Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation and Characterization of NCDs
2.3. Electrochemical Measurements
2.4. Weight Loss Measurement
2.5. Surface Analysis
3. Results and Discussions
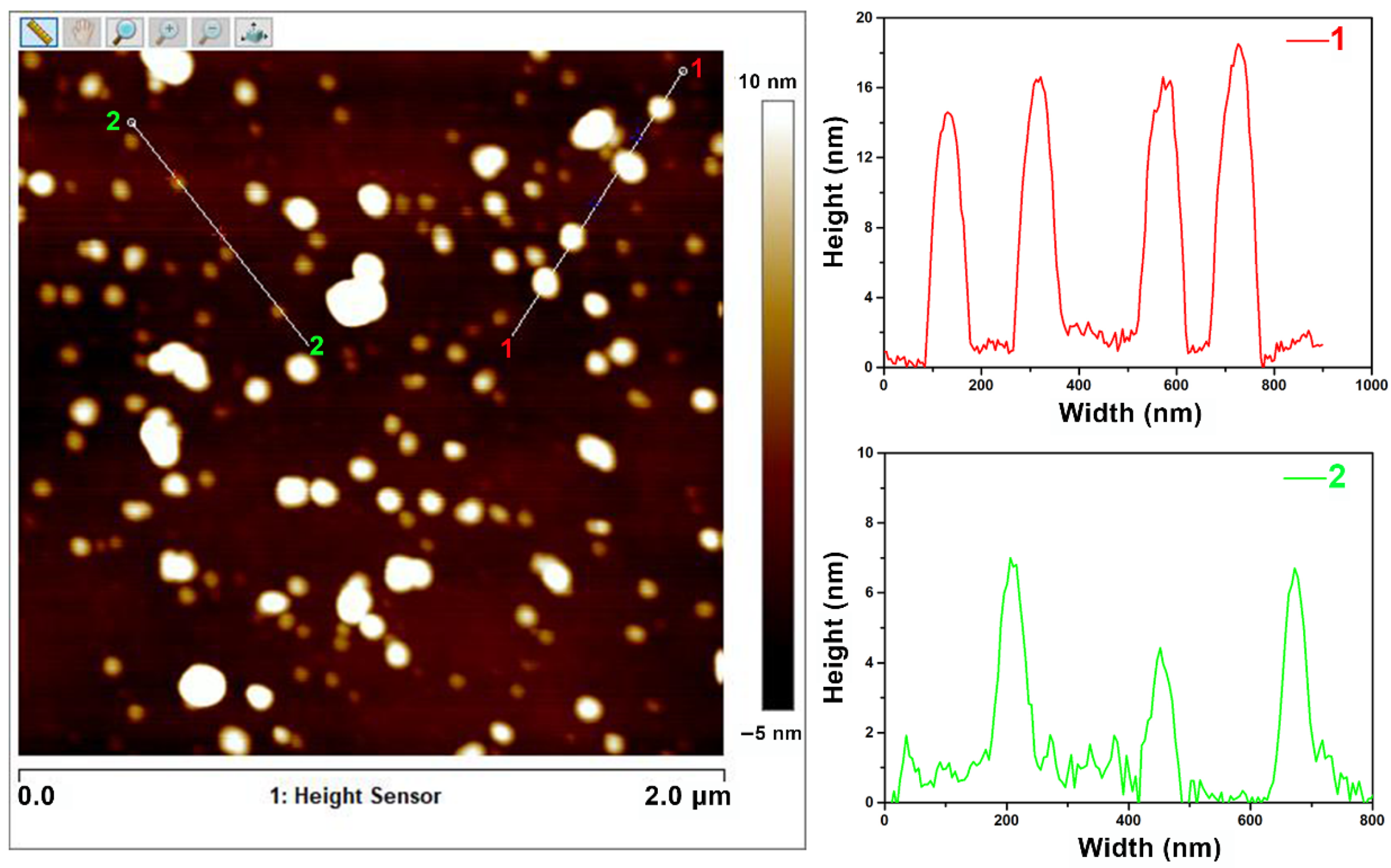
3.1. Characterization of NCDs
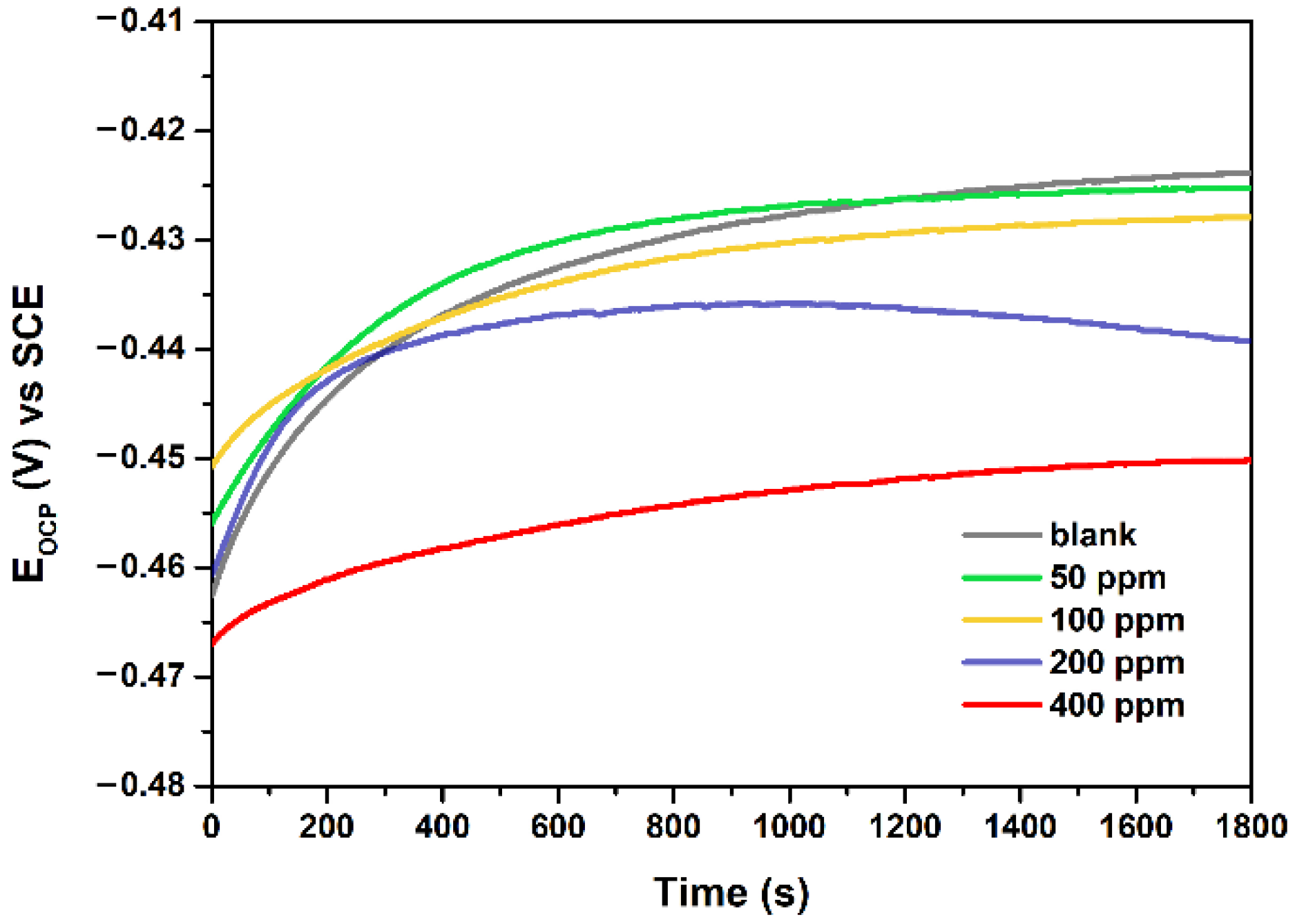
3.2. The Effect of NCD Concentration
3.3. Effect of Exposure Time and Temperature
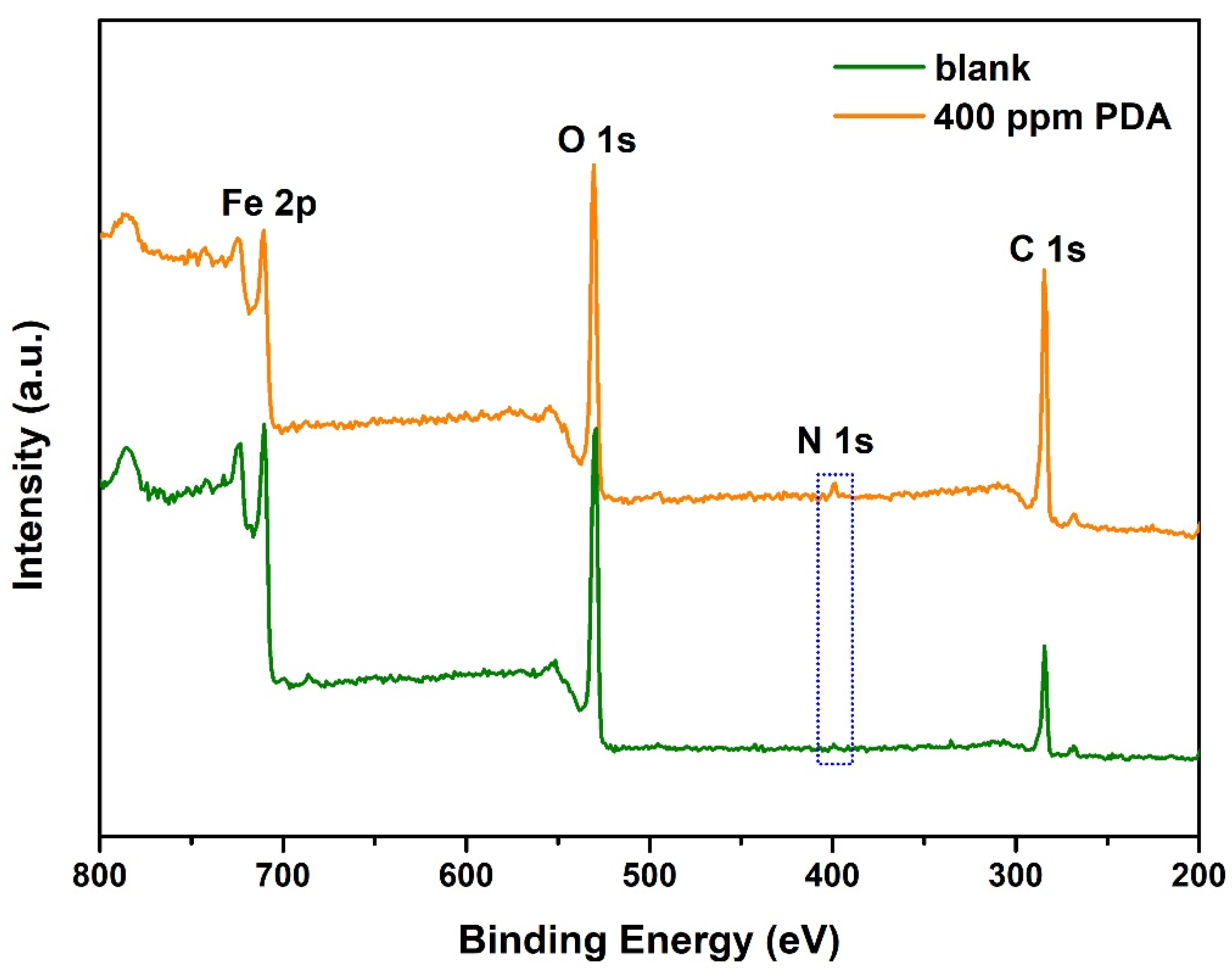
3.4. XPS Analysis on the Corroded Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandes, C.M.; Fagundes, T.D.S.F.; dos Santos, N.E.; Rocha, T.S.D.M.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.; Ponzio, E.A. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Erami, R.S.; Amirnasr, M.; Meghdadi, S.; Talebian, M.; Farrokhpour, H.; Raeissi, K. Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros. Sci. 2019, 151, 190–197. [Google Scholar] [CrossRef]
- Farahati, R.; Ghaffarinejad, A.; Mousavi-Khoshdel, S.M.; Rezania, J.; Behzadi, H.; Shockravi, A. Synthesis and potential applica-tions of some thiazoles as corrosion inhibitor of copper in 1 M HCl: Experimental and theoretical studies. Prog. Org. Coat. 2019, 132, 417–428. [Google Scholar] [CrossRef]
- Tan, B.; Xiang, B.; Zhang, S.; Qiang, Y.; Xu, L.; Chen, S.; He, J. Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J. Colloid Interface Sci. 2021, 582, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Potential of Borage flower aqueous extract as an environmen-tally sustainable corrosion inhibitor for acid corrosion of mild steel: Electrochemical and theoretical studies. J. Mol. Liq. 2019, 277, 895–911. [Google Scholar] [CrossRef]
- Sanaei, Z.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. Ind. Eng. Chem. 2019, 69, 18–31. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Ionic liquids as green and sustainable corrosion inhibitors for metals and alloys: An over-view. J. Mol. Liq. 2017, 233, 403–414. [Google Scholar] [CrossRef]
- Cao, S.; Liu, D.; Ding, H.; Wang, J.; Lu, H.; Gui, J. Task-specific ionic liquids as corrosion inhibitors on carbon steel in 0.5 M HCl solution: An experimental and theoretical study. Corros. Sci. 2019, 153, 301–313. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126863. [Google Scholar] [CrossRef]
- Abdallah, M. Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution. Corros. Sci. 2004, 46, 1981–1996. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A review of promising novel corrosion inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Jmiai, A.; El Ibrahimi, B.; Tara, A.; El Issami, S.; Jbara, O.; Bazzi, L. Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: Experimental and theoretical approaches. J. Mol. Struct. 2018, 1157, 408–417. [Google Scholar] [CrossRef]
- Shahini, M.; Ramezanzadeh, B.; Mohammadloo, H.E. Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: A review study from experimental and theoretical views. J. Mol. Liq. 2020, 325, 115110. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly photoluminescent car-bon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. 2013, 125, 4045–4049. [Google Scholar] [CrossRef]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M.; et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Xue, Q.; Zhao, H.; Wang, L. Carbon dots as new eco-friendly and effective corrosion inhibitor. J. Alloys Compd. 2017, 726, 680–692. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Wang, L.; Xue, Q. Novel nitrogen doped carbon dots for corrosion inhibition of carbon steel in 1 M HCl solution. Appl. Surf. Sci. 2018, 443, 145–156. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, D.; Chen, H.; Guo, S.; Yang, Q.; Chen, L.; Zhao, H.; Wang, L. A high-efficiency corrosion inhibitor of N-doped citric acid-based carbon dots for mild steel in hydrochloric acid environment. J. Hazard. Mater. 2019, 381, 121019. [Google Scholar] [CrossRef]
- Cui, M.; Qiang, Y.; Wang, W.; Zhao, H.; Ren, S. Microwave Synthesis of Eco-friendly Nitrogen Doped Carbon Dots for the Corro-sion Inhibition of Q235 Carbon Steel in 0.1 M HCl. Int. J. Electrochem. Sci. 2021, 16, 151019. [Google Scholar] [CrossRef]
- Ye, Y.; Zou, Y.; Jiang, Z.; Yang, Q.; Chen, L.; Guo, S.; Chen, H. An effective corrosion inhibitor of N doped carbon dots for Q235 steel in 1 M HCl solution. J. Alloys Compd. 2020, 815, 152338. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, D.; Chen, H. A green and effective corrosion inhibitor of functionalized carbon dots. J. Mater. Sci. Technol. 2019, 35, 2243–2253. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Zou, Y.; Zhao, H.; Chen, H. A feasible method to improve the protection ability of metal by functionalized car-bon dots as environment-friendly corrosion inhibitor. J. Clean. Prod. 2020, 264, 121682. [Google Scholar] [CrossRef]
- Cen, H.; Chen, Z.; Guo, X. N, S co-doped carbon dots as effective corrosion inhibitor for carbon steel in CO2-saturated 3.5% NaCl solution. J. Taiwan Inst. Chem. Eng. 2019, 99, 224–238. [Google Scholar] [CrossRef]
- Qu, K.; Wang, J.; Ren, J.; Qu, X. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sens-ing platform for the label-free detection of iron (iii) ions and dopamine. Chem. Eur. J. 2013, 19, 7243–7249. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Y.; Chen, B.; Zhang, L. Novel surfactants as green corrosion inhibitors for mild steel in 15% HCl: Experimental and theoretical studies. Chem. Eng. J. 2020, 402, 126219. [Google Scholar] [CrossRef]
- Pareek, S.; Jain, D.; Hussain, S.; Biswas, A.; Shrivastava, R.; Parida, S.K.; Kisan, H.K.; Lgaz, H.; Chung, I.-M.; Behera, D. A new insight into corrosion inhibition mechanism of copper in aerated 3.5 wt.% NaCl solution by eco-friendly Imidazopyrimidine Dye: Experimental and theoretical approach. Chem. Eng. J. 2019, 358, 725–742. [Google Scholar] [CrossRef]
- Khadiri, A.; Saddik, R.; Bekkouche, K.; Aouniti, A.; Hammouti, B.; Benchat, N.; Bouachrine, M.; Solmaz, R. Gravimetric, electro-chemical and quantum chemical studies of some pyridazine derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. J. Taiwan Inst. Chem. Eng. 2016, 58, 552–564. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Zhao, H.; Tan, B.; Wang, L. Enhanced anticorrosion performance of copper by novel N-doped carbon dots. Corros. Sci. 2019, 161, 108193. [Google Scholar] [CrossRef]
- Rella, S.; Mazzotta, E.; Caroli, A.; De Luca, M.; Bucci, C.; Malitesta, C. Investigation of polydopamine coatings by X-ray Photoe-lectron Spectroscopy as an effective tool for improving biomolecule conjugation. Appl. Surf. Sci. 2018, 447, 31–39. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Yuan, X.; Dong, S.; Zheng, Q.; Yang, W.; Huang, T. Novel and efficient curcumin based fluorescent polymer for scale and corro-sion inhibition. Chem. Eng. J. 2020, 389, 124296. [Google Scholar] [CrossRef]
- Bouknana, D.; Hammouti, B.; Messali, M.; Aouniti, A.; Sbaa, M. Phenolic and non-Phenolic Fractions of the Olive Oil Mill Wastewaters as Corrosion Inhibitor for Steel in HCl medium. Port. Electrochim. Acta 2014, 32, 1–19. [Google Scholar] [CrossRef]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum leave in 1 M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.; Soltani, N.; Salavati-Niasari, M. The inhibitive effect of some bis-N,S-bidentate Schiff bases on corrosion behaviour of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 2009, 51, 1073–1082. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Alvarez, L.X.; dos Santos, N.E.; Barrios, A.C.M.; Ponzio, E.A. Green synthesis of 1-benzyl-4-phenyl-1H-1, 2, 3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical anal-yses. Corros. Sci. 2019, 149, 185–194. [Google Scholar] [CrossRef]
- Bedair, M.; El-Sabbah, M.; Fouda, A.; Elaryian, H. Synthesis, electrochemical and quantum chemical studies of some prepared surfactants based on azodye and Schiff base as corrosion inhibitors for steel in acid medium. Corros. Sci. 2017, 128, 54–72. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Lakhlifi, T.; Traisnel, M.; Vezin, H.; Bentiss, F. New 1 H-pyrrole-2,5-dione derivatives as efficient organic inhibitors of carbon steel corrosion in hydrochloric acid medium: Electrochemical, XPS and DFT studies. Corros. Sci. 2015, 90, 572–584. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M.; Ganiyu, S.A.; Muhammad, Q. L-citrulline: An active corrosion inhibitor compo-nent of watermelon rind extract for mild steel in HCl medium. J. Taiwan Inst. Chem. Eng. 2015, 51, 177–185. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Singh, M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar] [CrossRef]
- Liao, L.L.; Mo, S.; Luo, H.Q.; Li, N.B. Longan seed and peel as environmentally friendly corrosion inhibitor for mild steel in acid solution: Experimental and theoretical studies. J. Colloid Interface Sci. 2017, 499, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Tavakkoli, N.; Kashani, M.K.; Mosavizadeh, A.E.E.O.; Oguzie, E.E.; Jalali, M.R. Silybum marianum extract as a natu-ral source inhibitor for 304 stainless steel corrosion in 1.0 M HCl. J. Ind. Eng. Chem. 2014, 20, 3217–3227. [Google Scholar] [CrossRef]
- Liao, L.L.; Mo, S.; Lei, J.L.; Luo, H.Q.; Li, N.B. Application of a cosmetic additive as an eco-friendly inhibitor for mild steel cor-rosion in HCl solution. J. Colloid Interface Sci. 2016, 474, 68–77. [Google Scholar] [CrossRef] [PubMed]

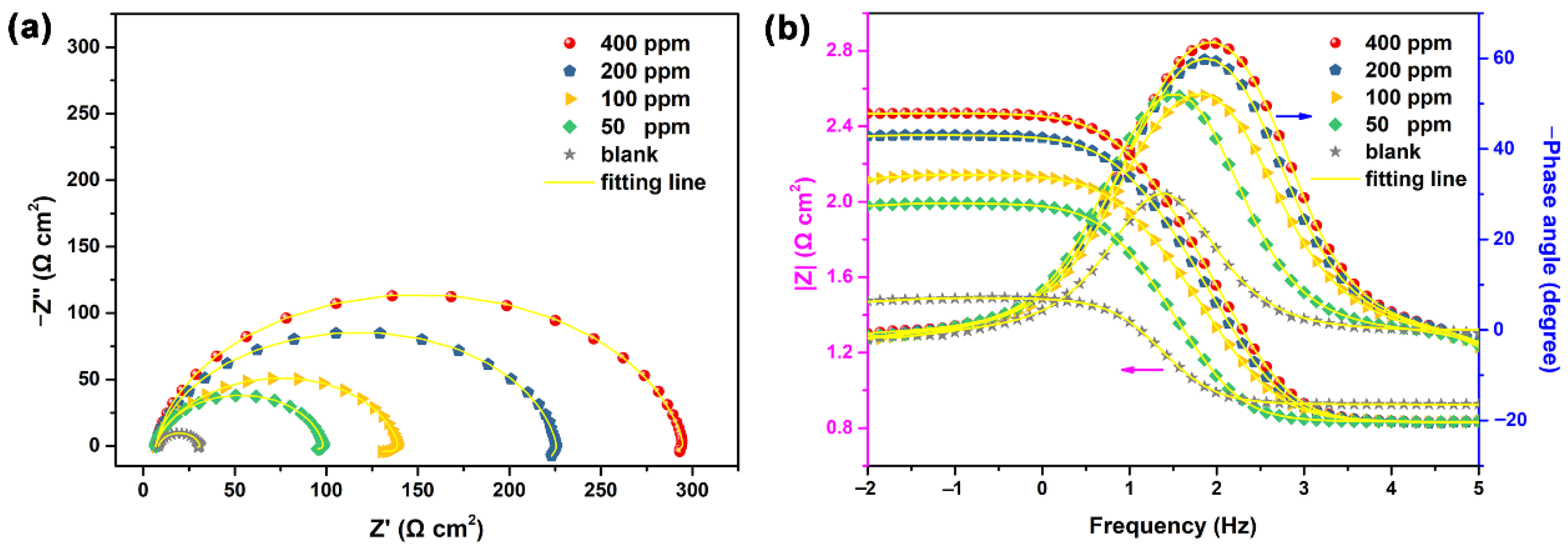
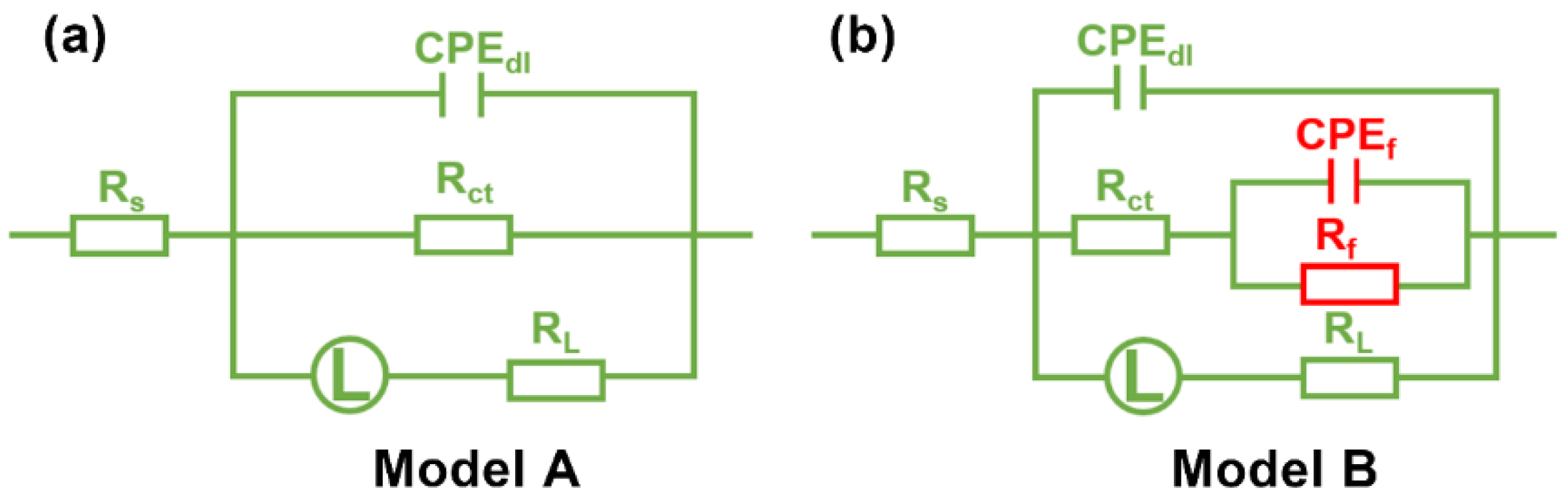


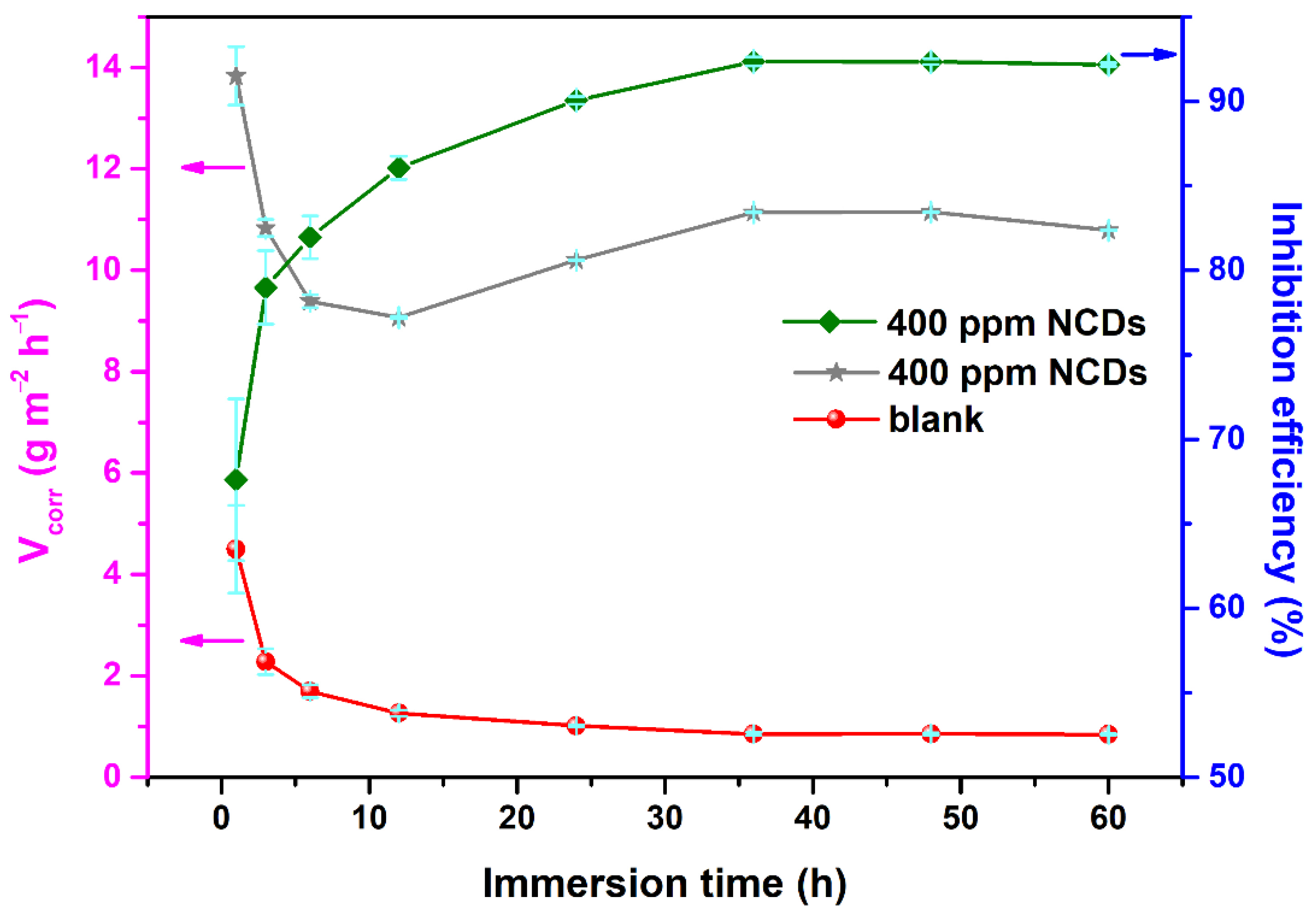



| Rs (Ω cm2) | Rct (Ω cm2) | CPEdl | L (H cm2) | RL (Ω cm2) | CPEf | Rf (Ω cm2) | η (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Y0 × 105 (Ω −1cm−2 sn) | n | Y0 × 105 (Ω−1 cm−2 sn) | n | |||||||
| blank | 7.074 | 13.52 | 92.29 | 0.8852 | 105.2 | 3.689 | / | / | / | / |
| 50 | 6.781 | 86.65 | 38.38 | 0.8904 | 53.69 | 5.044 | / | / | / | 84.4 |
| 100 | 6.725 | 124.3 | 24.79 | 0.8341 | 63.29 | 9.225 | / | / | / | 89.1 |
| 200 | 6.743 | 162.3 | 11.85 | 0.8914 | 816.7 | 22.5 | 91.2 | 0.9044 | 34.48 | 93.1 |
| 400 | 6.785 | 221.9 | 8.131 | 0.912 | 74.32 | 6.52 | 65.69 | 0.8152 | 60.43 | 95.2 |
| Ecorr (mV) | icorr (μA cm−2) | −βc (mV/dec) | βa (mV/dec) | Rp (Ω cm2) | θ | η (%) | |
|---|---|---|---|---|---|---|---|
| blank | −420 | 878 | 142.2 | 112.6 | 31 | / | / |
| 50 | −423 | 187 | 94.4 | 93.5 | 109 | 0.787 | 78.7 |
| 100 | −428 | 98.6 | 91.7 | 75.1 | 182 | 0.888 | 88.8 |
| 200 | −443 | 49.7 | 76.3 | 70.4 | 320 | 0.943 | 94.3 |
| 400 | −451 | 34.2 | 67.3 | 73.8 | 448 | 0.961 | 96.1 |
| T (K) | C (ppm) | Vcorr (g·m−2·h−1) | η |
|---|---|---|---|
| 298.15 | blank | 9.1 | / |
| 400 | 1.26 | 86.2 | |
| 308.15 | blank | 23 | / |
| 400 | 2.5 | 89.1 | |
| 318.15 | blank | 48 | / |
| 400 | 4.5 | 90.6 | |
| 328.15 | blank | 104 | / |
| 400 | 7.4 | 92.9 |
| C (ppm) | Ea (kJ·mol−1) | ΔH0 (kJ·mol−1) | ΔS0 (J·K−1·mol−1) |
|---|---|---|---|
| Blank | 65.38 | 62.79 | −15.71 |
| 400 | 47.94 | 45.34 | −90.62 |
| Elemental Composition (wt%) | Fe 2p | O 1s | N 1s | C 1s |
|---|---|---|---|---|
| 1 M HCl | 50.74 | 28.66 | 0.2 | 20.39 |
| 400 ppm PDA | 36.87 | 25.62 | 2.89 | 34.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, M.; Yu, Y.; Zheng, Y. Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots. Polymers 2021, 13, 1923. https://doi.org/10.3390/polym13121923
Cui M, Yu Y, Zheng Y. Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots. Polymers. 2021; 13(12):1923. https://doi.org/10.3390/polym13121923
Chicago/Turabian StyleCui, Mingjun, Yue Yu, and Yuxuan Zheng. 2021. "Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots" Polymers 13, no. 12: 1923. https://doi.org/10.3390/polym13121923
APA StyleCui, M., Yu, Y., & Zheng, Y. (2021). Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots. Polymers, 13(12), 1923. https://doi.org/10.3390/polym13121923





