Bioinspired Dielectric Film with Superior Mechanical Properties and Ultrahigh Electric Breakdown Strength Made from Aramid Nanofibers and Alumina Nanoplates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
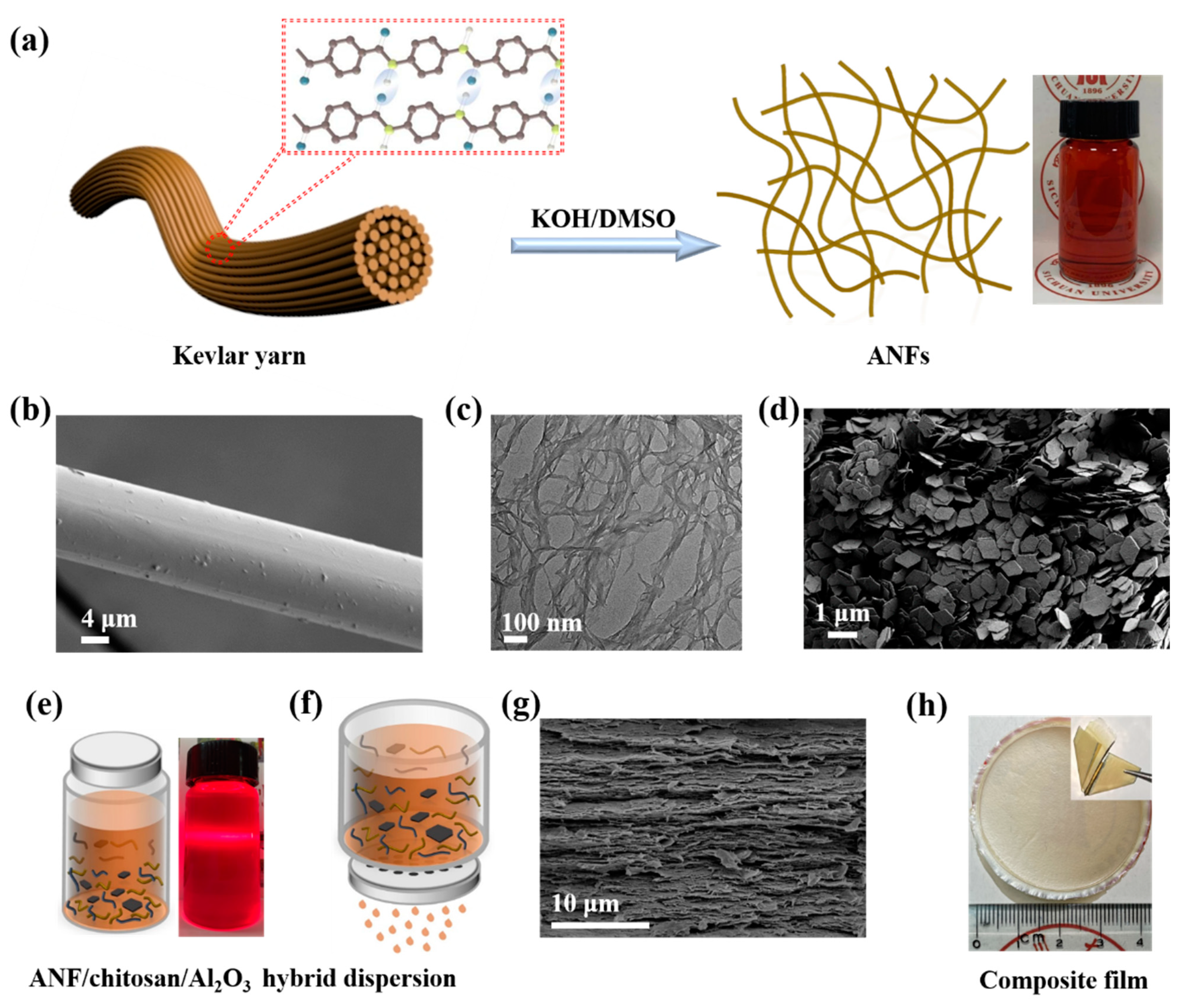
2.2. Preparation of ANFs, ANF/Chitosan, and ANF/Chitosan/Al2O3 Composite Films
2.3. Characterization
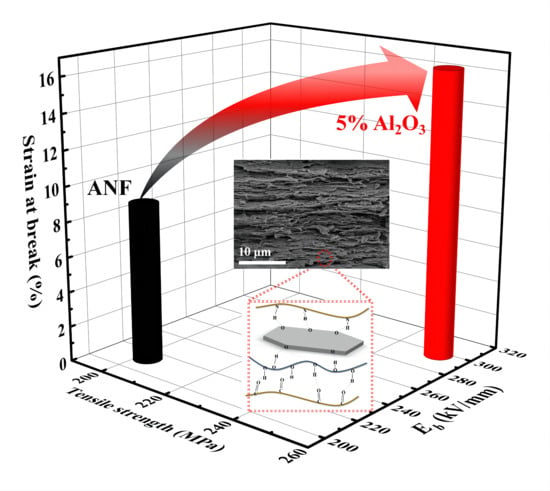
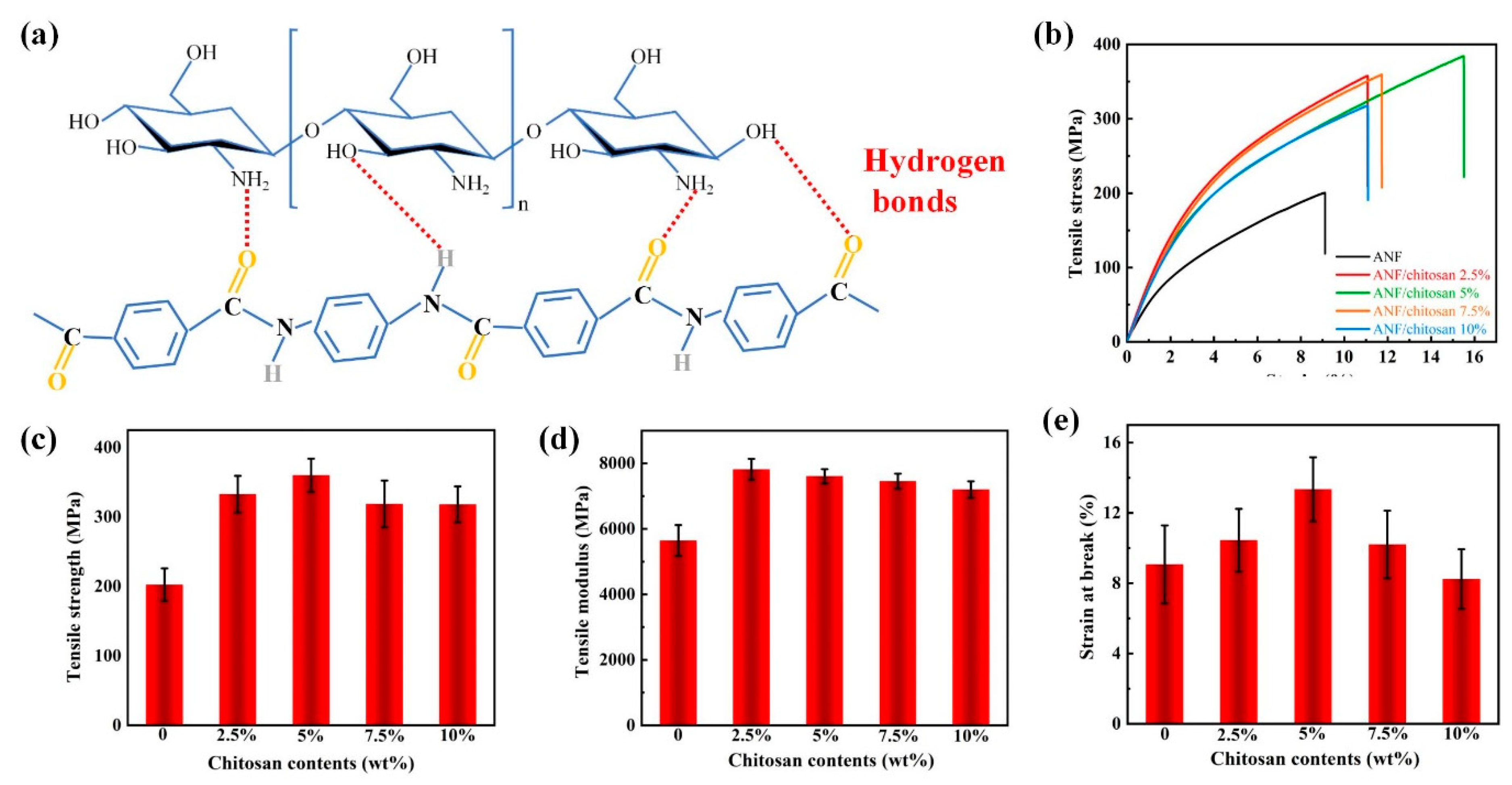
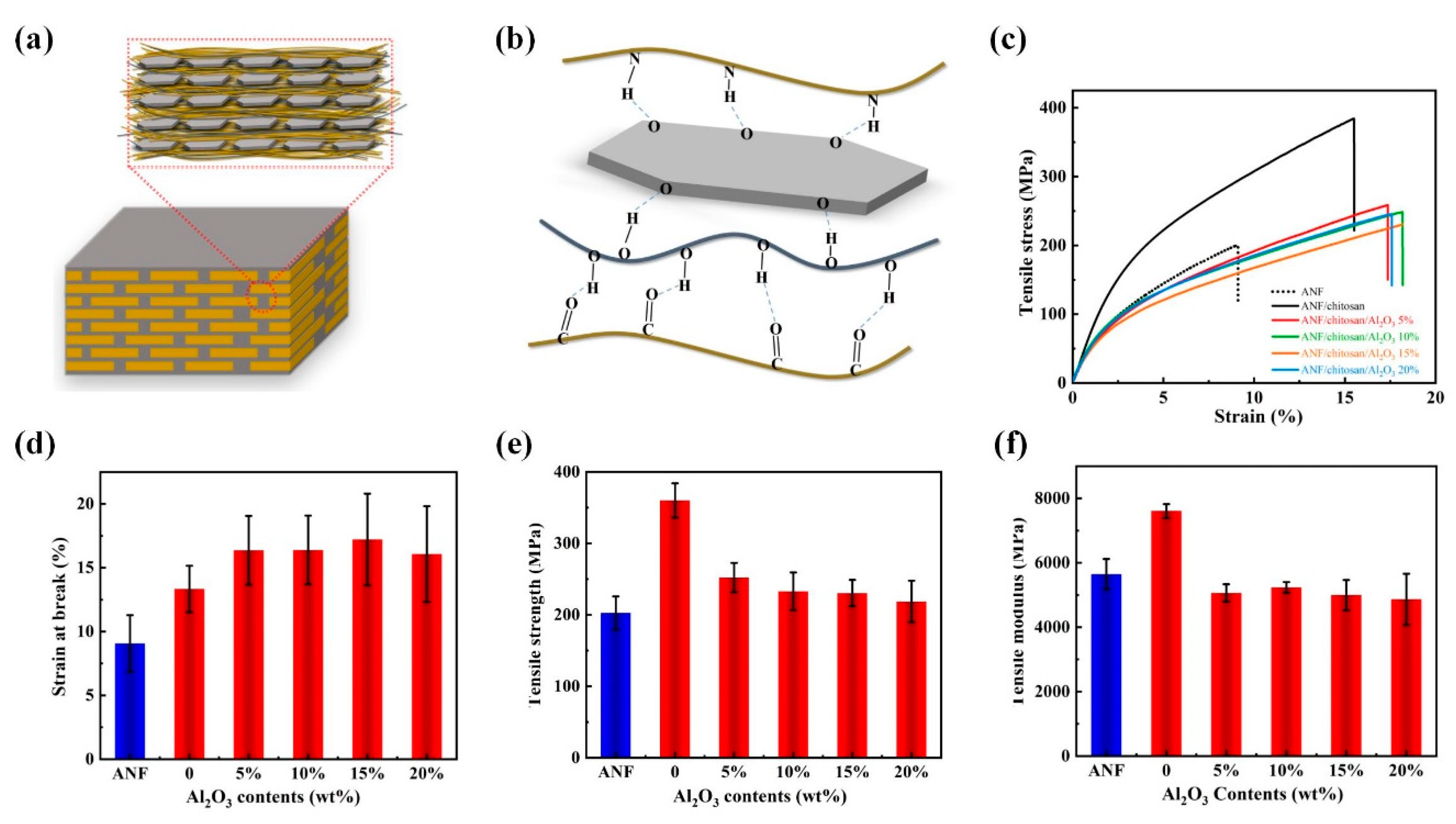
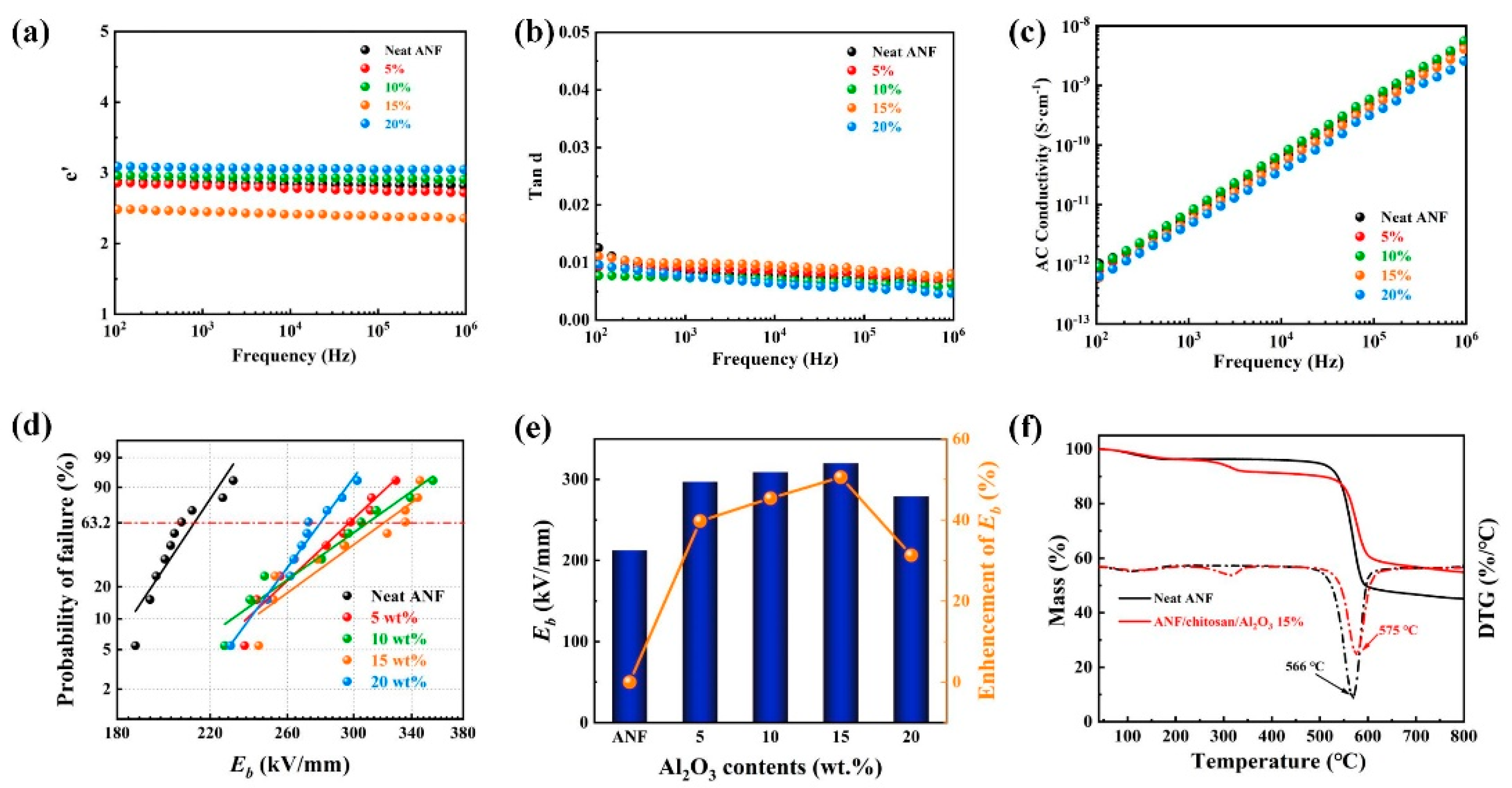
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, K.; Wang, J.; Liu, D.; Lei, C.; Liu, D.; Lei, W.; Fu, Q. Highly Thermoconductive, Thermostable, and Super-Flexible Film by Engineering 1D Rigid Rod-Like Aramid Nanofiber/2D Boron Nitride Nanosheets. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Gadinski, M.R.; Zhang, S.; Zhang, G.; Li, U.; Iagodkine, E.; Haque, A.; Chen, L.Q.; Jackson, N.; et al. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 2015, 523, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.P.; Chen, L.B.; Tian, G.L.; Wan, S.S.; Bai, L.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Yang, W. Multifunctional Thermal Management Materials with Excellent Heat Dissipation and Generation Capability for Future Electronics. ACS Appl. Mater. Interfaces 2019, 11, 18739–18745. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Yin, C.-G.; Cecen, V.; Fan, J.-C.; Shi, P.-H.; Xu, Q.-J.; Min, Y.-L. Polybenzimidazole thermal management composites containing functionalized boron nitride nanosheets and 2D transition metal carbide MXenes. Polymer 2019, 179, 121613. [Google Scholar] [CrossRef]
- Krause, B.; Rzeczkowski, P.; Potschke, P. Thermal Conductivity and Electrical Resistivity of Melt-Mixed Polypropylene Composites Containing Mixtures of Carbon-Based Fillers. Polymers 2019, 11, 1073. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, Z.; Bian, H.; Wu, W.; Xiao, H.; Dai, H. Thermally Conductive and Electrical Insulation BNNS/CNF Aerogel Nano-Paper. Polymers 2019, 11, 660. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Tuo, R.; Yang, W.; Zhang, Y.; Xie, Q.; Zha, J.; Lin, J.; He, S. Mechanical, Thermal, and Electrical Properties of BN-Epoxy Composites Modified with Carboxyl-Terminated Butadiene Nitrile Liquid Rubber. Polymers 2019, 11, 1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Hu, J.; Zhang, C.; Wang, J.; Chen, L.; Bian, X.; Lin, J.; Du, X. Performance improvement in nano-alumina filled silicone rubber composites by using vinyl tri-methoxysilane. Polym. Test. 2018, 67, 295–301. [Google Scholar] [CrossRef]
- Gupta, R.; Smith, L.; Njuguna, J.; Deighton, A.; Pancholi, K. Insulating MgO-Al2O3-LDPE nanocomposites for offshore medium-voltage DC cables. ACS Appl. Electron. Mater. 2020, 2, 1880–1891. [Google Scholar] [CrossRef]
- Huang, X.; Ding, Z.A.; Wang, J.; Wang, J.; Li, Q. The impacts of chemical modification on the initial surface creepage discharge behaviors of polyimide insulating film in power electronics. ACS Appl. Electron. Mater. 2020, 2, 3418–3425. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Z.; Luo, W.; Han, X.; Jang, S.H.; Dai, J.; Yang, B.; Hu, L. Thermally conductive, electrical insulating, optically transparent bi-layer nanopaper. ACS Appl. Mater. Interfaces 2016, 8, 28838–28843. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Wan, C.; Zheng, X.; Kormakov, S.; Gao, X.; Sun, J.; Zheng, X.; Wu, D. Enhancing thermal conductivity of polydimethylsiloxane composites through spatially confined network of hybrid fillers. Compos. Sci. Technol. 2019, 172, 163–171. [Google Scholar] [CrossRef]
- Li, L.; Ma, Z.; Xu, P.; Zhou, B.; Li, Q.; Ma, J.; He, C.; Feng, Y.; Liu, C. Flexible and alternant-layered cellulose nanofiber/graphene film with superior thermal conductivity and efficient electromagnetic interference shielding. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106134. [Google Scholar] [CrossRef]
- Xie, C.; He, L.; Shi, Y.; Guo, Z.X.; Qiu, T.; Tuo, X. From Monomers to a Lasagna-like Aerogel Monolith: An Assembling Strategy for Aramid Nanofibers. ACS Nano 2019, 13, 7811–7824. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, X.; Xiao, G.; Li, H.; Xia, S.; Wang, J. A Bioinspired Ultratough Multifunctional Mica-Based Nanopaper with 3D Aramid Nanofiber Framework as an Electrical Insulating Material. ACS Nano 2020, 14, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, Y.; Teng, C. Facial fabrication of aramid composite insulating paper with high strength and good thermal conductivity. Compos. Commun. 2020, 21, 100370. [Google Scholar] [CrossRef]
- Yang, M.; Cao, K.; Sui, L.; Qi, Y.; Zhu, J.A.; Waas, A.; Arruda, E.M.; Kieffer, J.; Thouless, M.D.; Kotov, N.A. Dispersions of Aramid Nanofibers: A New Nanoscale Building Block. ACS Nano 2011, 5, 6945–6954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.R.; Harris, J.; Zhou, T.; Loufakis, D.; Boyd, J.G.; Lutkenhaus, J.L. Mechanically Strong Graphene/Aramid Nanofiber Composite Electrodes for Structural Energy and Power. ACS Nano 2017, 11, 6682–6690. [Google Scholar] [CrossRef]
- Gong, Y.J.; Heo, J.W.; Lee, H.; Kim, H.; Cho, J.; Pyo, S.; Yun, H.; Kim, H.; Park, S.Y.; Yoo, J.; et al. Nonwoven rGO Fiber-Aramid Separator for High-Speed Charging and Discharging of Li Metal Anode. Adv. Energy Mater. 2020, 10. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Zhang, M.; Luo, J.; Lu, Z.; Ding, X. Fabrication, Applications, and Prospects of Aramid Nanofiber. Adv. Funct. Mater. 2020, 30, 2000186. [Google Scholar] [CrossRef]
- Zhao, L.; Liao, C.; Liu, Y.; Huang, X.; Ning, W.; Wang, Z.; Jia, L.; Ren, J. A combination of aramid nanofiber and silver nanoparticle decorated boron nitride for the preparation of a composite film with superior thermally conductive performance. Compos. Interfaces 2021, 1–17. [Google Scholar] [CrossRef]
- Wang, T.; Wei, C.; Yan, L.; Liao, Y.; Wang, G.; Zhao, L.; Fu, M.; Ren, J. Thermally conductive, mechanically strong dielectric film made from aramid nanofiber and edge-hydroxylated boron nitride nanosheet for thermal management applications. Compos. Interfaces 2020, 1–14. [Google Scholar] [CrossRef]
- Hu, P.; Lyu, J.; Fu, C.; Gong, W.B.; Liao, J.; Lu, W.; Chen, Y.; Zhang, X. Multifunctional Aramid Nanofiber/Carbon Nanotube Hybrid Aerogel Films. ACS Nano 2020, 14, 688–697. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Zhang, P.; Zhang, J.; Chen, G.; Feng, X. Mechanically strong MXene/Kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Zeng, X.; Ye, L.; Yu, S.; Li, H.; Sun, R.; Xu, J.; Wong, C.P. Artificial nacre-like papers based on noncovalent functionalized boron nitride nanosheets with excellent mechanical and thermally conductive properties. Nanoscale 2015, 7, 6774–6781. [Google Scholar] [CrossRef]
- Chen, S.-M.; Gao, H.-L.; Sun, X.-H.; Ma, Z.-Y.; Ma, T.; Xia, J.; Zhu, Y.-B.; Zhao, R.; Yao, H.-B.; Wu, H.-A.; et al. Superior Biomimetic Nacreous Bulk Nanocomposites by a Multiscale Soft-Rigid Dual-Network Interfacial Design Strategy. Matter 2019, 1, 412–427. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Zhao, Y.; Ruan, K.; Liu, X.; Zhang, J.; Guo, Y.; Yang, X.; Kong, J.; Gu, J. Highly Thermal Conductivities, Excellent Mechanical Robustness and Flexibility, and Outstanding Thermal Stabilities of Aramid Nanofiber Composite Papers with Nacre-Mimetic Layered Structures. ACS Appl Mater. Interfaces 2020, 12, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Liao, Y.; Jia, L.C.; Wang, Z.; Huang, X.L.; Ning, W.J.; Zhang, Z.X.; Ren, J.W. Ultra-Robust Thermoconductive Films Made from Aramid Nanofiber and Boron Nitride Nanosheet for Thermal Management Application. Polymers 2021, 13, 2028. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Thermal, mechanical, and dielectric properties of aluminium oxide and solid glass microsphere-reinforced epoxy composite for electronic packaging application. Polym. Compos. 2019, 40, 2573–2581. [Google Scholar] [CrossRef]
- Li, W.; Hillborg, H.; Gedde, U.W. Influence of process conditions and particle dispersion on the ac breakdown strength of polyethylene-aluminium oxide nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3536–3542. [Google Scholar] [CrossRef]
- Ren, J.; Yu, D. Effects of enhanced hydrogen bonding on the mechanical properties of poly (vinyl alcohol)/carbon nanotubes nanocomposites. Compos. Interfaces 2017, 25, 205–219. [Google Scholar] [CrossRef]
- E, S.; Ma, Q.; Huang, J.; Jin, Z.; Lu, Z. Enhancing mechanical strength and toughness of aramid nanofibers by synergetic interactions of covalent and hydrogen bonding. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106031. [Google Scholar] [CrossRef]
- Zhou, W.; Li, T.; Yuan, M.; Li, B.; Zhong, S.; Li, Z.; Liu, X.; Zhou, J.; Wang, Y.; Cai, H.; et al. Decoupling of inter-particle polarization and intra-particle polarization in core-shell structured nanocomposites towards improved dielectric performance. Energy Storage Mater. 2021, 42, 1–11. [Google Scholar] [CrossRef]
- On, S.Y.; Kim, M.S.; Kim, S.S. Effects of post-treatment of meta-aramid nanofiber mats on the adhesion strength of epoxy adhesive joints. Compos. Struct. 2017, 159, 636–645. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Huang, Y. High strength, thermostable and fast-drying hybrid transparent membranes with POSS nanoparticles aligned on aramid nanofibers. Compos. Part A Appl. Sci. Manuf. 2018, 110, 154–161. [Google Scholar] [CrossRef]
- Ren, J.; Li, Q.; Yan, L.; Jia, L.; Huang, X.; Zhao, X.; Ran, Q.; Fu, M. Enhanced thermal conductivity of epoxy composites by introducing graphene@boron nitride nanosheets hybrid nanoparticles. Mater. Des. 2020, 191, 108663. [Google Scholar] [CrossRef]
- Ren, J.; Yu, D.; Feng, L.; Wang, G.; Lv, G. Nanocable-structured polymer/carbon nanotube composite with low dielectric loss and high impedance. Compos. Part A Appl. Sci. Manuf. 2017, 98, 66–75. [Google Scholar] [CrossRef]

| Content (wt %) | β | Eb (kV/mm) |
|---|---|---|
| 0 | 16.3 | 212.6 |
| 5 | 10.2 | 297.1 |
| 10 | 7.7 | 309.1 |
| 15 | 7.9 | 320.1 |
| 20 | 14.7 | 279.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qing, Q.-W.; Wei, C.-M.; Li, Q.-H.; Liu, R.; Zhang, Z.-X.; Ren, J.-W. Bioinspired Dielectric Film with Superior Mechanical Properties and Ultrahigh Electric Breakdown Strength Made from Aramid Nanofibers and Alumina Nanoplates. Polymers 2021, 13, 3093. https://doi.org/10.3390/polym13183093
Qing Q-W, Wei C-M, Li Q-H, Liu R, Zhang Z-X, Ren J-W. Bioinspired Dielectric Film with Superior Mechanical Properties and Ultrahigh Electric Breakdown Strength Made from Aramid Nanofibers and Alumina Nanoplates. Polymers. 2021; 13(18):3093. https://doi.org/10.3390/polym13183093
Chicago/Turabian StyleQing, Qiu-Wanyu, Cheng-Mei Wei, Qi-Han Li, Rui Liu, Zong-Xi Zhang, and Jun-Wen Ren. 2021. "Bioinspired Dielectric Film with Superior Mechanical Properties and Ultrahigh Electric Breakdown Strength Made from Aramid Nanofibers and Alumina Nanoplates" Polymers 13, no. 18: 3093. https://doi.org/10.3390/polym13183093