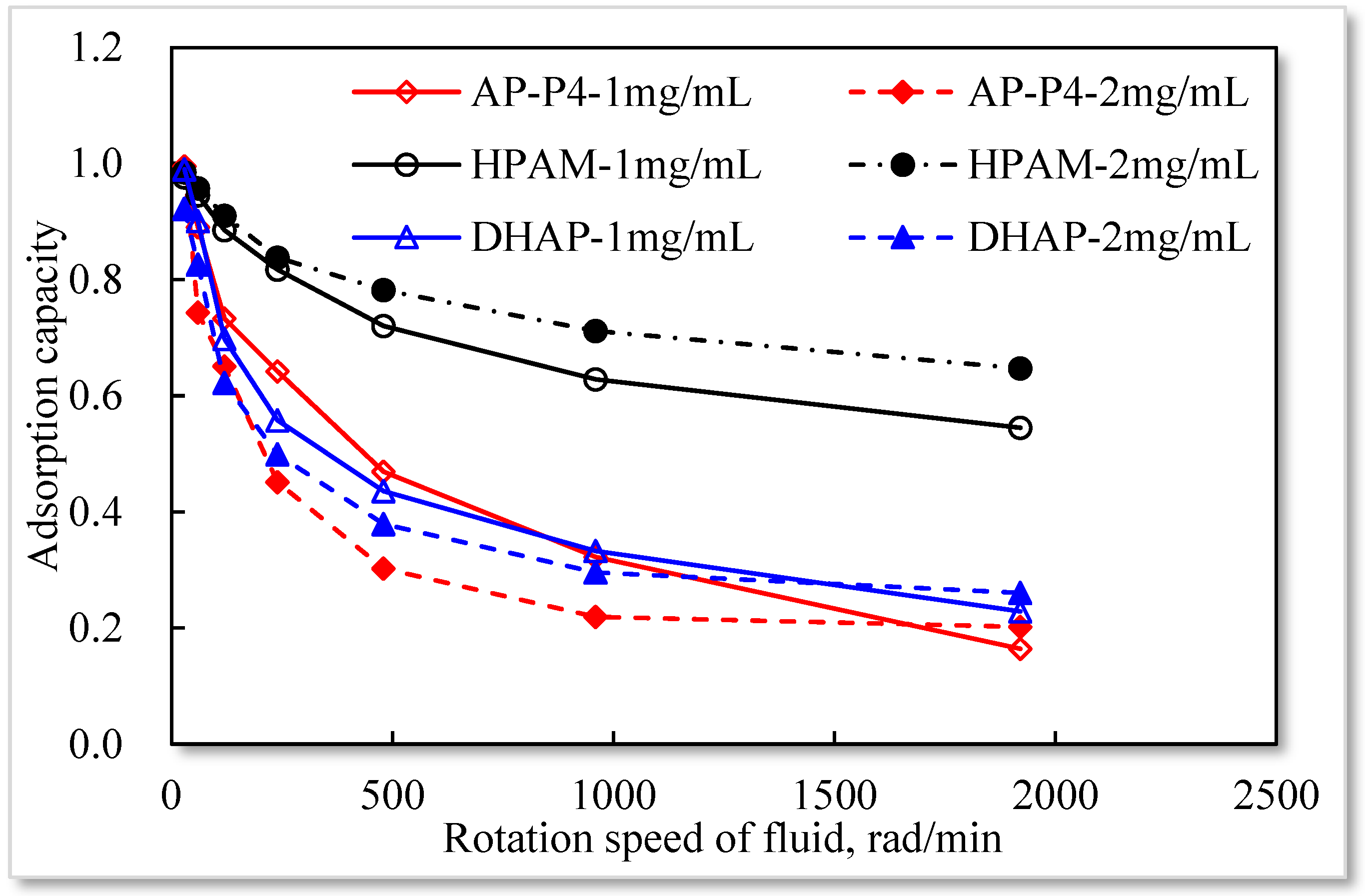
3.5. Influence of Fluid Movement on Adsorption Capacity
4. Conclusions
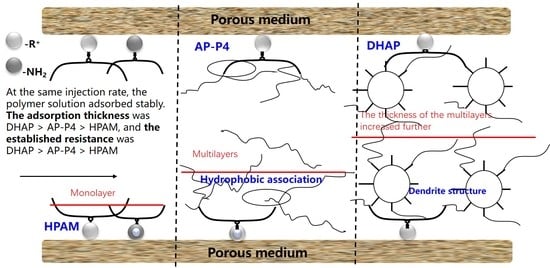
- The equilibrium adsorption of HPAM, AP-P4, and DHAP from solutions by quartz sand at optimal conditions are 200, 780, and 1100μg/g, respectively.
- The monomolecular adsorption of HPAM from its solutions on the sand surface is in good agreement with the Langmuir and quasi-second-order kinetic model.
- The adsorption of AP-P4 and DHAP polymers from solutions on the sand surface is multimolecular. Since the dendritic structure of polymers increases the thickness of the adsorption layer, the thickness of the adsorption layer of AP-P4 and DHAP is four and six times that of HPAM, respectively.
- To achieve maximum adsorption value, the solid–liquid ratio should be less than 3:7. The larger the effective adsorption area, the greater the adsorption capacity of the polymer solution. Additionally, the larger the fluid movement, the more apparent the decrease in the adsorption capacity. The greater the thickness of the adsorption layer, the more evident the fluid movement.
- Changing the flow state of polymer fluid in porous media can improve its adsorption capacity, laying the foundation for the technical guidance of changing mobility control through technological means.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Longde, S.U.; Xiaolin, W.U.; Wanfu, Z.H.; Xuejun, L.; Peihui, H. Technologies of enhancing oil recovery by chemical flooding in Daqing Oilfield, NE China. Pet. Explor. Dev. 2018, 04, 673–684. [Google Scholar]
- Zampieri, M.F.; Ferreira, V.H.; Quispe, C.C.; Sanches, K.K.; Moreno, R.B. History matching of experimental polymer flooding for enhanced viscous oil recovery. J. Braz. Soc. 2020, 42, 205. [Google Scholar] [CrossRef]
- Rock, A.; Hincapie, R.E.; Tahir, M.; Langanke, N.; Ganzer, L. On the Role of Polymer Viscoelasticity in Enhanced Oil Recovery: Extensive Laboratory Data and Review. Polymers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Branched polymers and nanoparticles flooding as separate processes for enhanced oil recovery. Fuel 2019, 257, 115996. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, C.; Yang, Y. Aggregating Thermodynamic Behavior of Amphiphilic Modified Xanthan Gum in Aqueous Solution and Oil-flooding Properties for Enhanced Oil Recovery. Chem. Eng. Sci. 2020, 115476. [Google Scholar] [CrossRef]
- Madhar, S.A.; Japan, J.T. Extensional Effects during Viscoelastic Polymer Flooding: Understanding Unresolved Challenges. SPE J. 2020, 25, 1827–1841. [Google Scholar]
- Ahsani, T.; Tamsilian, Y.; Rezaei, A. Molecular Dynamic Simulation and Experimental Study of Wettability Alteration by Hydrolyzed Polyacrylamide for Enhanced Oil Recovery: A New Finding for Polymer Flooding Process. J. Pet. Sci. Eng. 2020, 108029. [Google Scholar] [CrossRef]
- de O. Apolinário, F.; Pires, A.P. Oil displacement by multicomponent slug injection: An analytical solution for Langmuir adsorption isotherm. J. Pet. Sci. Eng. 2020, 107939. [Google Scholar] [CrossRef]
- Wang, F.; Yang, H.; Jiang, H.; Kang, X.; Hou, X.; Wang, T.; Kang, W. Formation mechanism and location distribution of blockage during polymer flooding. J. Pet. Sci. Eng. 2020, 194, 107503. [Google Scholar] [CrossRef]
- Bao, P.Y.; Li, A.F.; Luo, S.; Dang, X. Determination of adsorption parameters in numerical simulation for polymer flooding. In Proceedings of the IOP Conference Series Earth and Environmental Science, Harbin, China, 8–10 December 2017. [Google Scholar]
- Shi, L.; Zhu, S.; Ye, Z.; Xue, X.; Liu, C.; Lan, X. Effect of microscopic aggregation behavior on polymer shear resistance. J. Appl. Polym. Sci. 2019, 48670. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Experimental investigation of a natural surfactant adsorption on shale-sandstone reservoir rocks: Static and dynamic conditions. Fuel 2015, 159, 15–26. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, H.; You, Q.; Jia, Y. Distribution and Presence of Polymers in Porous Media. Energies 2017, 10, 2118. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Mahmood, S.M.; Akbari, S.; Abdulelah, H.; Yekeen, N.; Saraih, N. Experimental investigation and development of correlation for static and dynamic polymer adsorption in porous media. J. Pet. Sci. Eng. 2019, 106864. [Google Scholar] [CrossRef]
- Li, W.; Dai, C.; Ouyang, J.; Aziz, H.; Tao, J.; He, X.; Zhao, G. Adsorption and retention behaviors of heterogeneous combination flooding system composed of dispersed particle gel and surfactant. Colloid Surface A 2018, 538, 250–261. [Google Scholar] [CrossRef]
- Fu, Y.A.; Zou, J.A.; Zhang, L.A.; Liu, Y.A.; Guo, B.b.; Yuan, Z.B.; Yang, H.C. Adsorption Law of Polymer in Injection Well of Bohai SZ 36-1 Oilfield. Oilfield Chem. 2019, 36, 672–676. [Google Scholar]
- Li, Q.; Pu, W.; Wei, B.; Jin, F.; Li, K. Static adsorption and dynamic retention of an anti-salinity polymer in low permeability sandstone core. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Pan, J.; Huang, X.; Gao, L.; Peng, Y.; Liu, S.; Gu, R. Experimental investigation of a natural favonoid adsorption on macroporous polymers with intrinsic cis-diol moieties recognition function: Static and dynamic methods. Chem. Eng. J. 2017, 312, 263–274. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalili Nezhad, S.S.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int. Nano Lett. 2014, 4. [Google Scholar] [CrossRef]
- Stuart, M.A.C. Adsorbed Polymers in Colloidal Systems: From Statics to Dynamics. Polym. J. 1991, 23, 669–682. [Google Scholar] [CrossRef]
- Díaz, F.A.; Torné, J.P.; Prada, A.; Perez, G. Shear degradation model of HPAM solutions for the design of regulator valves in polymer flooding EOR. J. Pet Explor Prod. Technol. 2020, 10, 2587–2599. [Google Scholar] [CrossRef]
- Bessaies-Bey, H.; Fusier, J.; Hanafi, M.; Zhang, S.; Destarac, M.; Jouenne, S.; Passade-Boupat, N.; Lequeux, F.; de Lacaillerie, J.B.; Sanson, N. Competitive adsorption of PAM and HPAM on siliceous material. Colloids Surfaces A Phys. Eng. Asp. 2019, 579, 10–1016. [Google Scholar] [CrossRef]
- Wang, D.; Lai, N. Development and application of polymetric surfactant emulsification and viscosity reduction system. Petroleum. 2019, 5, 402–406. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Liu, Y.; Liu, G.; Xue, X.; Luo, P.; Ye, Z.; Zhang, X.; Liang, Y. Successful Scale Application of Associative Polymer Flooding for Offshore Heavy Oilfield in Bohai Bay of China. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, 17–19 October 2017. [Google Scholar]
- Shi, L.; Zhu, S.; Ye, Z.; Zhang, J.; Xue, X.; Zhao, W. The seepage flow characteristics of hydrophobically associated polymers with different aggregation behaviours in porous media. R. Soc. Open Sci. 2020, 7, 191270. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, L.; Ye, Z.; Yuan, N.; Li, X. Effect of micro-aggregation behaviour on the salt tolerance of polymer solutions. J. Appl. Polym. Sci. 2020, 50277. [Google Scholar]
- Cui, Z.; Qi, D.; Song, B.; Pei, X.; Hu, X. Inhibiting Hydrophobization of Sandstones via Adsorption of Alkyl Carboxyl Betaines in Surfactant–Polymer Flooding Using Poly Alkylammonium Bromides. Energy Fuels 2016, 30, 2043–2051. [Google Scholar] [CrossRef]
- Li, S.; Qiao, C.; Li, Z.; Wanambwa, S. Properties of Carbon Dioxide Foam Stabilized by Hydrophilic Nanoparticles and Hexadecyltrimethylammonium Bromide. Energy Fuels 2017, 31, 1478–1488. [Google Scholar] [CrossRef]
- Quan, H.; Lu, Q.; Chen, Z.; Huang, Z.; Jiang, Q. Adsorption–desorption behavior of the hydrophobically associating copolymer AM/APEG/C-18/SSS. RSC Adv. 2019, 9, 12300–12309. [Google Scholar] [CrossRef]
- Dupuis, G.; Rousseau, D.; Tabary, R.; Grassl, B. Flow of Hydrophobically Modified Water-Soluble-Polymer Solutions in Porous Media: New Experimental Insights in the Diluted Regime. SPE Journal. 2011, 16, 43–54. [Google Scholar] [CrossRef]
- Lai, N.; Zhang, Y.; Zhou, Q.; Ye, Z.; Wei, C.; Zeng, F. Interaction of Dendrimer-Based Polymer with Sodium Dodecyl Benzenesulfonate: Characterization and Effect on Properties of Composites. Energy Fuels 2016, 30, 9362–9371. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R.; Saini, D. Synthesis, optimization and characterization of PVA-co-poly(methacrylic acid) green adsorbents and applications in environmental remediation. Polym. Bull. 2020, 77, 3079–3100. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Wang, J.-X. Preparation of phosphorus based hyper cross-linked polymers and adsorption of salicylic acid from aqueous solution. J. Mol. Struct. 2020, 1221, 128804. [Google Scholar] [CrossRef]
- Arfi, R.B.; Karoui, S.; Mougin, K.; Ghorbal, A. Cetyltrimethylammonium bromide-treated Phragmites australis powder as novel polymeric adsorbent for hazardous Eriochrome Black T removal from aqueous solutions. Polym. Bull. 2019, 76. [Google Scholar] [CrossRef]
- Landarani, M.; Arab Chamjangali, M.; Bahramian, B. Preparation and Characterization of a Novel Chemically Modified PVC Adsorbent for Methyl Orange Removal: Optimization, and Study of Isotherm, Kinetics, and Thermodynamics of Adsorption Process. Water Air Soil Pollut. 2020, 231. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Removal of Methylene Blue in Aqueous Solution by Economic Adsorbent Derived from Apricot Stone Activated Carbon. Fibers Polym. 2020, 21, 810–820. [Google Scholar] [CrossRef]
- Wadhera, P.; Jindal, R.; Dogra, R. Insight into adsorption kinetics and isotherms for adsorption of methylene blue using gum rosin alcohol/psyllium-based green adsorbent. Iran. Polym. J. 2020, 29, 501–514. [Google Scholar] [CrossRef]
- Culita, D.C.; Simonescu, C.M.; Patescu, R.E.; Preda, S.; Stanica, N.; Munteanu, C.; Oprea, O. Polyamine Functionalized Magnetite Nanoparticles as Novel Adsorbents for Cu(II) Removal from Aqueous Solutions. J. Inorg. Organomet. Polym. Mater. 2017, 27, 490–502. [Google Scholar] [CrossRef]
- Aghabozorgi, S.; Rostami, B. An Investigation of Polymer Adsorption in Porous Media Using Pore Network Modelling. Transp. Porous Media 2016, 115, 169–187. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wang, J.; Ren, Y.; Xuan, C.; Liu, C.; Shen, C. Superhydrophobic and superoleophilic porous reduced graphene oxide/polycarbonate monoliths for high-efficiency oil/water separation. J. Hazard. Mater. 2018, 344, 849–856. [Google Scholar] [CrossRef]
- Zhang, G.; Seright, R. Effect of Concentration on HPAM Retention in Porous Media. SPE J. 2014, 19, 373–380. [Google Scholar] [CrossRef]
- Cohen, Y.; Christ, F.R. Polymer Retention and Adsorption in the Flow of Polymer Solutions Through Porous Media. SPE Reserv. 1986, 1, 113–118. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Spotlight on the New Natural Surfactant Flooding in Carbonate Rock Samples in Low Salinity Condition. Sci. Rep. 2018, 8, 10985. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.A.; Shadizadeh, S.R. Experimental and Theoretical Study of a New Plant Derived Surfactant Adsorption on Quartz Surface: Kinetic and Isotherm Methods. J. Disper. Sci. Technol. 2015, 36, 441–452. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Adsorption of Novel Nonionic Surfactant and Particles Mixture in Carbonates: Enhanced Oil Recovery Implication. Energy Fuels 2012, 26, 4655–4663. [Google Scholar] [CrossRef]
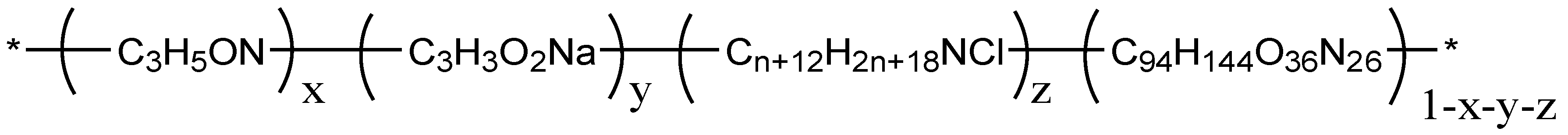





| Category | Model and Formula | Physical Meaning of Formula |
|---|---|---|
| Isothermal adsorption model | Linear expression of Langmuir model | The model assumes that only one adsorbate molecule is adsorbed on each activator site. The attachment sites are identical and undifferentiated. The adsorption force is strong enough and belongs to chemical or physical force. The adsorbate does not move on the surface of adsorbent. The adsorption capacity is maximized when the adsorbate is saturated on the surface of adsorbent. |
| Linear expression of Temkin | The model is suitable for adsorbents with heterogeneous surfaces and is often used to describe adsorption processes in which there are strong intermolecular interactions between adsorbate and adsorbent, such as strong electrostatic interaction or ion exchange interaction | |
| Dubinin–Radushkevich model | The model assumes that the adsorption energy is heterogeneous and the distribution of adsorption energy is Gaussian | |
| Adsorption kinetic model | Pseudo-second order adsorption kinetics model | The model assumes that the adsorption rate is directly proportional to the square of the adsorbate concentration, and the limiting factor of the adsorption rate is the adsorption mechanism. Chemisorption is the only or the most essential adsorption mechanism, and the adsorption reaction occurs by sharing or gaining/losing electrons between the adsorbent and adsorbate. |
| Elovich model | The model assumes that the adsorption energy is not uniform and increases linearly with the increase in surface coverage. The adsorption rate is not uniform, but decreases exponentially with the increase in adsorption capacity. | |
| Particle diffusion model | The model assumes that the driving force of the adsorption process is from the concentration gradient of the adsorbate in the solution. | |
| Pseudo-first order adsorption kinetic model | The model assumes that the adsorption rate is directly proportional to the concentration of adsorbate, and the factor limiting the adsorption rate is the mass transfer resistance in the particle. |
| Mesh | 20–40 | 40–60 | 60–70 | 80–100 | 100–120 | >160 |
|---|---|---|---|---|---|---|
| Radius, cm | 0.0295 | 0.015 | 0.011 | 0.008 | 0.007 | 0.0045 |
| Surface area, cm3, of 1 g quartz sand | 38.375 | 75.472 | 102.916 | 141.510 | 161.725 | 251.572 |
| Concentration (mg/mL) | 0.1 | 0.25 | 0.5 | 0.75 | 0.8 | 1 | 1.4 | 1.8 | 2 | 2.5 |
|---|---|---|---|---|---|---|---|---|---|---|
| HPAM, μg/g | 14.6 | 33.6 | 70.7 | 87.2 | 92.4 | 132.4 | 189.2 | 197.3 | 201.2 | 201.1 |
| AP-P4, μg/g | 42.3 | 84.1 | 121.8 | 157.3 | 170.0 | 209.1 | 502.5 | 642.7 | 764.2 | 784.2 |
| DHAP, μg/g | 52.4 | 104.6 | 156.2 | 184.3 | 194.2 | 236.8 | 347.2 | 890.1 | 1087.3 | 1105.7 |
| Contact Time (min) | 1 | 5 | 15 | 30 | 45 | 60 | 360 | 720 | 1440 | 2880 |
|---|---|---|---|---|---|---|---|---|---|---|
| HPAM, μg/g | 5.9 | 28.6 | 85.4 | 154.8 | 198.2 | 199.2 | 192.3 | 198.1 | 200.5 | 199.3 |
| AP-P4, μg/g | 6.9 | 35.2 | 96.3 | 185.3 | 259.6 | 342.3 | 613.1 | 754.2 | 765.2 | 763.1 |
| DHAP, μg/g | 13.9 | 55.2 | 154.3 | 285.3 | 379.6 | 612.3 | 913.1 | 1095.2 | 1100.5 | 1092.6 |
| Mesh | 20–40 | 40–60 | 60–70 | 80~100 | 100–120 | >160 |
|---|---|---|---|---|---|---|
| HPAM, μg/cm2 | 1.62 | 1.66 | 1.63 | 1.51 | 1.52 | 1.17 |
| AP-P4, μg/cm2 | 6.67 | 5.86 | 5.38 | 5.31 | 4.93 | 4.26 |
| DHAP, μg/cm2 | 8.45 | 7.78 | 7.73 | 7.76 | 7.43 | 6.44 |
| Polymer | Adsorption Capacity, μg/g | Rotation Speed of Fluid, rad/min | ||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 240 | 480 | 960 | 1920 | ||
| Concentration, mg/mL | Linear Speed Corresponding to Different Rotation Speed, m/s | |||||||
| 0.01 | 0.02 | 0.04 | 0.08 | 0.16 | 0.32 | 0.64 | ||
| HPAM | 1 | 129.3 | 125.2 | 117.2 | 108.2 | 95.4 | 83.2 | 72.1 |
| 2 | 198.2 | 192.6 | 183.2 | 168.6 | 157.3 | 143.2 | 130.2 | |
| AP-P4 | 1 | 206 | 186.2 | 153.2 | 134.2 | 98.2 | 67.3 | 34.3 |
| 2 | 760.4 | 567.8 | 497.2 | 345.1 | 231 | 167.8 | 154.2 | |
| DHAP | 1 | 234.5 | 213.2 | 165.2 | 132.1 | 103.2 | 78.7 | 54.2 |
| 2 | 1002.3 | 898.2 | 676.3 | 543.2 | 412.3 | 321.3 | 283.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Ye, Z.; Liu, Z.; Chen, Z.; Li, J.; Xiang, Z. Adsorption Characteristics of Polymer Solutions on Media Surfaces and Their Main Influencing Factors. Polymers 2021, 13, 1774. https://doi.org/10.3390/polym13111774
Zhu S, Ye Z, Liu Z, Chen Z, Li J, Xiang Z. Adsorption Characteristics of Polymer Solutions on Media Surfaces and Their Main Influencing Factors. Polymers. 2021; 13(11):1774. https://doi.org/10.3390/polym13111774
Chicago/Turabian StyleZhu, Shijie, Zhongbin Ye, Zhezhi Liu, Zhonghua Chen, Jun Li, and Zuping Xiang. 2021. "Adsorption Characteristics of Polymer Solutions on Media Surfaces and Their Main Influencing Factors" Polymers 13, no. 11: 1774. https://doi.org/10.3390/polym13111774
APA StyleZhu, S., Ye, Z., Liu, Z., Chen, Z., Li, J., & Xiang, Z. (2021). Adsorption Characteristics of Polymer Solutions on Media Surfaces and Their Main Influencing Factors. Polymers, 13(11), 1774. https://doi.org/10.3390/polym13111774