Thermal and Dielectric Properties of Cyanate Ester Cured Main Chain Rigid-Rod Epoxy Resin
Abstract
:1. Introduction
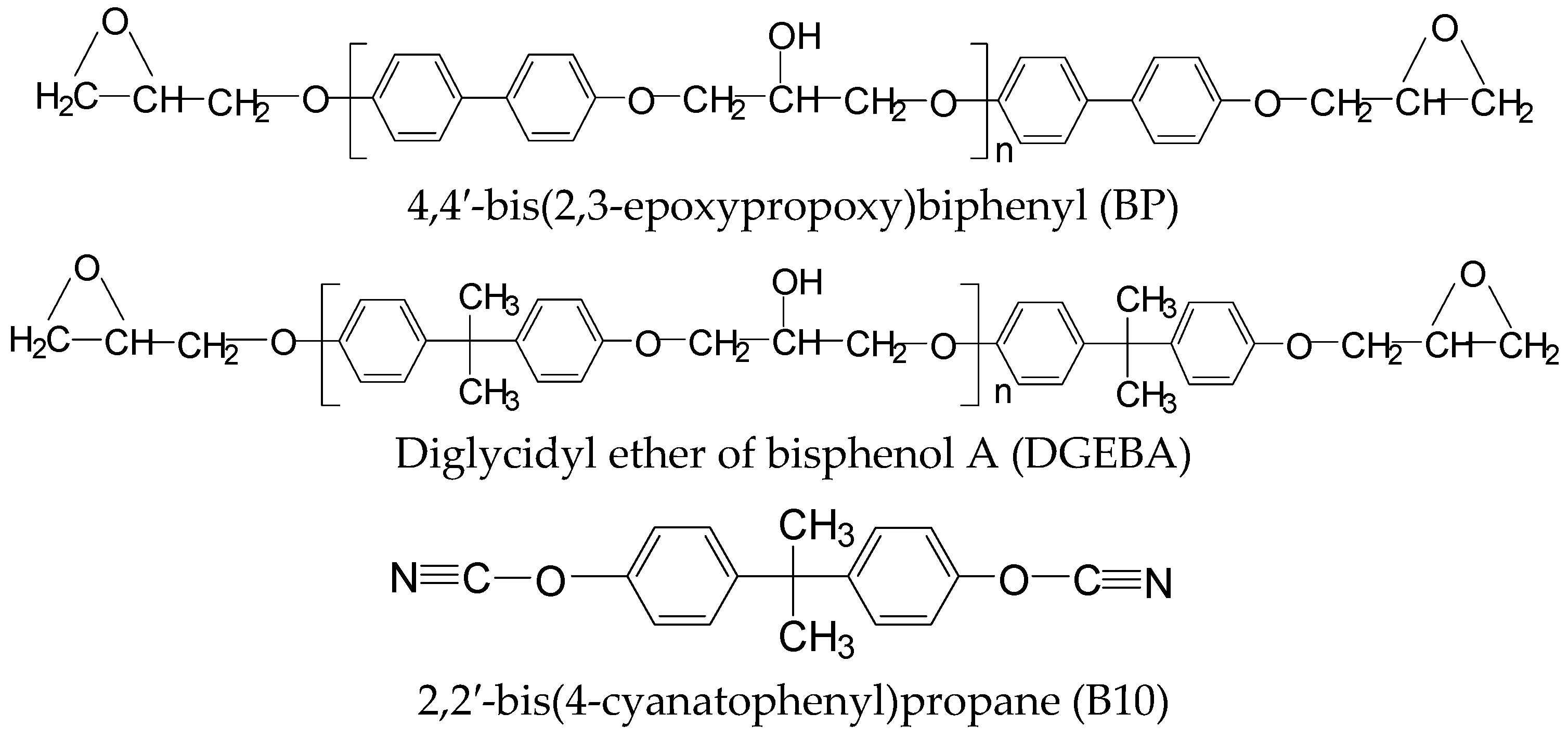
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cured Epoxy
2.2.1. DGEBA/B10 System
2.2.2. BP/B10 System
2.3. Morphological Observation of the Epoxy Resin
2.4. XRD Measurement
2.5. Thermal Properties
2.6. Dielectric Properties
3. Results and Discussion
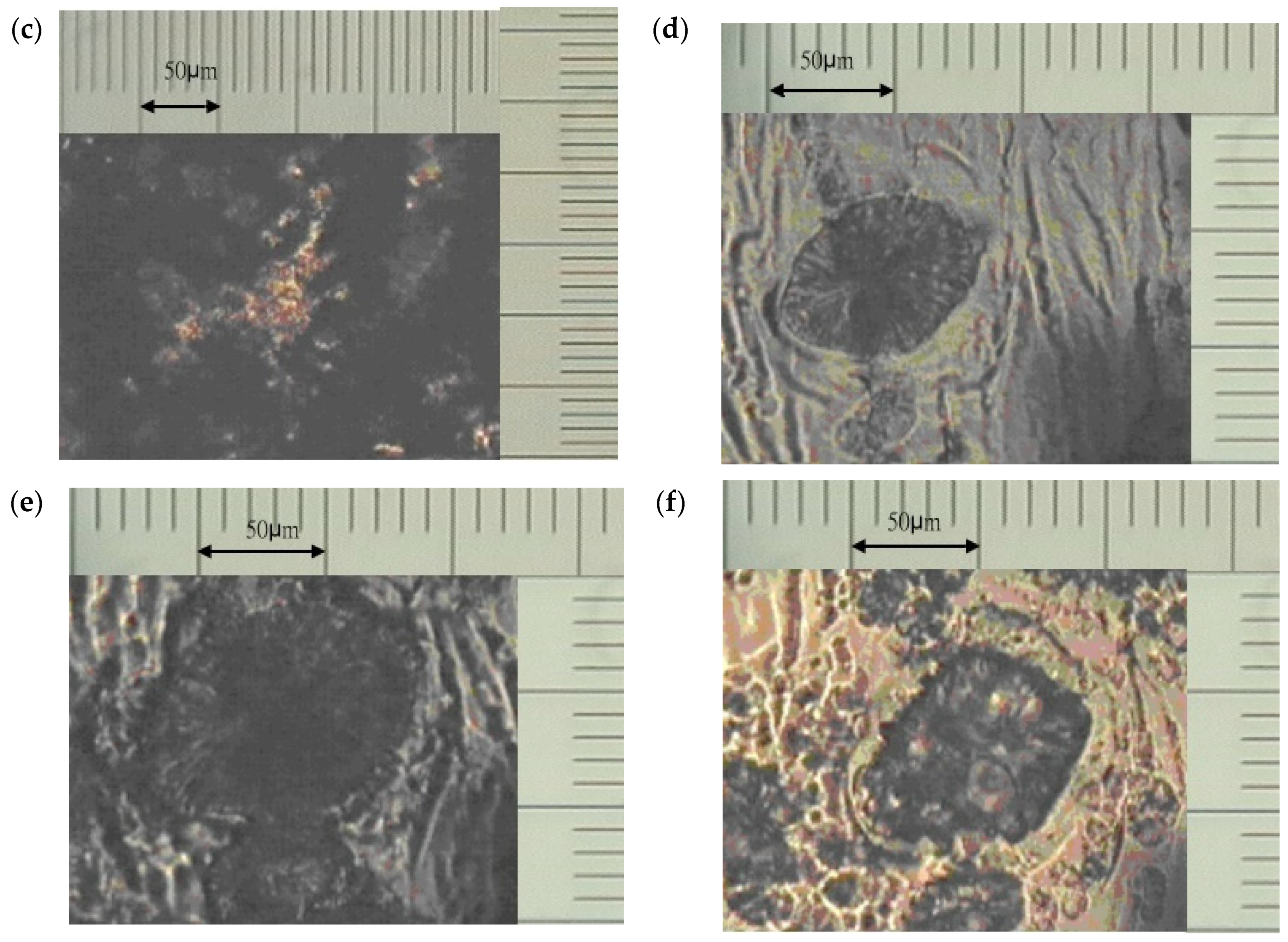
3.1. Morphological Observation
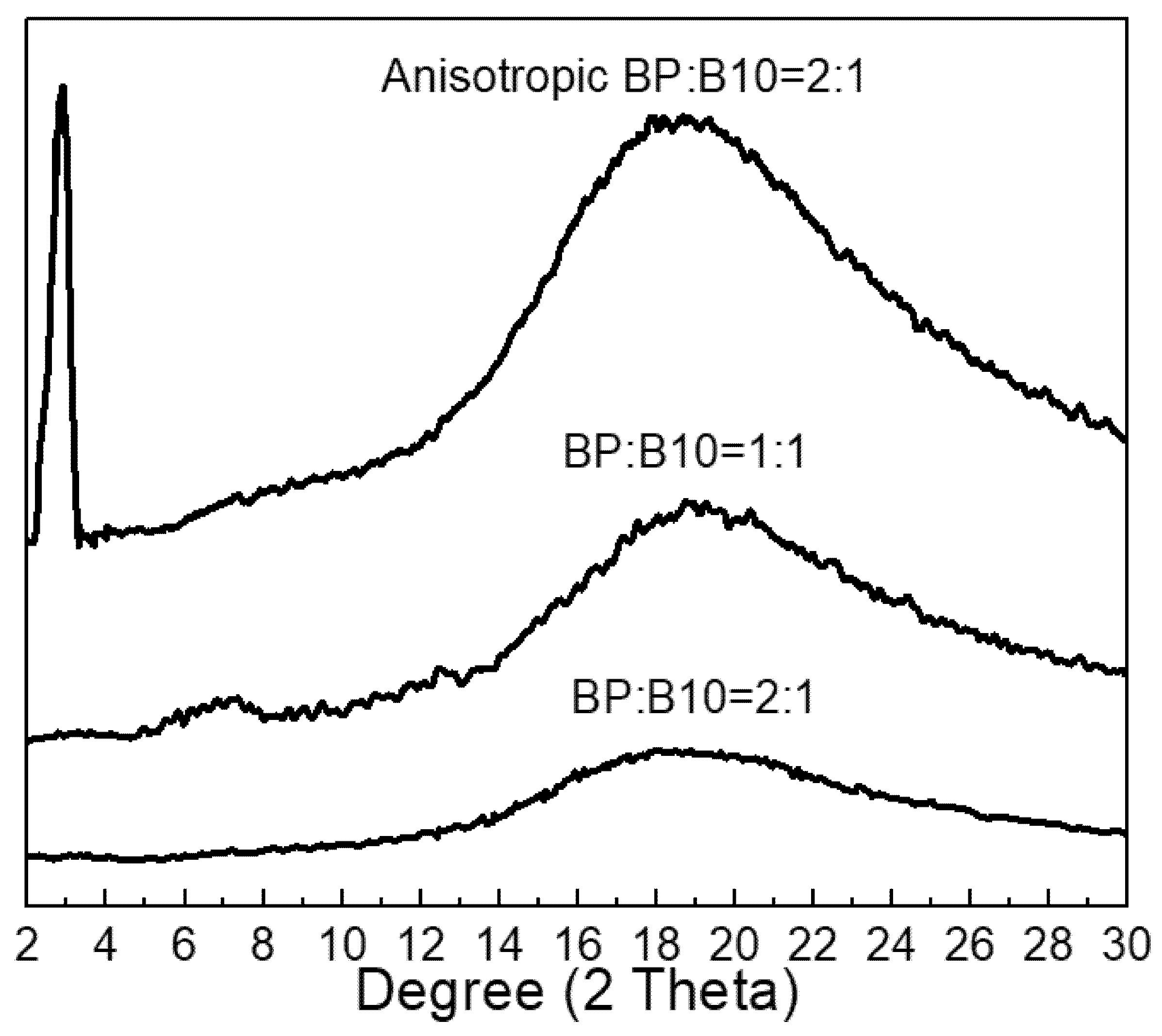
3.2. XRD Analysis
3.3. TGA Analysis
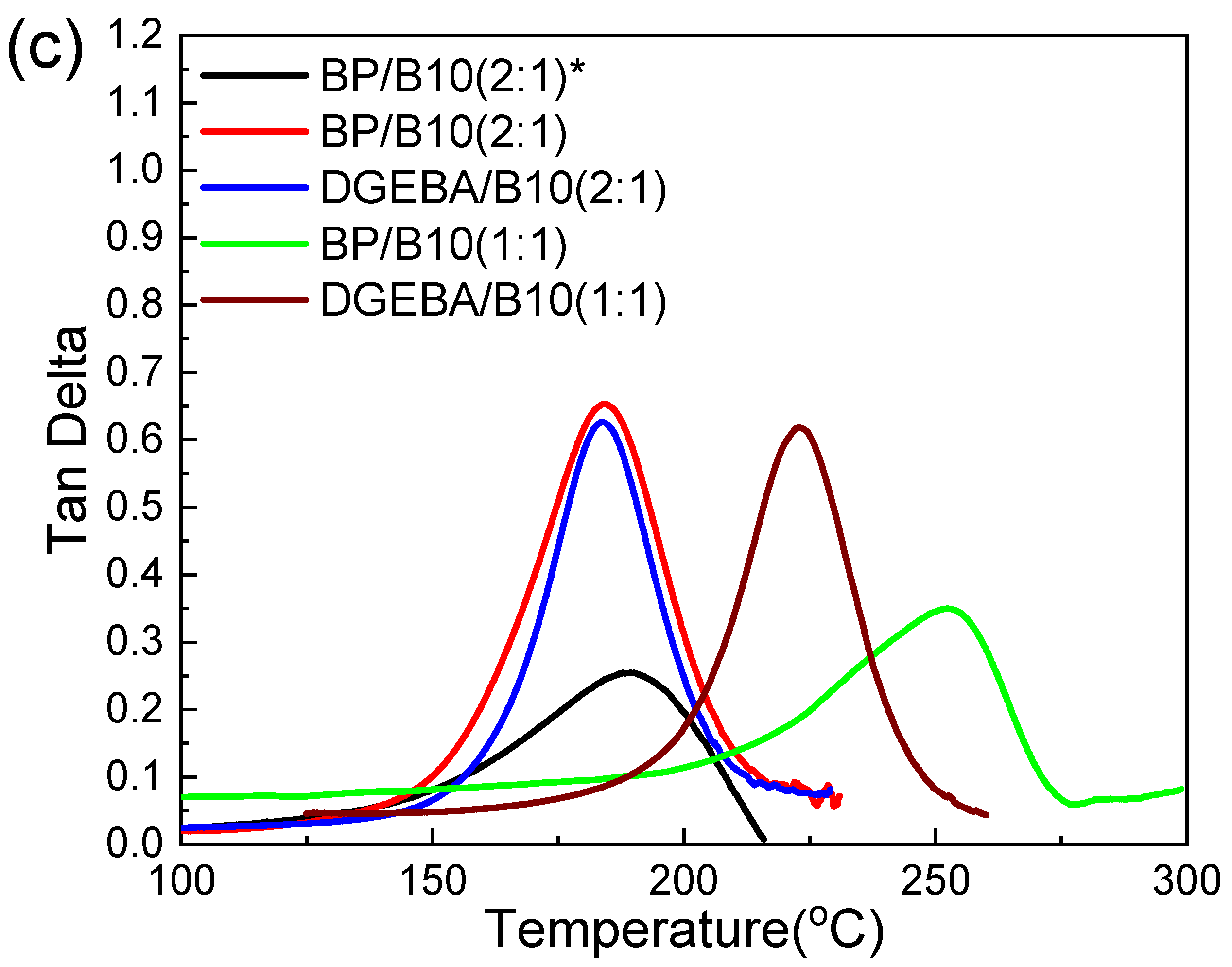
3.4. DMA Analysis
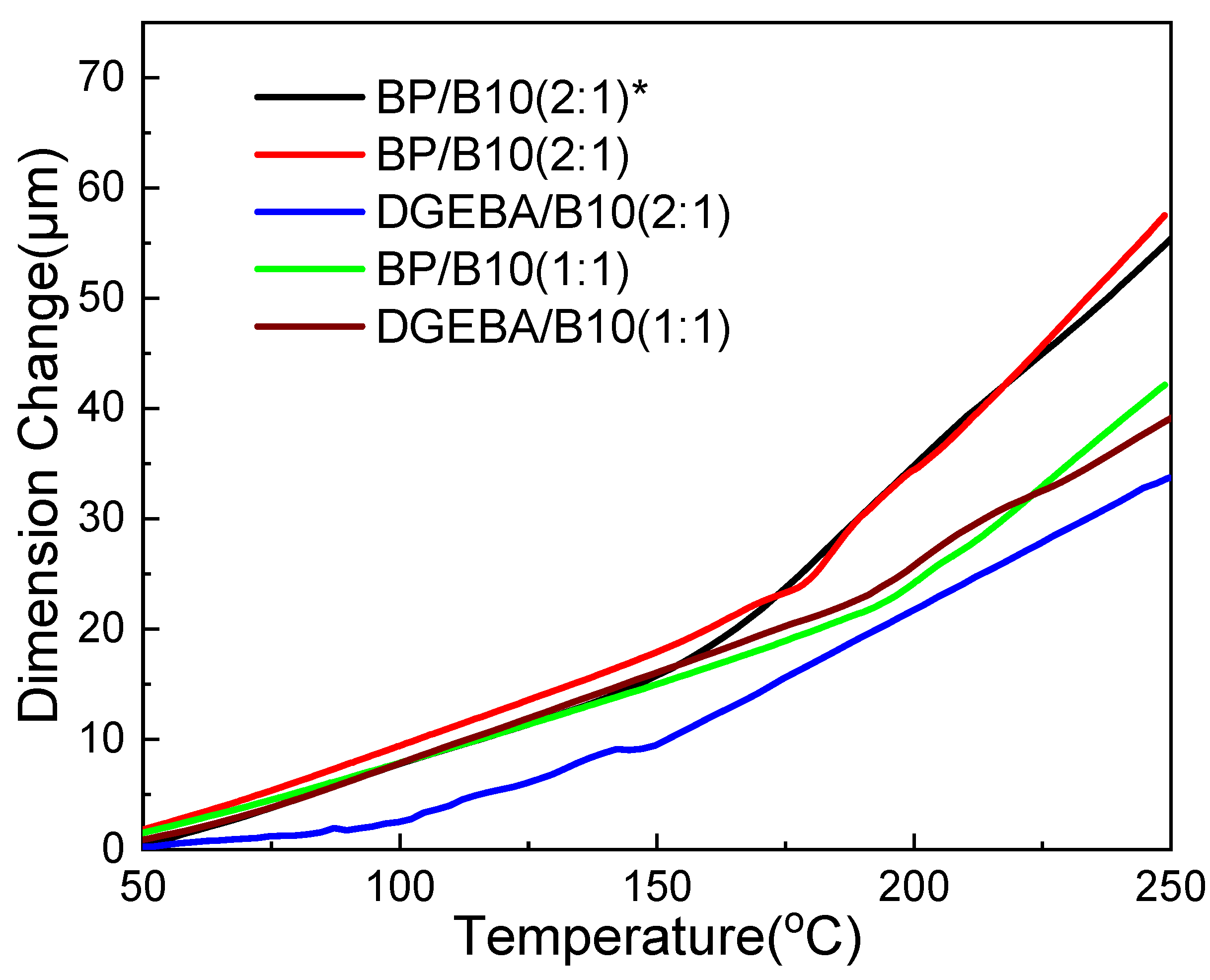
3.5. TMA Analysis
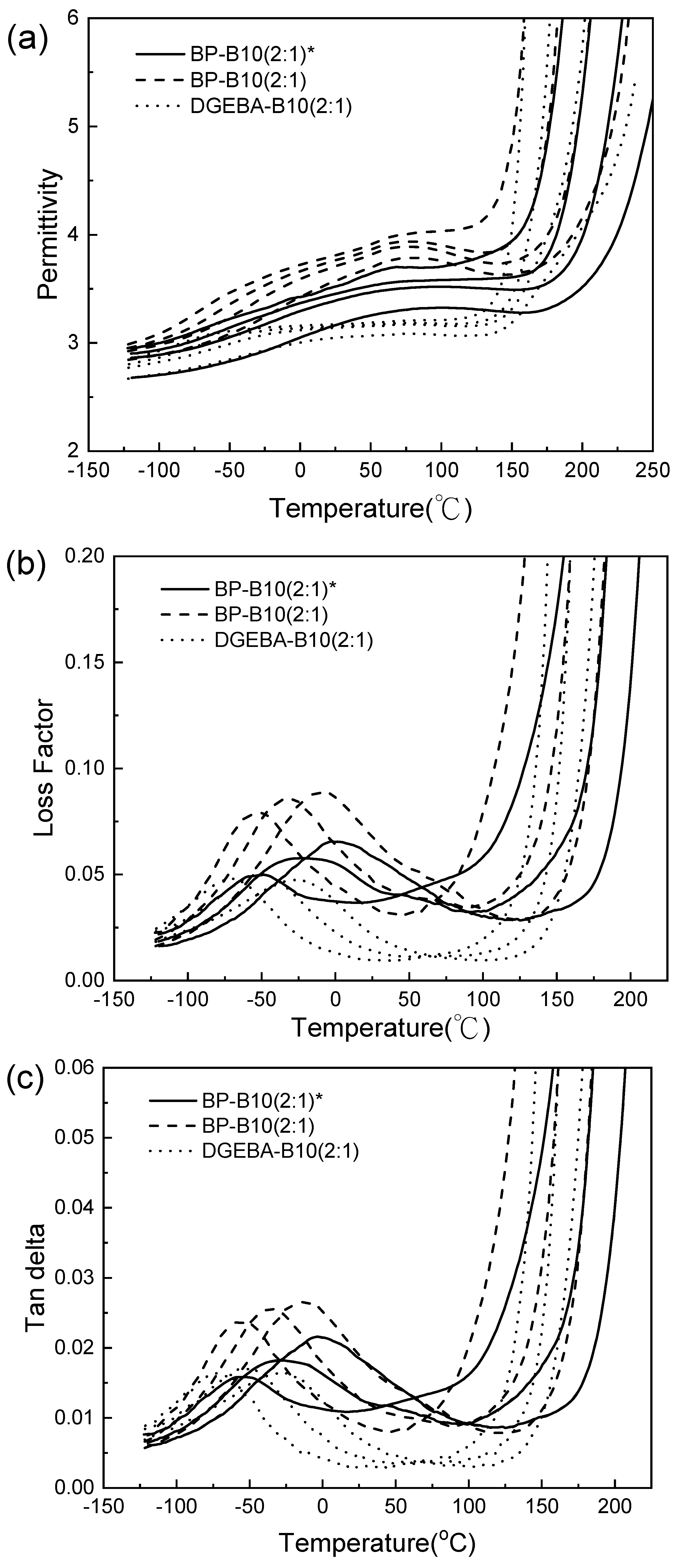
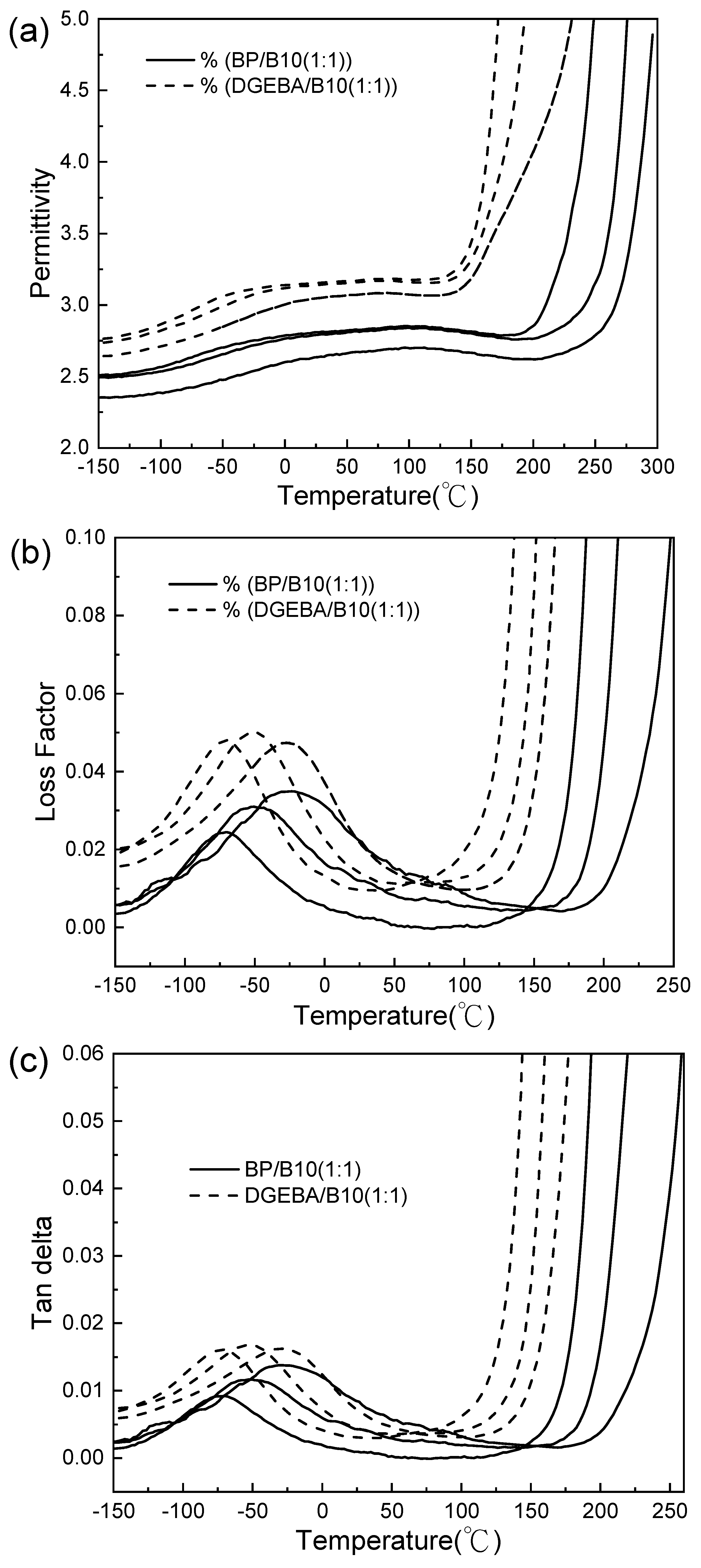
3.6. DEA Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gu, J.; Liang, C.; Zhao, X.; Gan, B.; Qiu, H.; Guo, Y.; Yang, X.; Zhang, Q.; Wang, D.Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Huo, S.; Zhang, B.; Yang, S. Preparation and flame retardancy of DOPO-based epoxy resin containing bismaleimide. High Perform. Polym. 2016, 28, 1090–1095. [Google Scholar] [CrossRef]
- Gong, Y.; Zhou, W.; Sui, X.; Kou, Y.; Xu, L.; Cai, H.; Liu, X.; Chen, Q. Mechanical and dielectric properties of epoxy composites filled with hybrid aluminum particles with binary size distribution. High Perform. Polym. 2019, 31, 124–134. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, J.; Tang, Y. Synthesis of a phosphorus/nitrogen-containing compound based on maleimide and cyclotriphosphazene and its flame-retardant mechanism on epoxy resin. Polym. Degrad. Stab. 2016, 126, 9–16. [Google Scholar] [CrossRef]
- da Silva, T.T.; da Silveira, P.H.P.M.; Ribeiro, M.P.; Lemos, M.F.; da Silva, A.P.; Monteiro, S.N.; Nascimento, L.F.C. Thermal and Chemical Characterization of Kenaf Fiber (Hibiscus cannabinus) Reinforced Epoxy Matrix Composites. Polymers 2021, 13, 2016. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Ali, M.U.; Hussain, S.J.; Ahmad, M.S.; Zafar, A.; Ghafoor, U.; Subhani, T. Improved Ablative Properties of Nanodiamond-Reinforced Carbon Fiber–Epoxy Matrix Composites. Polymers 2021, 13, 2035. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a Novel Flame Retardant Containing Phosphorus, Nitrogen and Boron and Its Application in Flame-Retardant Epoxy Resin; Elsevier: Amsterdam, The Netherlands, 2016; Volume 133, ISBN 8627876517. [Google Scholar]
- Morkavuk, S.; Köklü, U.; Bağcı, M.; Gemi, L. Cryogenic machining of carbon fiber reinforced plastic (CFRP) composites and the effects of cryogenic treatment on tensile properties: A comparative study. Compos. Part B Eng. 2018, 147, 1–11. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Vertuccio, L.; Simonet, B.; Santos, B.; Zarrelli, M.; Arena, M.; Viscardi, M. Different methods of dispersing carbon nanotubes in epoxy resin and initial evaluation of the obtained nanocomposite as a matrix of carbon fiber reinforced laminate in terms of vibroacoustic performance and flammability. Materials 2019, 12, 2998. [Google Scholar] [CrossRef] [Green Version]
- Barra, G.; Vertuccio, L.; Naddeo, C.; Arena, M.; Viscardi, M.; Guadagno, L. Thermal degradation and fire properties of epoxy modified resins. AIP Conf. Proc. 2018, 1981, 20149. [Google Scholar]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Toughening of Petroleum Based (DGEBA) Epoxy Resins with Various Renewable Resources Based Flexible Chains for High Performance Applications: A Review. Ind. Eng. Chem. Res. 2018, 57, 2711–2726. [Google Scholar] [CrossRef]
- Shirude, S.R.; Shambharkar, S.Y.; Bhosale, H.B.; Patil, V.U.; Patil, A.G. A Survey on Epoxy Resins. Int. J. Innov. Res. Sci. Eng. Technol. ISO 2007, 3297, 14861–14867. [Google Scholar]
- Su, W.F.; Huang, H.W.; Pan, W.P. Thermal properties of rigid rod epoxies cured with diaminodiphenylsulfone and dicyandiamide. Thermochim. Acta 2002, 392, 391–394. [Google Scholar] [CrossRef]
- Su, W.F.A.; Schoch, K.F.; Smith, J.D.B. Comparison of cure conditions for rigid rod epoxy and bisphenol A epoxy using thermomechanical analysis. J. Appl. Polym. Sci. 1998, 70, 2163–2167. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Wu, K.; Shi, J.; Liang, L.; Lu, M. Biphenyl liquid crystal epoxy containing flexible chain: Synthesis and thermal properties. J. Appl. Polym. Sci. 2020, 137, 49143. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, X.; Zhang, J.; Gu, J. Intrinsic high thermal conductive liquid crystal epoxy film simultaneously combining with excellent intrinsic self-healing performance. J. Mater. Sci. Technol. 2021, 68, 209–215. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Yang, D.; Zhang, J.; Guo, Y.; Zhong, X.; Kong, J.; Gu, J. High-efficiency improvement of thermal conductivities for epoxy composites from synthesized liquid crystal epoxy followed by doping BN fillers. Compos. Part B Eng. 2020, 185, 107784. [Google Scholar] [CrossRef]
- Su, W.A. Thermoplastic and thermoset main chain liquid crystal polymers prepared from biphenyl mesogen. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 3251–3256. [Google Scholar] [CrossRef]
- Su, W.F.; Fu, Y.C.; Pan, W.P. Thermal properties of high refractive index epoxy resin system. Thermochim. Acta 2002, 392–393, 385–389. [Google Scholar] [CrossRef]
- Su, W.F.A.; Chen, K.C.; Tseng, S.Y. Effects of chemical structure changes on thermal, mechanical, and crystalline properties of rigid rod epoxy resins. J. Appl. Polym. Sci. 2000, 78, 446–451. [Google Scholar] [CrossRef]
- Zeng, L.; Liang, G.; Gu, A.; Yuan, L.; Zhuo, D.; Hu, J.T. High performance hybrids based on a novel incompletely condensed polyhedral oligomeric silsesquioxane and bismaleimide resin with improved thermal and dielectric properties. J. Mater. Sci. 2012, 47, 2548–2558. [Google Scholar] [CrossRef]
- Goertzen, W.K.; Kessler, M.R. Thermal and mechanical evaluation of cyanate ester composites with low-temperature processability. Compos. Part A Appl. Sci. Manuf. 2007, 38, 779–784. [Google Scholar] [CrossRef]
- Crawford, A.O.; Howlin, B.J.; Cavalli, G.; Hamerton, I. Examining the thermo-mechanical properties of novel cyanate ester blends through empirical measurement and simulation. React. Funct. Polym. 2012, 72, 596–605. [Google Scholar] [CrossRef]
- Yan, H.Q.; Chen, S.; Qi, G.R. Synthesis, cure kinetics and thermal properties of the 2,7- dihydroxynaphthalene dicyanate. Polymer 2003, 44, 7861–7867. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, S.; Xie, Y.; Gu, J.; Song, Z.; Gao, F.; Kong, J. Interfacial RAFT polymerization induced ultra low dielectric loss ceramic/cyanate ester composites. Compos. Sci. Technol. 2016, 124, 10–16. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, Y.; Wang, K.; Wang, Y.; Feng, A. Fabrication and characterization of OMMt/BMI/CE composites with low dielectric properties and high thermal stability for electronic packaging. J. Mater. Sci. Mater. Electron. 2016, 27, 5592–5599. [Google Scholar] [CrossRef]
- Venkatesh, M.; Gouthaman, S.; Kanemoto, S.O.; Lakshmi, M.S.; Hamerton, I. Development of epoxy-cyanate ester-clay nanocomposites offering enhanced thermally stability. J. Appl. Polym. Sci. 2019, 136, 47754. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.S. Effect of cyanate ester on the cure behavior and thermal stability of epoxy resin. J. Appl. Polym. Sci. 1997, 65, 85–90. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, G.; Ren, P.; Wang, Y.; Cui, X. In situ polymerization of graphene oxide and cyanate ester-epoxy with enhanced mechanical and thermal properties. Appl. Surf. Sci. 2014, 316, 549–557. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Liu, Y.; Teng, C.; Cui, W. Curing Mechanism and Mechanical Properties of Al2O3/Cyanate Ester–Epoxy Composites. J. Electron. Mater. 2020, 49, 1473–1481. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, M.; Jiang, M.; Huang, Y.; Liu, X. Curing behaviors of cyanate ester/epoxy copolymers and their dielectric properties. High Perform. Polym. 2017, 29, 1175–1184. [Google Scholar] [CrossRef]
- Hwang, H.J.; Hsu, S.W.; Chung, C.L.; Wang, C.S. Low dielectric epoxy resins from dicyclopentadiene-containing poly(phenylene oxide) novolac cured with dicyclopentadiene containing epoxy. React. Funct. Polym. 2008, 68, 1185–1193. [Google Scholar] [CrossRef]
- Hwang, H.J.; Wang, C.S. Thermal behavior and properties of naphthalene containing bismaleimide-triazine resins. J. Appl. Polym. Sci. 1998, 68, 1199–1207. [Google Scholar] [CrossRef]
- Lin, C.H. Synthesis of novel phosphorus-containing cyanate esters and their curing reaction with epoxy resin. Polymer 2004, 45, 7911–7926. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar]
- Krishnadevi, K.; Selvaraj, V. Biowaste material reinforced cyanate ester based epoxy composites for flame retardant applications. High Perform. Polym. 2015, 28, 881–894. [Google Scholar] [CrossRef]
- Wu, F.; Tuan, C.C.; Song, B.; Moon, K.S.; Wong, C.P. Controlled synthesis and evaluation of cyanate ester/epoxy copolymer system for high temperature molding compounds. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1337–1345. [Google Scholar] [CrossRef]
- Ariraman, M.; Sasikumar, R.; Alagar, M. Hybridization of PDMS based cyanate ester and DGEBA for radiation resistant and microelectronics applications. RSC Adv. 2015, 5, 63641–63649. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Liang, G.; Ren, P.; Zhang, Z.; Lu, T. Effect of the epoxy molecular weight on the properties of a cyanate ester/epoxy resin system. J. Appl. Polym. Sci. 2006, 101, 1744–1750. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, L.; Wang, Z.; Gu, A.; Liang, G. Self-constructed nanodomain structure in thermosetting blend based on the dynamic reactions of cyanate ester and epoxy resins and its related property. Compos. Part B 2019, 177, 107438. [Google Scholar] [CrossRef]
- Wang, G.; Wang, R.; Fu, G.; Gao, T.; Fu, C.; Kuang, H.; Yang, F.; Jiao, W.; Hao, L.; Liu, W. Study on Phenolphthalein Poly ( ether sulfone ) -Modified Cyanate Ester Resin and Epoxy Resin Blends. Polym. Eng. Sci. 2015, 55, 2591–2602. [Google Scholar] [CrossRef]
- Wang, C.S.; Lee, M.C. Synthesis, Characterization, and Properties of Multifunctional Naphthalene-Containing Epoxy Resins Cured with Cyanate Ester. J. Appl. Polym. Sci. 1999, 73, 1611–1622. [Google Scholar] [CrossRef]
- Ho, T.H.; Hwang, H.J.; Shieh, J.Y.; Chung, M.C. Thermal, physical and flame-retardant properties of phosphorus-containing epoxy cured with cyanate ester. React. Funct. Polym. 2009, 69, 176–182. [Google Scholar] [CrossRef]
- Su, W.A.; Chuang, C. Effects of Chemical Structure Changes on Curing Reactions and Thermal Properties of Cyanate Ester-Cured Rigid-Rod. J. Appl. Polym. Sci. 2002, 85, 2419–2422. [Google Scholar] [CrossRef]
- Hsu, S.H.; Wu, M.C.; Chen, S.; Chuang, C.M.; Lin, S.H.; Su, W.F. Synthesis, morphology and physical properties of multi-walled carbon nanotube/biphenyl liquid crystalline epoxy composites. Carbon N. Y. 2012, 50, 896–905. [Google Scholar] [CrossRef]
- Shiota, A.; Ober, C.K. Analysis of smectic structure formation in liquid crystalline thermosets. Polymer 1997, 38, 5857–5867. [Google Scholar] [CrossRef]
- Nielsen, L.E. Cross-Linking Effect on Physical Properties of Polymers. J. Macromol. Sci. Part C Polym. Rev. 2008, 3, 69–103. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, K.; Wu, G.; Feng, A.; Zhuo, L. The effect of bis allyl benzoxazine on the thermal, mechanical and dielectric properties of bismaleimide–cyanate blend polymers. RSC Adv. 2015, 5, 58821–58831. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, H.; Geng, C.; Wu, Y.; Dai, G.; Teng, C. Effect of poly(ether ether ketone) and allyl compounds on microstructure and properties of bismaleimide. J. Mater. Sci. Mater. Electron. 2019, 30, 991–1000. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, K.; Wu, G.; Zhuo, L.; Li, J.; Zhang, Y. The curing reaction of benzoxazine with bismaleimide/cyanate ester resin and the properties of the terpolymer. Polymer 2015, 77, 354–360. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Liu, Y.; Teng, C.; Cui, W. Effect of Al2O3 on microstructure and dielectric properties of epoxy-cyanate ester composite material. J. Mater. Sci. Mater. Electron. 2019, 30, 20614–20623. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, X.; Wu, G.; Feng, A. Thermal conductivity and dielectric properties of bismaleimide/cyanate ester copolymer. High Volt. 2017, 2, 167–171. [Google Scholar] [CrossRef]
- Yu, J.; Huo, R.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Influence of interface structure on dielectric properties of epoxy/alumina nanocomposites. Macromol. Res. 2012, 20, 816–826. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.; Wu, G.; Wu, W.; Wang, Y.; Wu, K.; Cheng, Y. Investigation of dielectric and thermal conductive properties of epoxy resins modified by core-shell structured PS@SiO2. Compos. Part A Appl. Sci. Manuf. 2017, 97, 76–82. [Google Scholar] [CrossRef]



| Cured Epoxy | Epoxy:B10 Ratio | 5% Weight Loss Temp (°C) | Residual Weight (%) | |||
|---|---|---|---|---|---|---|
| At 350 °C | At 400 °C | At 450 °C | At 500 °C | |||
| BP * | 2:1 | 362.7 | 96.65 | 79.85 | 45.26 | 40.30 |
| BP | 304.4 | 74.62 | 46.48 | 28.77 | 26.49 | |
| DGEBA | 344.3 | 94.31 | 65.62 | 16.80 | 14.86 | |
| BP | 1:1 | 335.1 | 92.35 | 62.53 | 42.77 | 38.96 |
| DGEBA | 344.7 | 94.21 | 60.13 | 34.40 | 21.23 | |
| Cured Epoxy | Epoxy:B10 Ratio | Highest Rate of Weight Loss Temp (°C) | Deriv. Weight at the Highest Rate of Weight Loss Temp (%/°C) |
|---|---|---|---|
| BP * | 2:1 | 414.3 | 0.93 |
| BP | 400.7 | 0.68 | |
| DGEBA | 418.5 | 1.57 | |
| BP | 1:1 | 392.2 | 0.77 |
| DGEBA | 394.3 | 1.40 |
| Cured Epoxy | Epoxy:B10 Ratio | Peak Value of DMA’s tanδ (Tg, °C) |
|---|---|---|
| BP * | 2:1 | 190 |
| BP | 183 | |
| DGEBA | 184 | |
| BP | 1:1 | 253 |
| DGEBA | 223 |
| Cured Epoxy | Epoxy:B10 Ratio | Tg (°C) | CTE (μm/m°C) | |
|---|---|---|---|---|
| α1 | α2 | |||
| BP * | 2:1 | 174.2 | 60.6 | 150 |
| BP | 179.5 | 80.0 | 218 | |
| DGEBA | 149.8 | 65.4 | 171 | |
| BP | 1:1 | 197.3 | 73.8 | 182 |
| DGEBA | 202.5 | 85.9 | 134 | |
| Frequency (Hz) | Temperature (°C) | Permittivity | Loss Factor |
|---|---|---|---|
| 10 | −125 | 2.597 | 0.012 |
| 30 | 3.707 | 0.263 | |
| 150 | 3.497 | 0.620 | |
| 100 | −125 | 2.575 | 0.009 |
| 30 | 3.451 | 0.088 | |
| 150 | 3.252 | 0.141 | |
| 1000 | −125 | 2.555 | 0.010 |
| 30 | 3.374 | 0.054 | |
| 150 | 3.150 | 0.060 | |
| 10,000 | −125 | 2.465 | 0.009 |
| 30 | 3.334 | 0.066 | |
| 150 | 3.014 | 0.038 |
| Frequency (Hz) | Temperature (°C) | Permittivity | Loss Factor |
|---|---|---|---|
| 10 | −125 | 2.929 | 0.024 |
| 30 | 3.826 | 0.064 | |
| 150 | 4.819 | 0.016 | |
| 100 | −125 | 2.903 | 0.613 |
| 30 | 3.781 | 0.045 | |
| 150 | 3.929 | 0.013 | |
| 1000 | −125 | 2.884 | 0.110 |
| 30 | 3.728 | 0.033 | |
| 150 | 3.749 | 0.012 | |
| 10,000 | −125 | 2.811 | 0.046 |
| 30 | 3.602 | 0.029 | |
| 150 | 3.632 | 0.011 |
| Frequency (Hz) | Temperature (°C) | Permittivity | Loss Factor |
|---|---|---|---|
| 10 | −125 | 2.837 | 0.028 |
| 30 | 3.172 | 0.020 | |
| 150 | 4.037 | 1.856 | |
| 100 | −125 | 2.797 | 0.024 |
| 30 | 3.155 | 0.013 | |
| 150 | 3.431 | 0.312 | |
| 1000 | −125 | 2.765 | 0.023 |
| 30 | 3.142 | 0.010 | |
| 150 | 3.312 | 0.074 | |
| 10,000 | −125 | 2.072 | 0.018 |
| 30 | 3.051 | 0.011 | |
| 150 | 3.184 | 0.032 |
| Frequency (Hz) | Temperature (°C) | Permittivity | Loss Factor |
|---|---|---|---|
| 10 | −125 | 2.548 | 0.005 |
| 30 | 2.821 | 0.020 | |
| 150 | 2.833 | 0.040 | |
| 100 | −125 | 2.525 | 0.284 |
| 30 | 2.812 | 0.011 | |
| 150 | 2.814 | 0.006 | |
| 1000 | −125 | 2.505 | 0.044 |
| 30 | 2.795 | 0.002 | |
| 150 | 2.802 | 0.004 | |
| 10,000 | −125 | 2.362 | 0.009 |
| 30 | 2.638 | 0.001 | |
| 150 | 2.665 | 0.040 |
| Frequency (Hz) | Temperature (°C) | Permittivity | Loss Factor |
|---|---|---|---|
| 10 | −125 | 2.802 | 0.016 |
| 30 | 3.064 | 0.008 | |
| 150 | 3.075 | 0.035 | |
| 100 | −125 | 2.757 | 0.014 |
| 30 | 3.048 | 0.011 | |
| 150 | 3.052 | 0.013 | |
| 1000 | −125 | 2.756 | 0.014 |
| 30 | 3.031 | 0.015 | |
| 150 | 3.038 | 0.011 | |
| 10,000 | −125 | 2.652 | 0.014 |
| 30 | 2.943 | 0.020 | |
| 150 | 2.963 | 0.008 |
| Cured Epoxy | 10 Hz | 100 Hz | 1000 Hz | 10,000 Hz |
|---|---|---|---|---|
| BP/B10 (2:1) * | −85.08 | −55.45 | −23.64 | 1.57 |
| BP/B10 (2:1) | −70.09 | −50.19 | −34.27 | −6.44 |
| DGEBA/B10 (2:1) | −82.29 | −70.20 | −49.95 | −29.58 |
| BP/B10 (1:1) | −87.74 | −70.35 | −50.60 | −21.05 |
| DGEBA/B10 (1:1) | −77.79 | −61.95 | −41.80 | −21.89 |
| Cured Epoxy | Apparent Activation Energy (KJ/mol) |
|---|---|
| BP/B10 (2:1) * | 61.78 |
| BP/B10 (2:1) | 44.54 |
| DGEBA/B10 (2:1) | 42.33 |
| BP/B10 (1:1) | 51.88 |
| DGEBA/B10 (1:1) | 42.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-P.; Chuang, C.-M. Thermal and Dielectric Properties of Cyanate Ester Cured Main Chain Rigid-Rod Epoxy Resin. Polymers 2021, 13, 2917. https://doi.org/10.3390/polym13172917
Li C-P, Chuang C-M. Thermal and Dielectric Properties of Cyanate Ester Cured Main Chain Rigid-Rod Epoxy Resin. Polymers. 2021; 13(17):2917. https://doi.org/10.3390/polym13172917
Chicago/Turabian StyleLi, Chi-Ping, and Chih-Min Chuang. 2021. "Thermal and Dielectric Properties of Cyanate Ester Cured Main Chain Rigid-Rod Epoxy Resin" Polymers 13, no. 17: 2917. https://doi.org/10.3390/polym13172917






