Facile Synthesis of Thermoplastic Polyamide Elastomers Based on Amorphous Polyetheramine with Damping Performance
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
2.2. Characterization
2.2.1. Intrinsic Viscosity
2.2.2. Density Measurement
2.2.3. Nuclear Magnetic Resonance (NMR)
2.2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.2.5. Gel Permeation Chromatography (GPC)
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. Wide-Angle X-ray Diffraction (WAXD)
2.2.8. Small-Angle X-ray Scattering (SAXS)
2.2.9. Atomic Force Microscopy (AFM)
2.2.10. Transmission Electron Microscopy (TEM)
2.2.11. Dynamic Thermomechanical Analysis (DMA)
2.2.12. Mechanical Properties
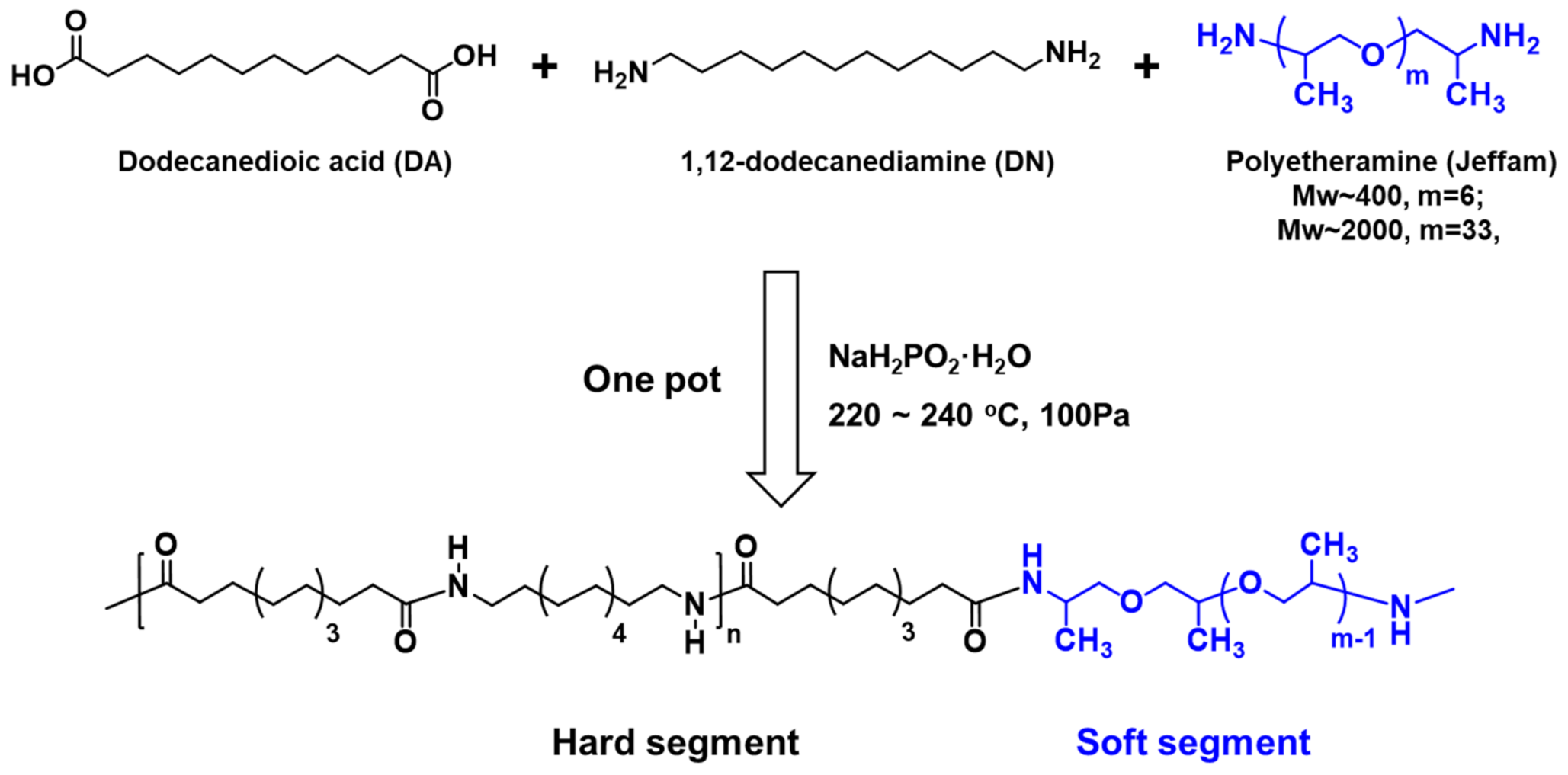
2.3. Synthesis of TPAEs
3. Results and Discussion
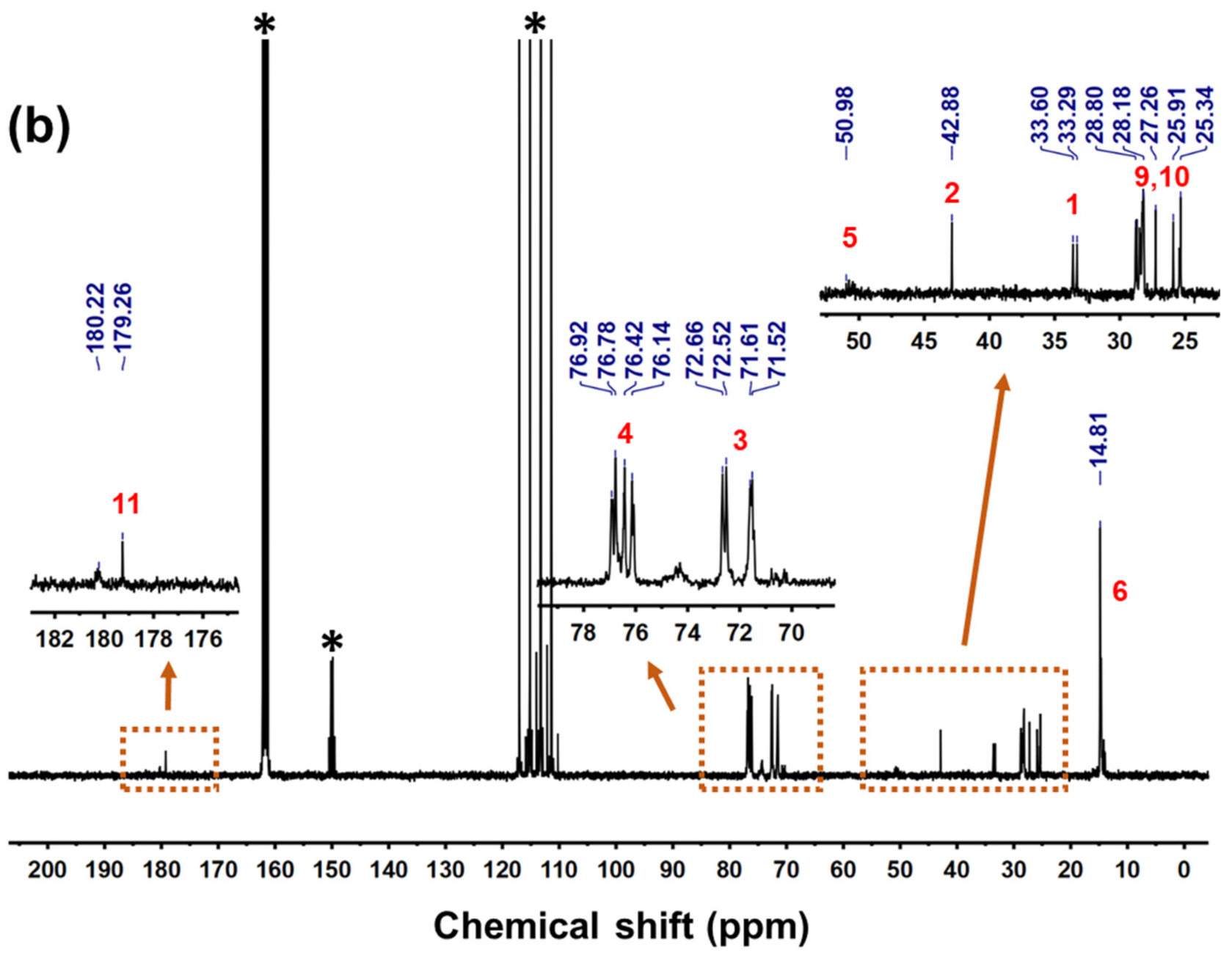
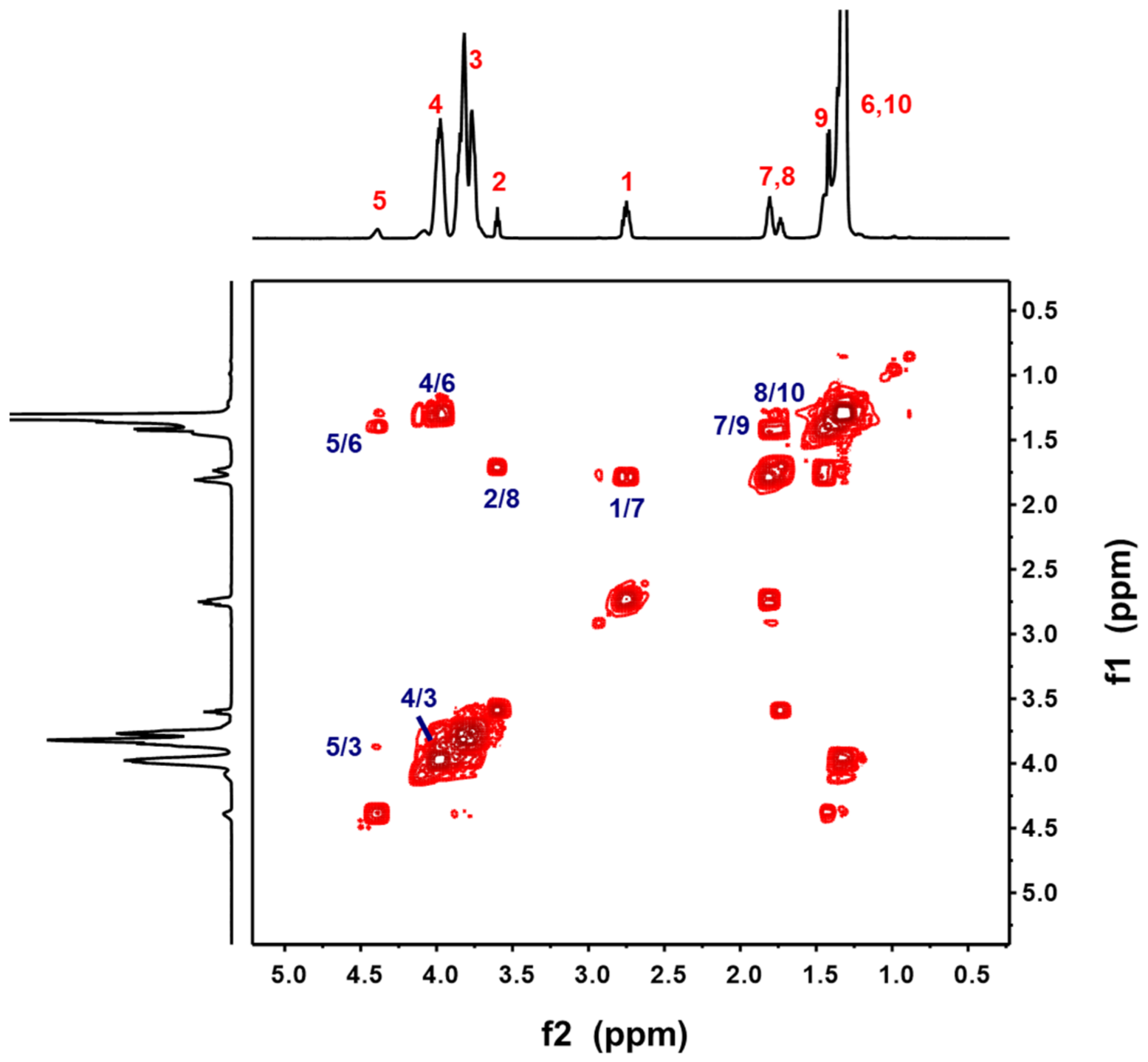
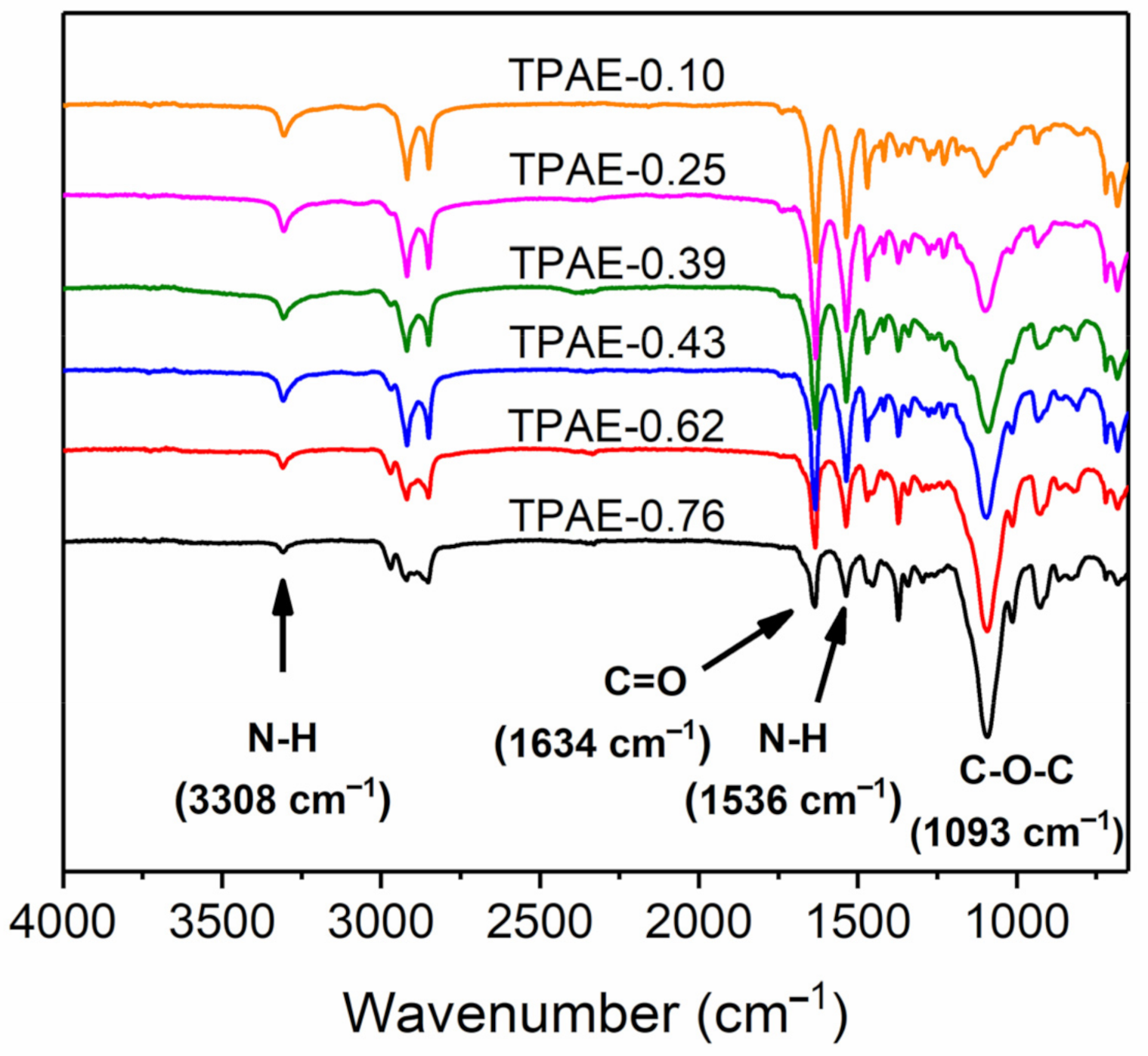
3.1. Synthesis and Structure Characterization of TPAEs
3.2. Thermal Characterization
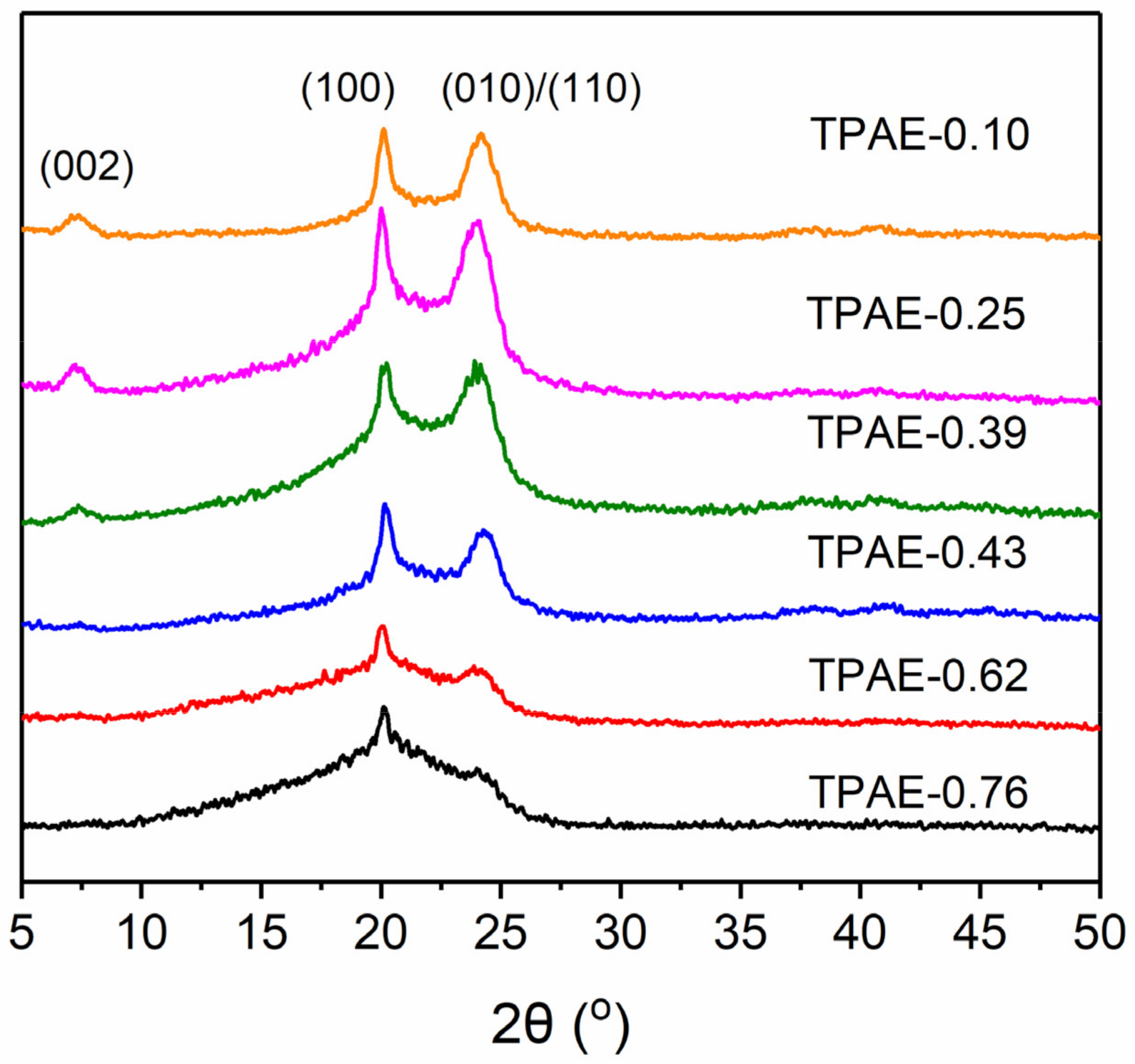
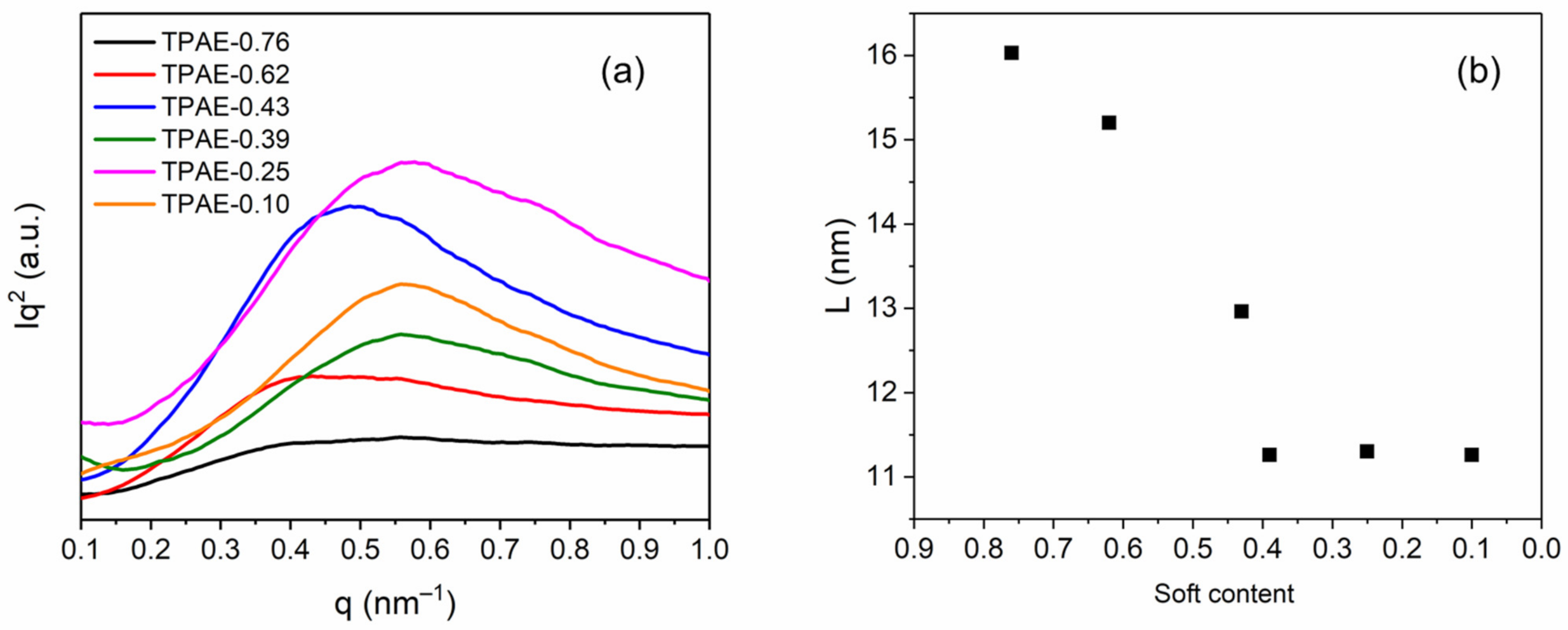
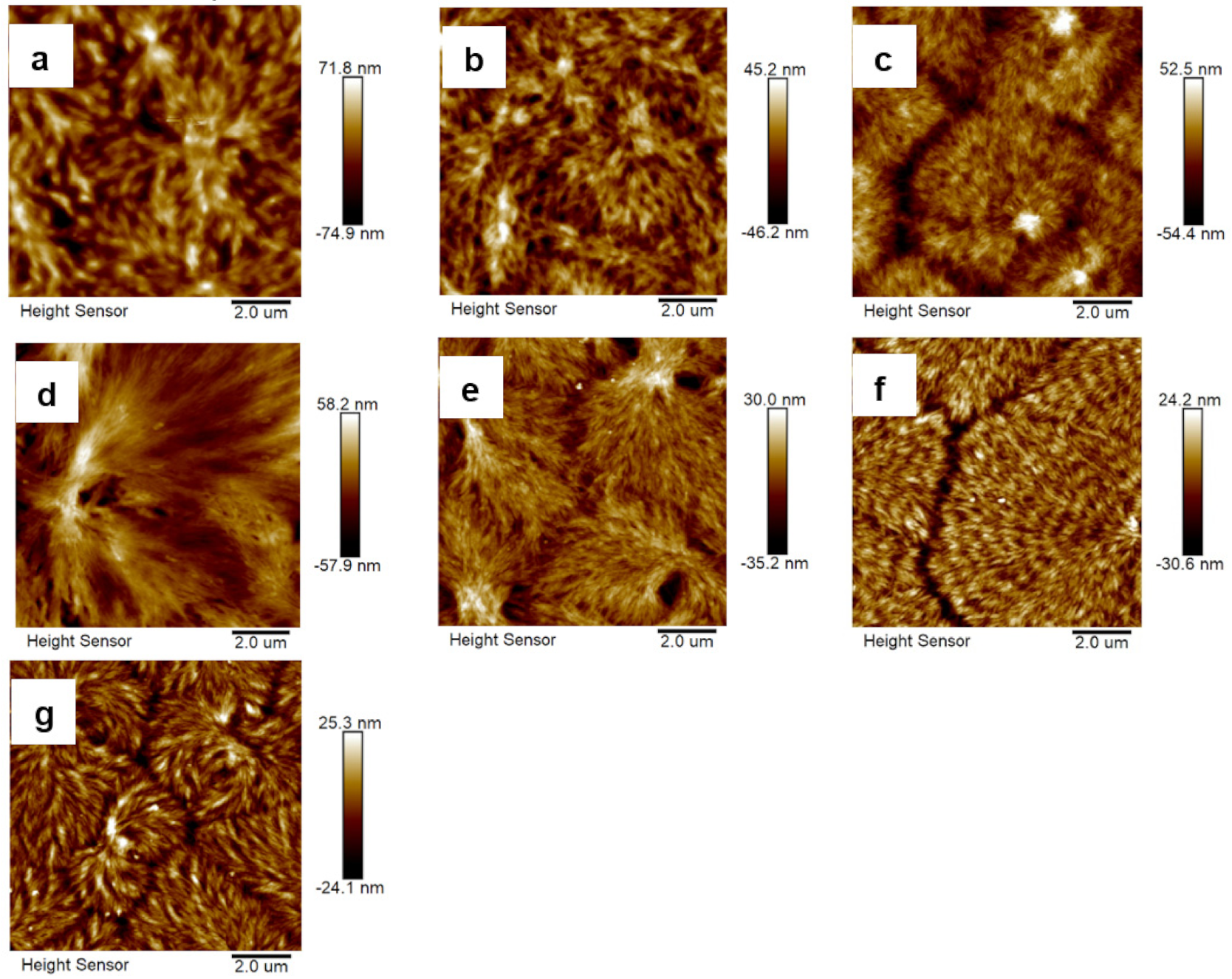
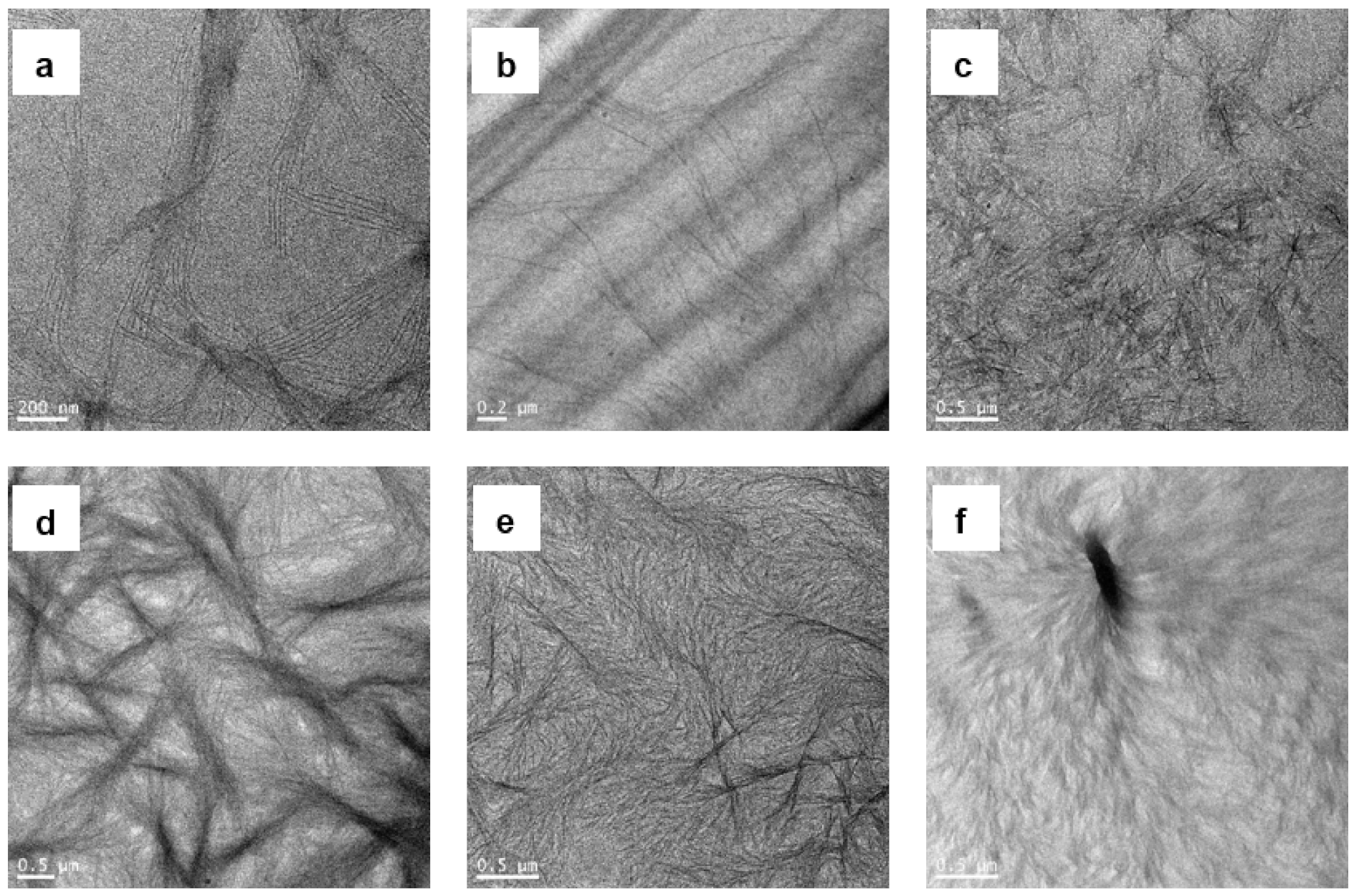
3.3. Morphological Characterizations
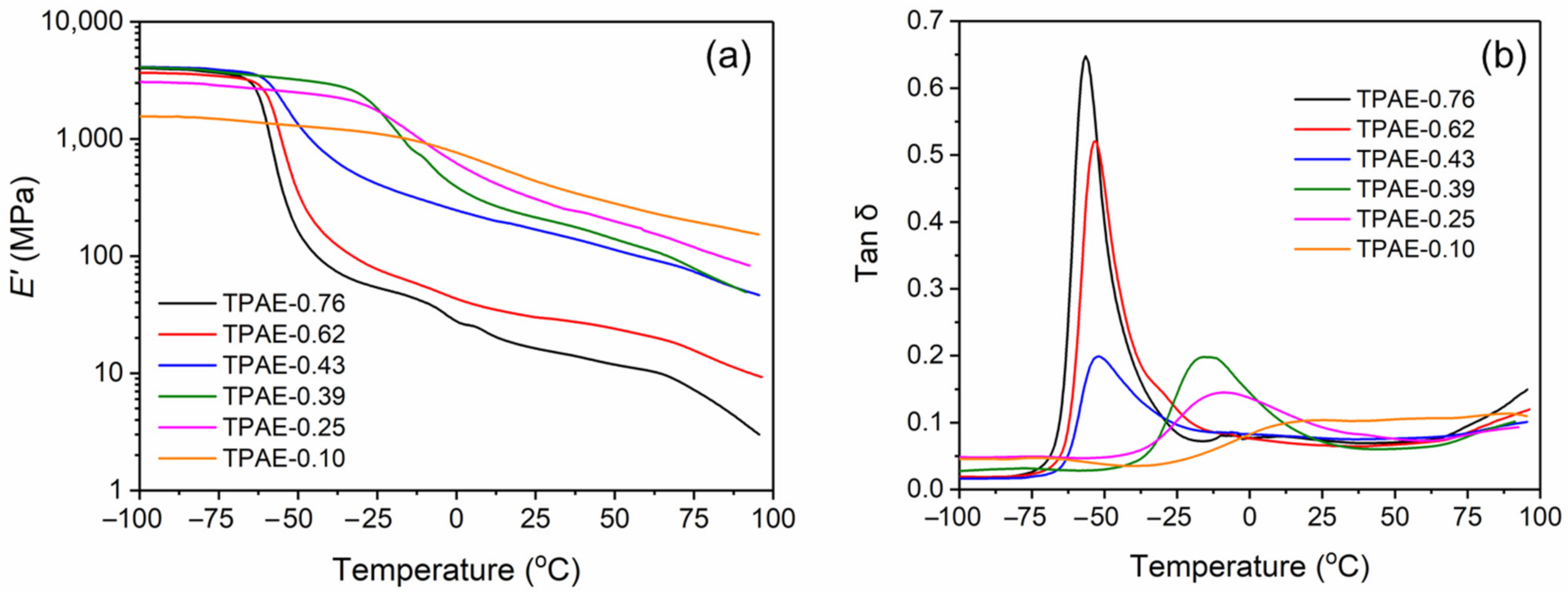
3.4. Dynamic Thermomechanical Analysis
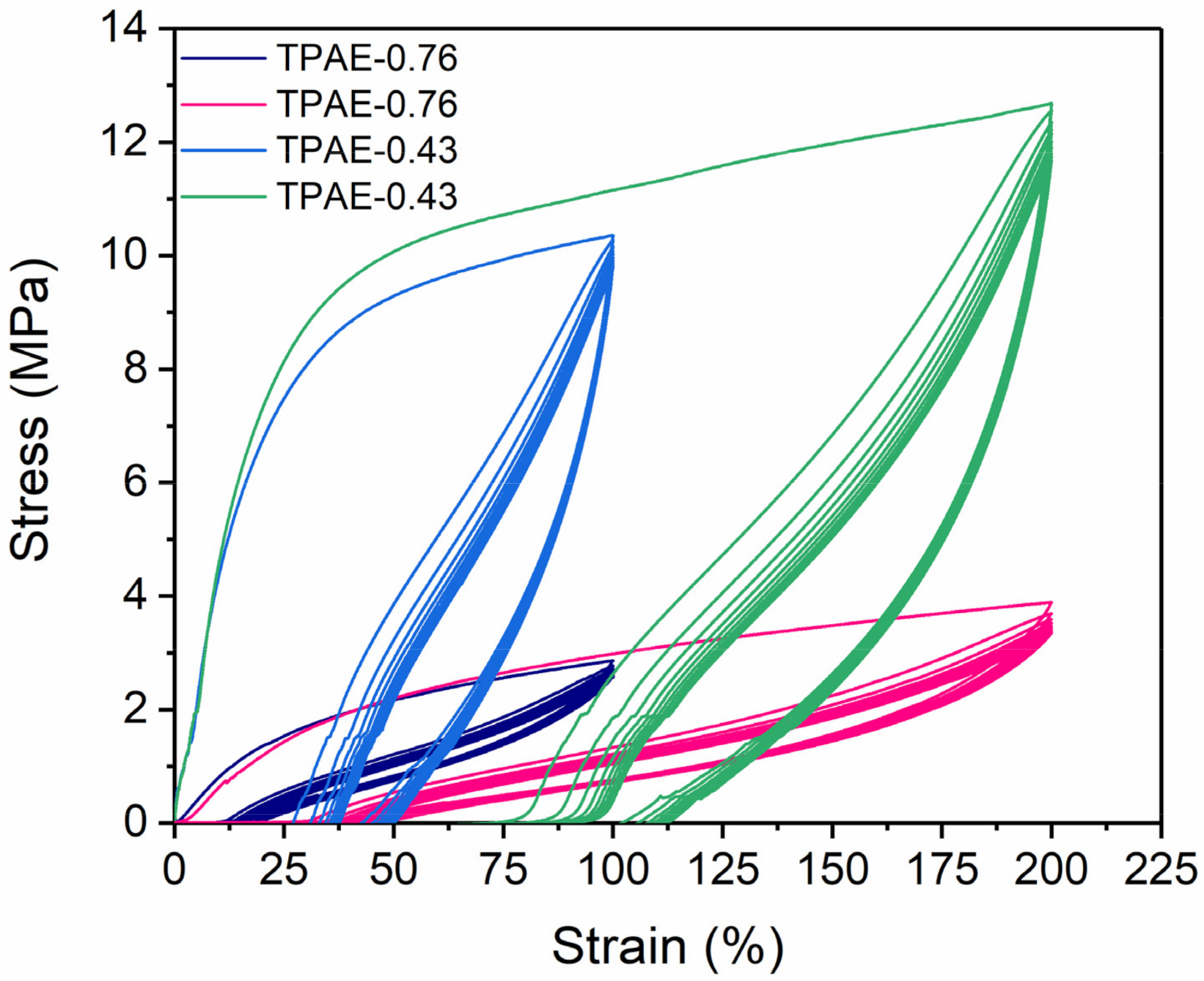
3.5. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Husken, D.; Feijen, J.; Gaymans, R.J. Hydrophilic segmented block copolymers based on poly(ethylene oxide) and monodisperse amide segments. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4522–4535. [Google Scholar] [CrossRef]
- Boulares, A.; Tessier, M.; Marechal, E. Synthesis and characterization of poly (copolyethers-block-polyamides) II. Characterization and properties of the multiblock copolymers. Polymer 2000, 41, 3561–3580. [Google Scholar] [CrossRef]
- Boulares, A.; Tessier, M.; MarÉChal, E. Synthesis and Characterization of Poly(Copolyethers-block-Polyamides). I. Structural Study of Polyether Precursors. J. Macromol. Sci. Part A 1998, 35, 933–953. [Google Scholar] [CrossRef]
- Barbi, V.; Funari, S.S.; Gehrke, R.; Scharnagl, N.; Stribeck, N. SAXS and the Gas Transport in Polyether-b lock-polyamide Copolymer Membranes. Macromolecules 2003, 36, 749–758. [Google Scholar] [CrossRef]
- Sheth, J.P.; Xu, J.; Wilkes, G.L. Solid state structure–property behavior of semicrystalline poly (ether-block-amide) PEBAX® thermoplastic elastomers. Polymer 2003, 44, 743–756. [Google Scholar] [CrossRef]
- Bai, L.; Hong, Z.; Wang, D.; Li, J.; Wang, X.; Pan, G.; Li, L.; Li, X. Deformation-induced phase transitions of polyamide 12 in its elastomer segmented copolymers. Polymer 2010, 51, 5604–5611. [Google Scholar] [CrossRef]
- Tavernier, B.; Mewis, J.; Van Puyvelde, P.; Takenaka, M.; Ernst, B.; Hashimoto, T. Effect of thermomechanical history on the crystallization of poly(ether-block-amide). Polym. Eng. Sci. 2008, 48, 2418–2425. [Google Scholar] [CrossRef] [Green Version]
- Sauer, B.B.; McLean, R.S.; Brill, D.J.; Londono, D.J. Morphology and orientation during the deformation of segmented elastomers studied with small-angle X-ray scattering and atomic force microscopy. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1727–1740. [Google Scholar] [CrossRef]
- Song, Y.; Yamamoto, H.; Nemoto, N. Segmental orientations and deformation mechanism of poly (ether-block-amide) films. Macromolecules 2004, 37, 6219–6226. [Google Scholar] [CrossRef]
- Fakirov, S. Handbook of Condensation Thermoplastic Elastomers; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; Chapter 9; pp. 243–257. [Google Scholar]
- Burns, G.T.; Tam, N. Is it the shoes? A simple proposal for regulating footwear in road running. Br. J. Sports Med. 2020, 54, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Shi, Z.; Li, N.; Qiu, J.; Xing, H.; Jiang, Z.; Li, M.; Tang, T. Novel Method for Preparing a High-Performance Auxetic Foam Directly from Polymer Resin by a One-Pot CO2 Foaming Process. ACS Appl. Mater. Interfaces 2020, 12, 48040–48048. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, A.; Li, Y.; Shao, L.; Zhang, H.; Xiang, X.; Wang, J.; Ren, Q.; Kang, S.; Dong, D. Lightweight, flexible and highly sensitive segregated microcellular nanocomposite piezoresistive sensors for human motion detection. Compos. Sci. Technol. 2021, 203, 108571. [Google Scholar] [CrossRef]
- Barzegari, M.R.; Hossieny, N.; Jahani, D.; Park, C.B. Characterization of hard-segment crystalline phase of poly(ether-block-amide) (PEBAX®) thermoplastic elastomers in the presence of supercritical CO2 and its impact on foams. Polymer 2017, 114, 15–27. [Google Scholar] [CrossRef]
- Armstrong, S.; Freeman, B.; Hiltner, A.; Baer, E. Gas permeability of melt-processed poly(ether block amide) copolymers and the effects of orientation. Polymer 2012, 53, 1383–1392. [Google Scholar] [CrossRef]
- Bondar, V.; Freeman, B.D.; Pinnau, I. Gas sorption and characterization of poly (ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2463–2475. [Google Scholar] [CrossRef]
- Liu, S.L.; Shao, L.; Chua, M.L.; Lau, C.H.; Wang, H.; Quan, S. Recent progress in the design of advanced PEO-containing membranes for CO2 removal. Prog. Polym. Sci. 2013, 38, 1089–1120. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Wang, Y.; Qiu, Y.; Li, H.; Hua, K.; Li, X.; Ji, J.; Deng, M. High CO2 separation performance of Pebax®/CNTs/GTA mixed matrix membranes. J. Membr. Sci. 2017, 521, 104–113. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separation. J. Membr. Sci. 2008, 307, 88–95. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Dong, G.; Scholes, C.; Chen, V. Effect of Fabrication and Operation Conditions on CO2 Separation Performance of PEO–PA Block Copolymer Membranes. Ind. Eng. Chem. Res. 2015, 54, 7273–7283. [Google Scholar] [CrossRef]
- Wang, J.; Bao, L.; Zhao, H.; Lei, J. Preparation and characterization of permanently anti-static packaging composites composed of high impact polystyrene and ion-conductive polyamide elastomer. Compos. Sci. Technol. 2012, 72, 976–981. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, Q.; Pan, X.; Xi, Z.; Zhao, L.; Yuan, W. Structure and Morphology of Thermoplastic Polyamide Elastomer Based on Long-Chain Polyamide 1212 and Renewable Poly(trimethylene glycol). Ind. Eng. Chem. Res. 2020, 59, 17502–17512. [Google Scholar] [CrossRef]
- Xu, J.; Chen, W.; Wang, C.; Zheng, M.; Ding, C.; Jiang, W.; Tan, L.; Fu, J. Extremely stretchable, self-healable elastomers with tunable mechanical properties: Synthesis and applications. Chem. Mater. 2018, 30, 6026–6039. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Chen, J.; Hu, P.; Wang, Y.; Jiang, W.; Fu, J. Transparent, Mechanically Strong, Extremely Tough, Self-Recoverable, Healable Supramolecular Elastomers Facilely Fabricated via Dynamic Hard Domains Design for Multifunctional Applications. Adv. Funct. Mater. 2019, 30, 1907109. [Google Scholar] [CrossRef]
- Nohales, A.; Solar, L.; Porcar, I.; Vallo, C.I.; Gómez, C.M. Morphology, flexural, and thermal properties of sepiolite modified epoxy resins with different curing agents. Eur. Polym. J. 2006, 42, 3093–3101. [Google Scholar] [CrossRef]
- Strachota, A.; Kroutilová, I.; Kovářová, J.; Matějka, L. Epoxy networks reinforced with polyhedral oligomeric silsesquioxanes (POSS). Thermomechanical properties. Macromolecules 2004, 37, 9457–9464. [Google Scholar] [CrossRef]
- Mo, R.; Song, L.; Hu, J.; Sheng, X.; Zhang, X. An acid-degradable biobased epoxy-imine adaptable network polymer for the fabrication of responsive structural color film. Polym. Chem. 2020, 11, 974–981. [Google Scholar] [CrossRef]
- Deliballi, Z.; Kiskan, B.; Yagci, Y. Main-chain benzoxazine precursor block copolymers. Polym. Chem. 2018, 9, 178–183. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, H.; Sablong, R.J.; Koning, C.E.; van Benthem, R. t-Butyl-Oxycarbonylated Diamines as Building Blocks for Isocyanate-Free Polyurethane/Urea Dispersions and Coatings. Macromol. Rapid Commun. 2018, 39, 1800004. [Google Scholar] [CrossRef]
- Xu, S.; Ye, L. Synthesis and properties of monomer cast nylon-6-b-polyether amine copolymers with different structures. RSC Adv. 2015, 5, 32460–32468. [Google Scholar] [CrossRef]
- Van Hutten, P.; Walch, E.; Veeken, A.; Gaymans, R. Segmented block copolymers based on polyamide-4, 6 and poly (propylene oxide). Polymer 1990, 31, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.C.; Jo, W.H. Segmented block copolyetheramides based on nylon 6 and polyoxypropylene. I. Synthesis and characterization. J. Appl. Polym. Sci. 1994, 54, 585–591. [Google Scholar] [CrossRef]
- Yu, Y.C.; Jo, W.H. Segmented block copolyetheramides based on nylon 6 and polyoxypropylene. II. Structure and properties. J. Appl. Polym. Sci. 1995, 56, 895–904. [Google Scholar] [CrossRef]
- Yu, Y.C.; Jo, W.H.; Lee, M.S. Segmented block copolyetheramides based on nylon 6 and polyoxypropylene. III. SAXS analysis. J. Appl. Polym. Sci. 1997, 64, 2155–2163. [Google Scholar]
- Mori, T.; Masukawa, S.; Kikkawa, T.; Fujimori, A.; Satoh, A.; Matsumoto, K.; Jikei, M.; Oishi, Y.; Shibasaki, Y. Rapid synthesis and properties of segmented block copolymers based on monodisperse aromatic poly(N-methyl benzamide) and poly(propylene oxide). RSC Adv. 2017, 7, 33812–33821. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Yang, X.; Zhang, Y.; Zheng, Y.; He, X.; Luo, J. Random and block copolymer membranes based on flexible etheric-aliphatic soft segments designed for CO2/CH4 separation. J. Nat. Gas Sci. Eng. 2018, 54, 92–101. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, X.; Zheng, Y.; He, X.; Chen, F.; Luo, J. Preparation of poly(ether-block-amide)/poly(amide-co-poly(propylene glycol)) random copolymer blend membranes for CO2/N2 separation. Polym. Eng. Sci. 2019, 59, E14–E23. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Q.; Wang, Y.; Zhang, C.; Mo, Z.; Cao, S. Melting behaviors, isothermal and non-isothermal crystallization kinetics of nylon 1212. Polymer 2003, 44, 2537–2545. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, S.; Wu, D.; Wang, X.; Yu, J.; Chen, L.; Li, F. Facile synthesis of polyamide 6 (PA6)-based thermoplastic elastomers with a well-defined microphase separation structure by melt polymerization. Polym. Chem. 2018, 9, 1327–1336. [Google Scholar] [CrossRef]
- Ren, M.; Mo, Z.; Chen, Q.; Song, J.; Wang, S.; Zhang, H.; Zhao, Q. Crystallization kinetics and morphology of nylon 1212. Polymer 2004, 45, 3511–3518. [Google Scholar] [CrossRef]
- Zhu, P.; Dong, X.; Cao, Y.; Wang, L.; Liu, X.; Wang, Z.; Wang, D. The Brill transition in polyether-b-amide segmented copolymers and composition dependence. Eur. Polym. J. 2017, 93, 334–346. [Google Scholar] [CrossRef]
- Song, J.; Zhang, H.; Ren, M.; Chen, Q.; Sun, X.; Wang, S.; Zhang, H.; Mo, Z. Crystal Transition of Nylon-12,12 under Drawing and Annealing. Macromol. Rapid Commun. 2005, 26, 487–490. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.; Luo, J.; Zhao, C.; Han, C.C. Multilength Scale Studies of the Confined Crystallization in Poly(l-lactide)-block-Poly(ethylene glycol) Copolymer. Macromolecules 2012, 45, 4254–4261. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, P.; Wang, Z.; Zhou, Y.; Chen, H.; Müller, A.J.; Wang, D.; Dong, X. Influence of soft block crystallization on microstructural variation of double crystalline poly(ether-mb-amide) multiblock copolymers. Polym. Cryst. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Chi, D.; Liu, F.; Na, H.; Chen, J.; Hao, C.; Zhu, J. Poly(neopentyl glycol 2,5-furandicarboxylate): A Promising Hard Segment for the Development of Bio-based Thermoplastic Poly(ether-ester) Elastomer with High Performance. ACS Sustain. Chem. Eng. 2018, 6, 9893–9902. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. New hybrid latexes from a soybean oil-based waterborne polyurethane and acrylics via emulsion polymerization. Biomacromolecules 2007, 8, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, X.; Tao, G.; Guo, J.; Jiang, H. Damping elastomer with broad temperature range based on irregular networks formed by end-linking of hydroxyl-terminated poly(dimethylsiloxane). Polym. Eng. Sci. 2016, 56, 97–102. [Google Scholar] [CrossRef]
- Zhao, X.; Shou, T.; Liang, R.; Hu, S.; Yu, P.; Zhang, L. Bio-based thermoplastic polyurethane derived from polylactic acid with high-damping performance. Ind. Crop. Prod. 2020, 154, 112619. [Google Scholar] [CrossRef]
- Sijbrandi, N.J.; Kimenai, A.J.; Mes, E.P.C.; Broos, R.; Bar, G.; Rosenthal, M.; Odarchenko, Y.; Ivanov, D.A.; Dijkstra, P.J.; Feijen, J. Synthesis, Morphology, and Properties of Segmented Poly(ether amide)s with Uniform Oxalamide-Based Hard Segments. Macromolecules 2012, 45, 3948–3961. [Google Scholar] [CrossRef]



| Sample Code | Feed Ratio of DA/DN/Jeffam (mol/mol) | MW of Jeffam (g/mol) | Calculated MW of PA1212 Segment (g/mol) | [η] (dL/g) | Density (g/cm3) | Soft Content in feed | Soft Content by NMR | Mn a (×104) (g/mol) | Mw a (×104) (g/mol) | PDI a |
|---|---|---|---|---|---|---|---|---|---|---|
| TPAE-0.76 | 2/1/1 | 2000 | 624 | 1.26 | 1.01 | 0.77 | 0.76 | 2.34 | 5.48 | 2.34 |
| TPAE-0.62 | 3/2/1 | 2000 | 1018 | 1.39 | 1.01 | 0.66 | 0.62 | 3.02 | 7.87 | 2.61 |
| TPAE-0.43 | 8/7/1 | 2000 | 2988 | 1.36 | 1.02 | 0.45 | 0.43 | 3.82 | 8.83 | 2.30 |
| TPAE-0.39 | 2/1/1 | 400 | 624 | 1.32 | 1.03 | 0.40 | 0.39 | 3.55 | 7.56 | 2.13 |
| TPAE-0.25 | 3/2/1 | 400 | 1018 | 1.43 | 1.02 | 0.27 | 0.25 | 4.50 | 11.09 | 2.47 |
| TPAE-0.10 | 8/7/1 | 400 | 2988 | 1.17 | 1.02 | 0.12 | 0.10 | 3.75 | 9.22 | 2.46 |
| Sample Code | Tm (°C) | ΔHm J/g | Tc (°C) | ΔHc J/g | Xc (%) | Tg (DSC) (°C) | Tg (DMA) (°C) | E’ at −25 °C (MPa) | E’ at 25 °C (MPa) |
|---|---|---|---|---|---|---|---|---|---|
| TPAE-0.76 | 170.8 | 6.65 | 133.7 | 6.88 | 9.5 | −60.3 | −56.4 | 54.0 | 16.3 |
| TPAE-0.62 | 177.4 | 16.06 | 137.9 | 16.22 | 14.5 | −60.1 | −53.6 | 77.4 | 30.0 |
| TPAE-0.43 | 176.9 | 37.65 | 147.1 | 36.67 | 22.6 | −60.8 | −51.8 | 414.4 | 168.7 |
| TPAE-0.39 | 156.2 | 35.00 | 111.2 | 35.37 | 19.6 | −19.5 | −15.7 | 2378.1 | 213.8 |
| TPAE-0.25 | 163.3 | 43.48 | 127.9 | 43.46 | 19.8 | −10.2 | −9.2 | 2008.5 | 306.7 |
| TPAE-0.10 | 176.8 | 54.03 | 140.6 | 55.70 | 20.5 | N.D. | N.D. | 1154.1 | 436.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Tang, Q.; Pan, X.; Li, J.; Zhao, L.; Xi, Z.; Yuan, W. Facile Synthesis of Thermoplastic Polyamide Elastomers Based on Amorphous Polyetheramine with Damping Performance. Polymers 2021, 13, 2645. https://doi.org/10.3390/polym13162645
Jiang J, Tang Q, Pan X, Li J, Zhao L, Xi Z, Yuan W. Facile Synthesis of Thermoplastic Polyamide Elastomers Based on Amorphous Polyetheramine with Damping Performance. Polymers. 2021; 13(16):2645. https://doi.org/10.3390/polym13162645
Chicago/Turabian StyleJiang, Jie, Qiuyu Tang, Xun Pan, Jinjin Li, Ling Zhao, Zhenhao Xi, and Weikang Yuan. 2021. "Facile Synthesis of Thermoplastic Polyamide Elastomers Based on Amorphous Polyetheramine with Damping Performance" Polymers 13, no. 16: 2645. https://doi.org/10.3390/polym13162645
APA StyleJiang, J., Tang, Q., Pan, X., Li, J., Zhao, L., Xi, Z., & Yuan, W. (2021). Facile Synthesis of Thermoplastic Polyamide Elastomers Based on Amorphous Polyetheramine with Damping Performance. Polymers, 13(16), 2645. https://doi.org/10.3390/polym13162645








