Recent Advances on Cellulose Nanocrystals and Their Derivatives
Abstract
:1. Introduction
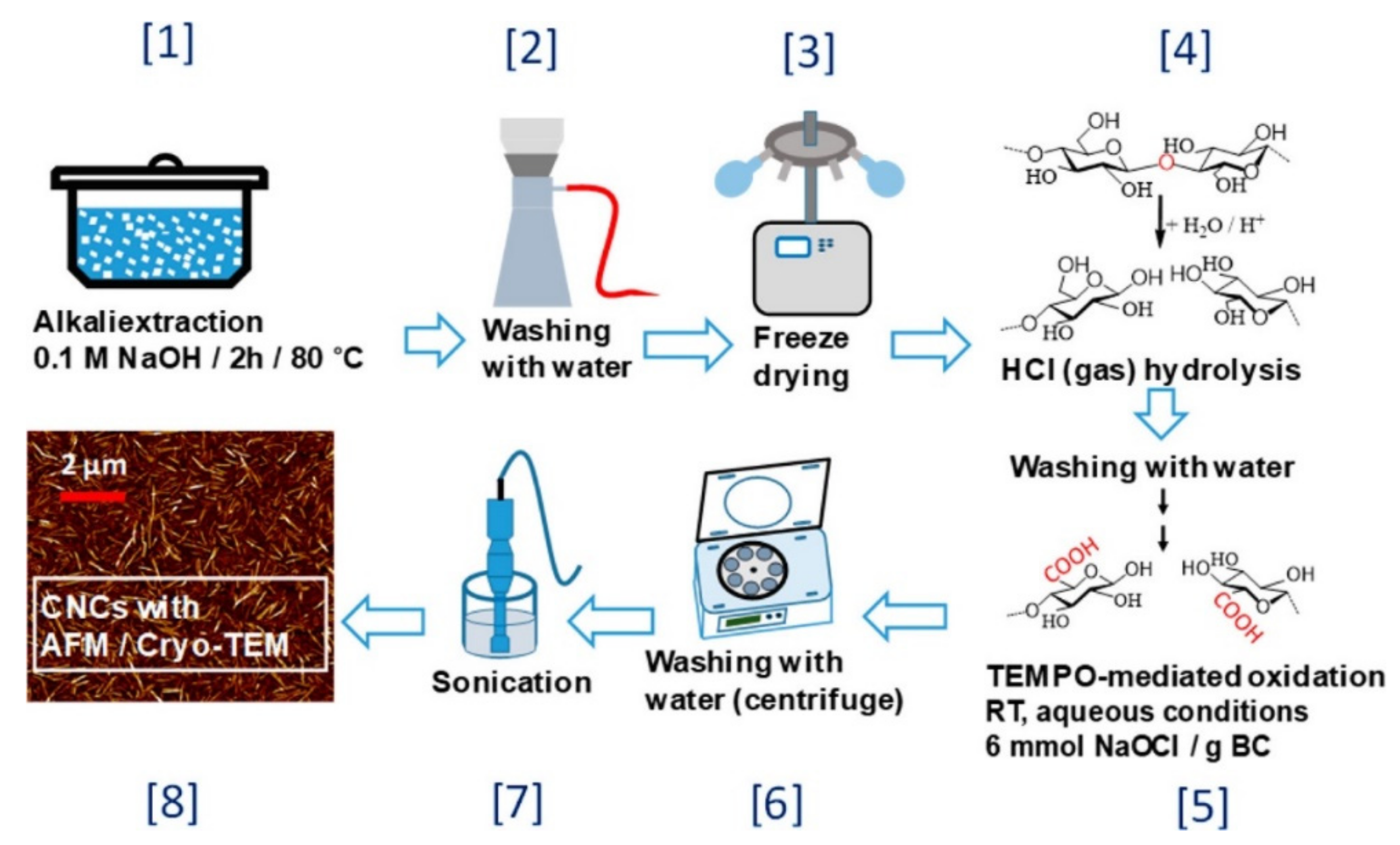
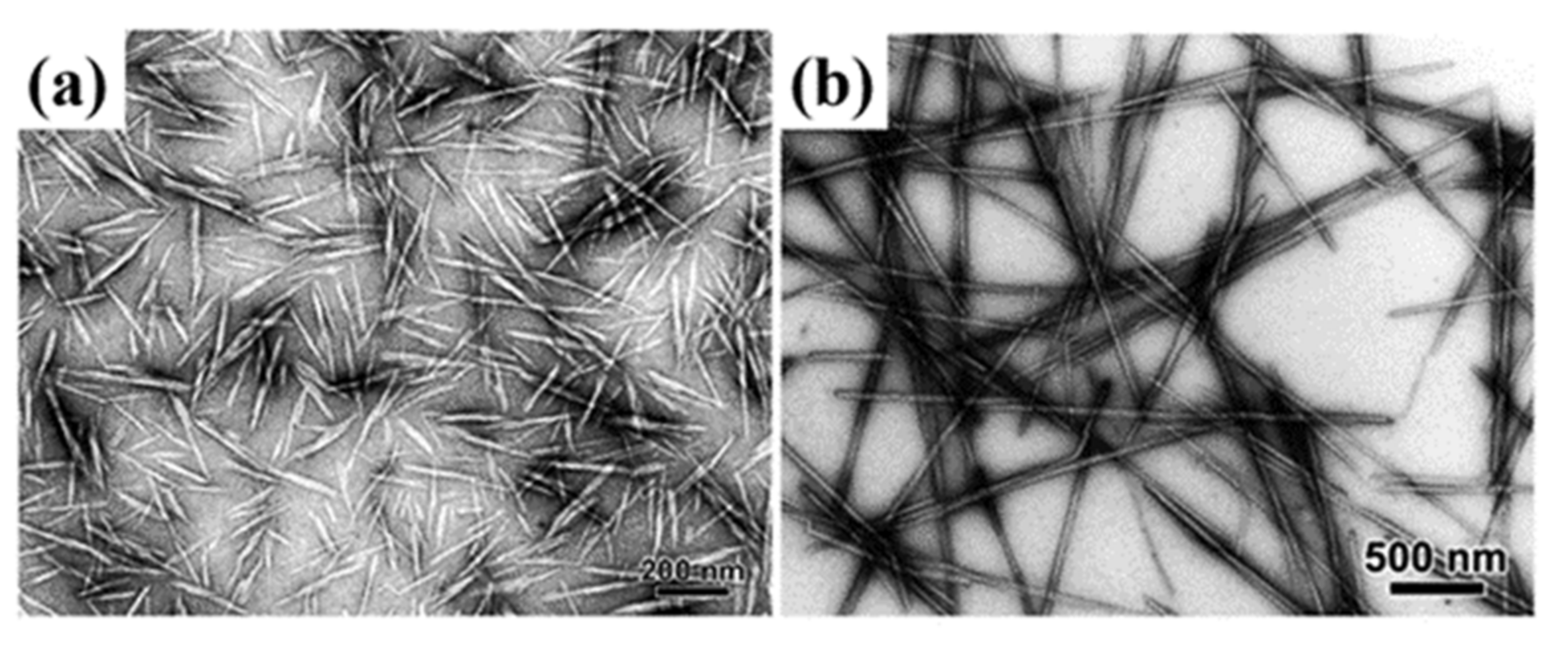
2. Isolation of CNCs
2.1. Inorganic Acid Hydrolysis
2.2. Other Methods
3. Surface Modification of CNCs
3.1. Small Molecules Chemically “Grafted to” CNCs
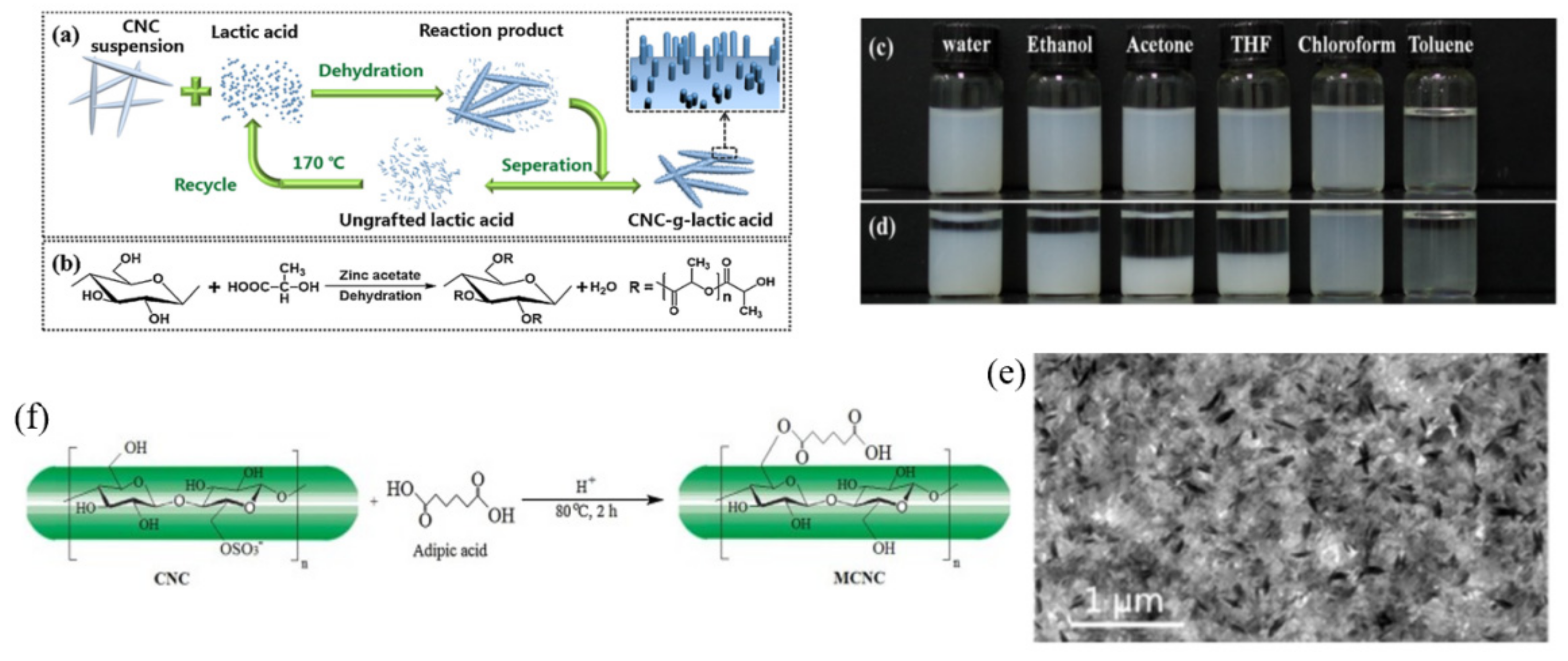
3.1.1. Esterification
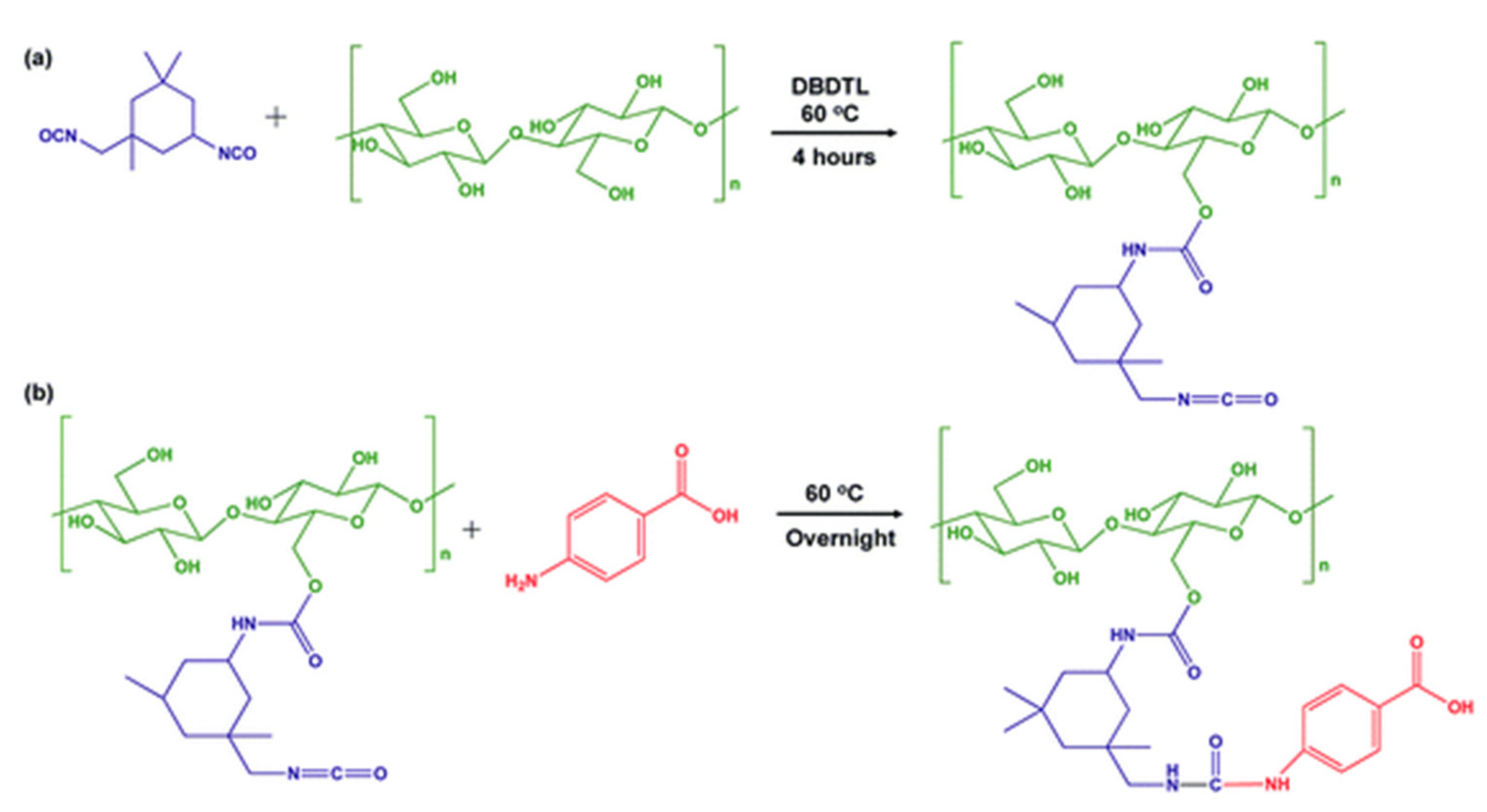
3.1.2. Isocyanate
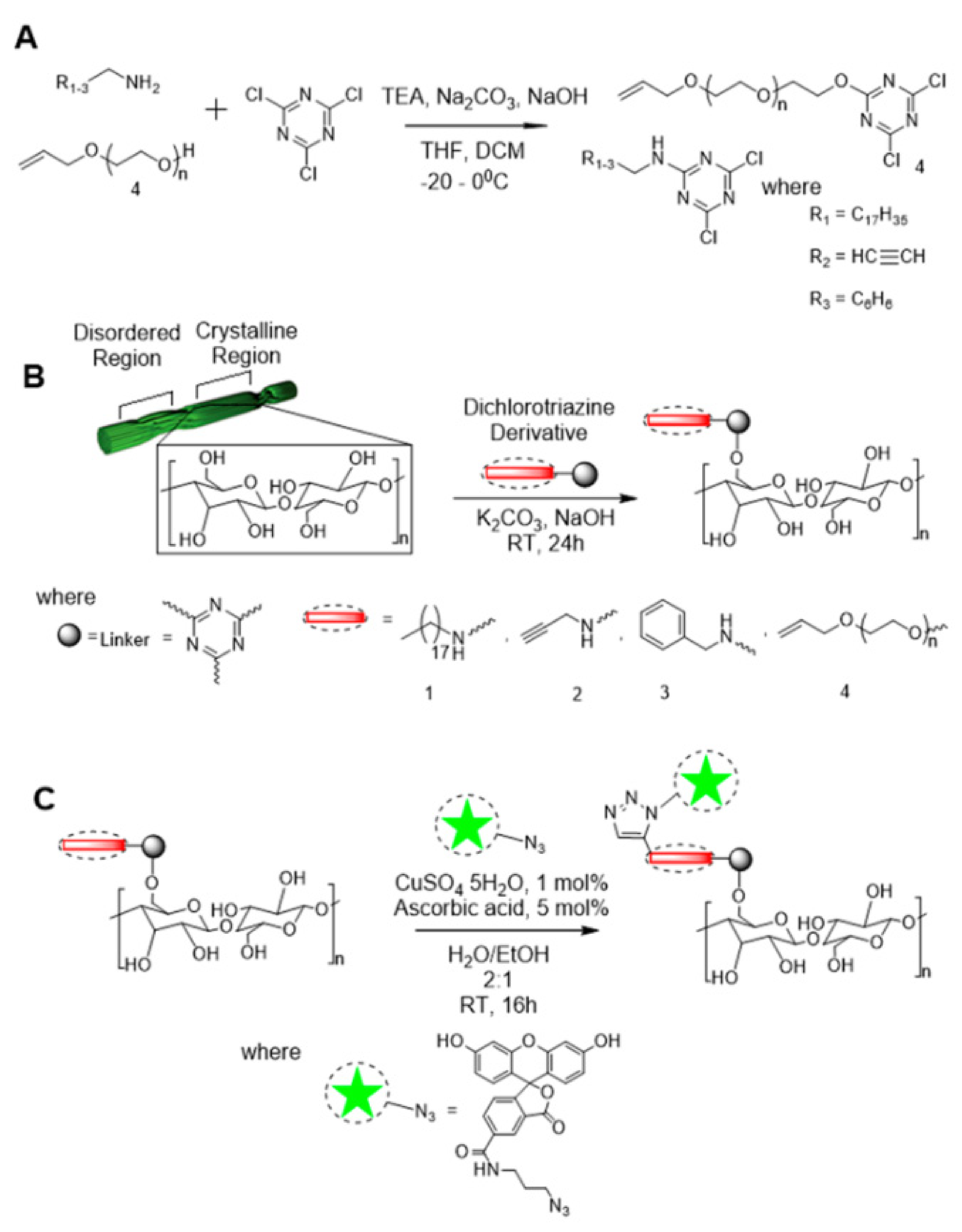
3.1.3. Triazinyl
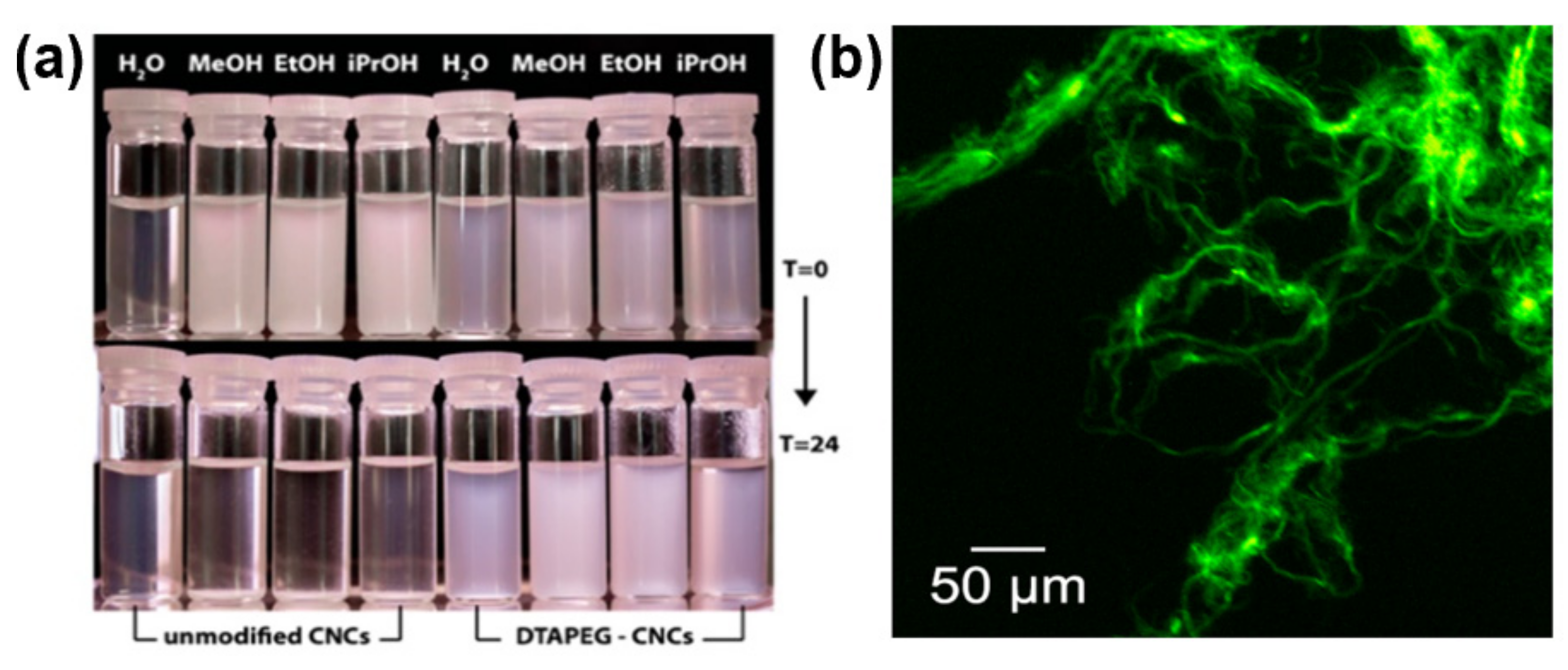
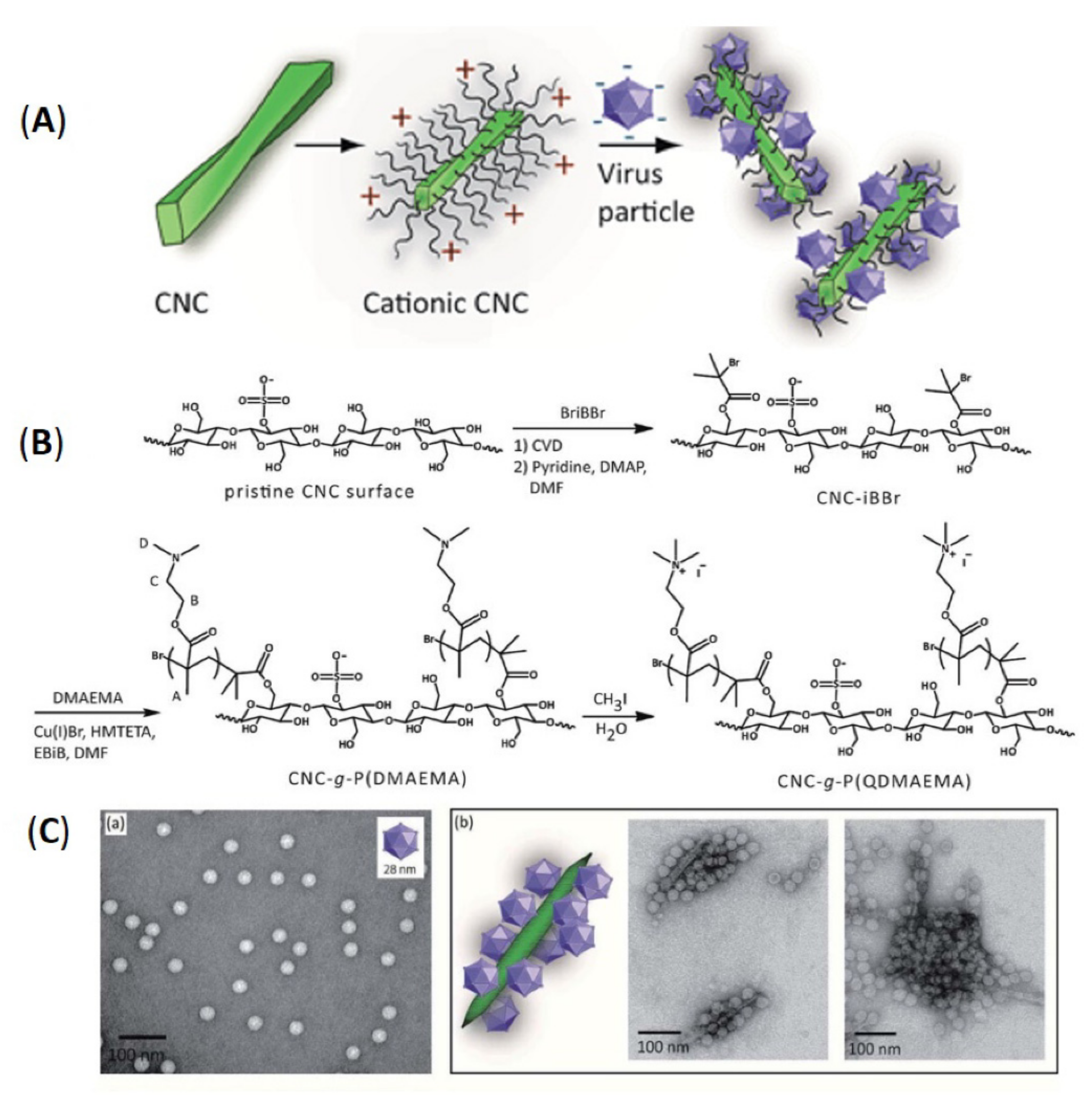
3.1.4. Cationization
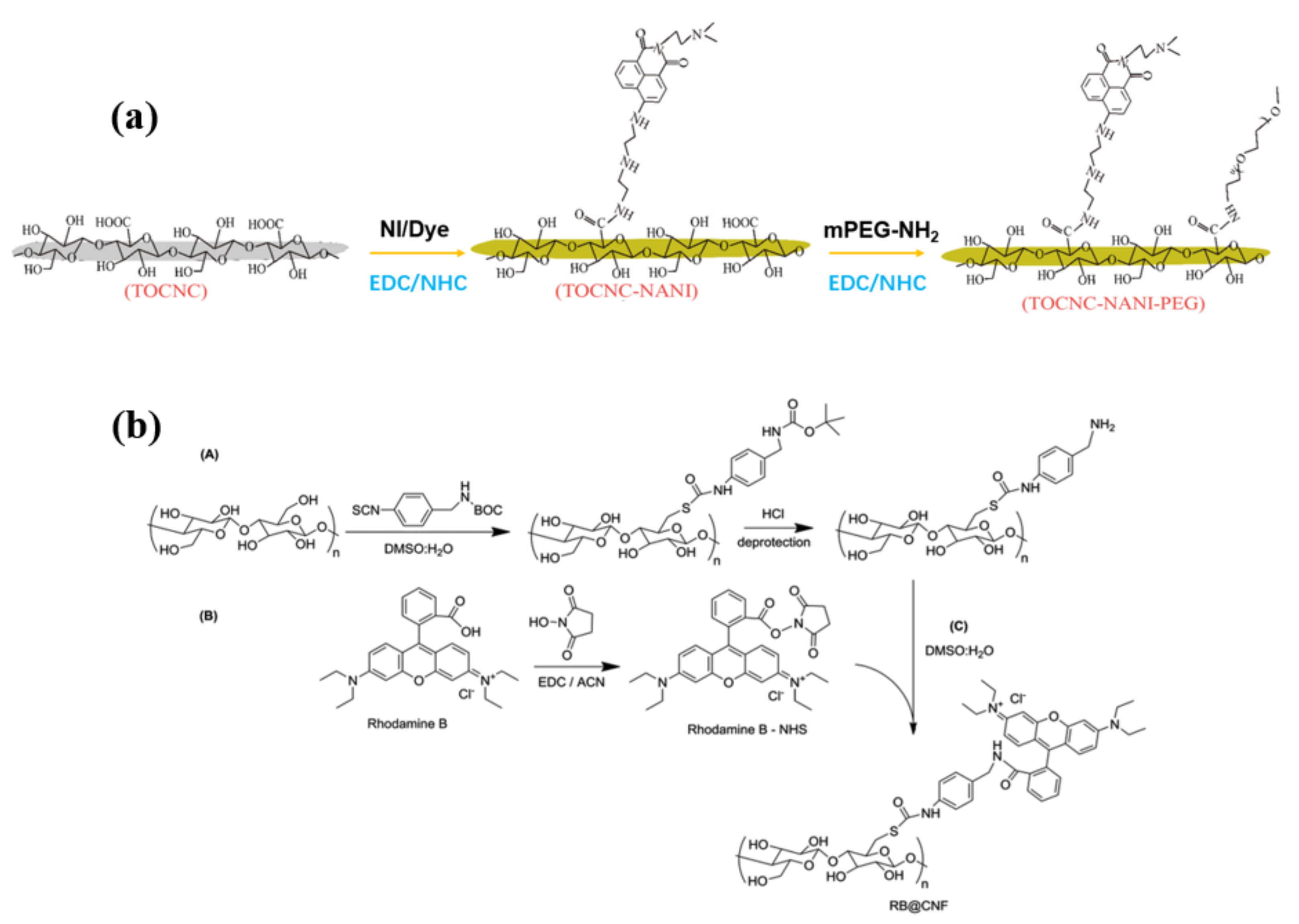
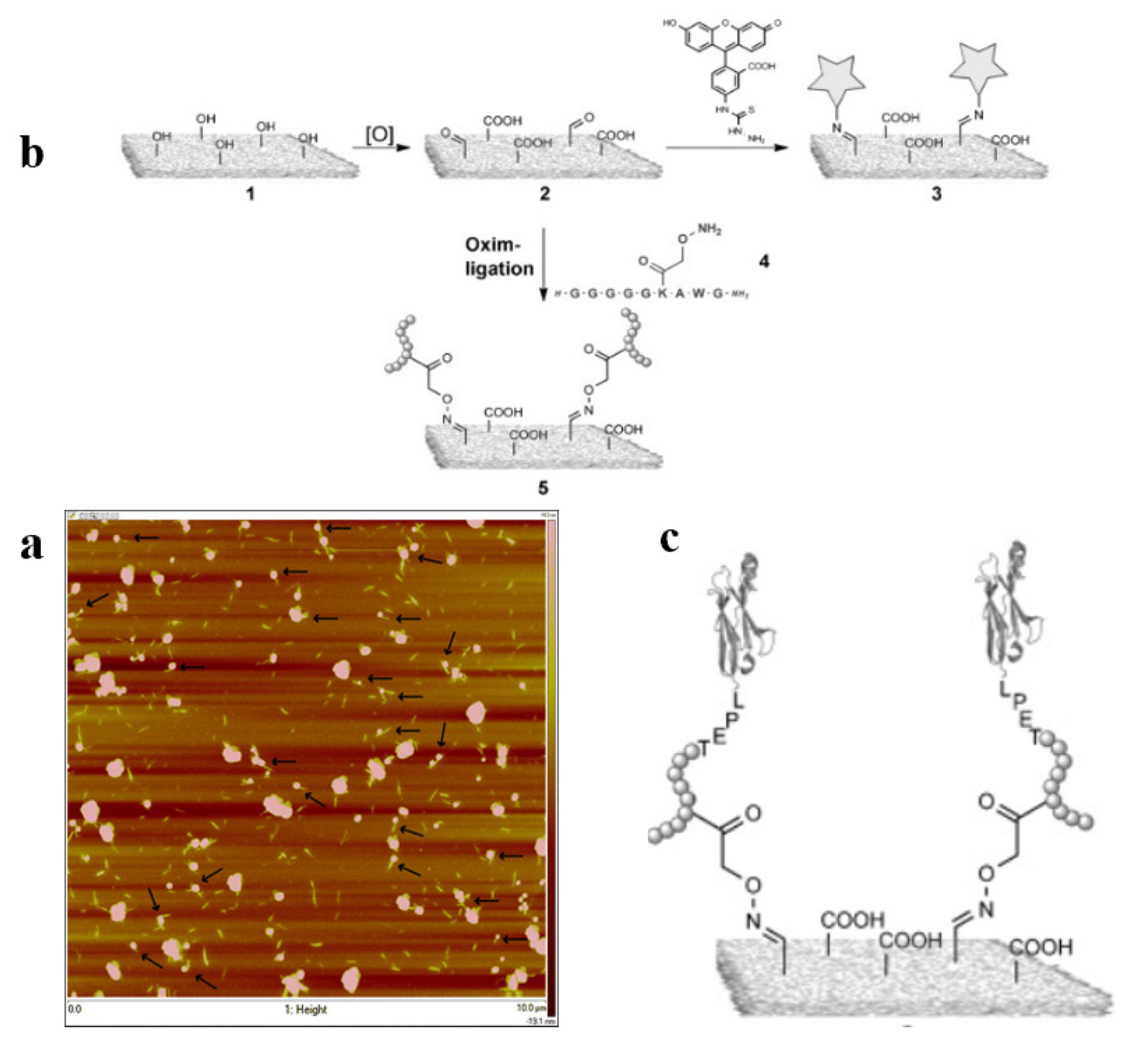
3.1.5. Amidation and Imidization
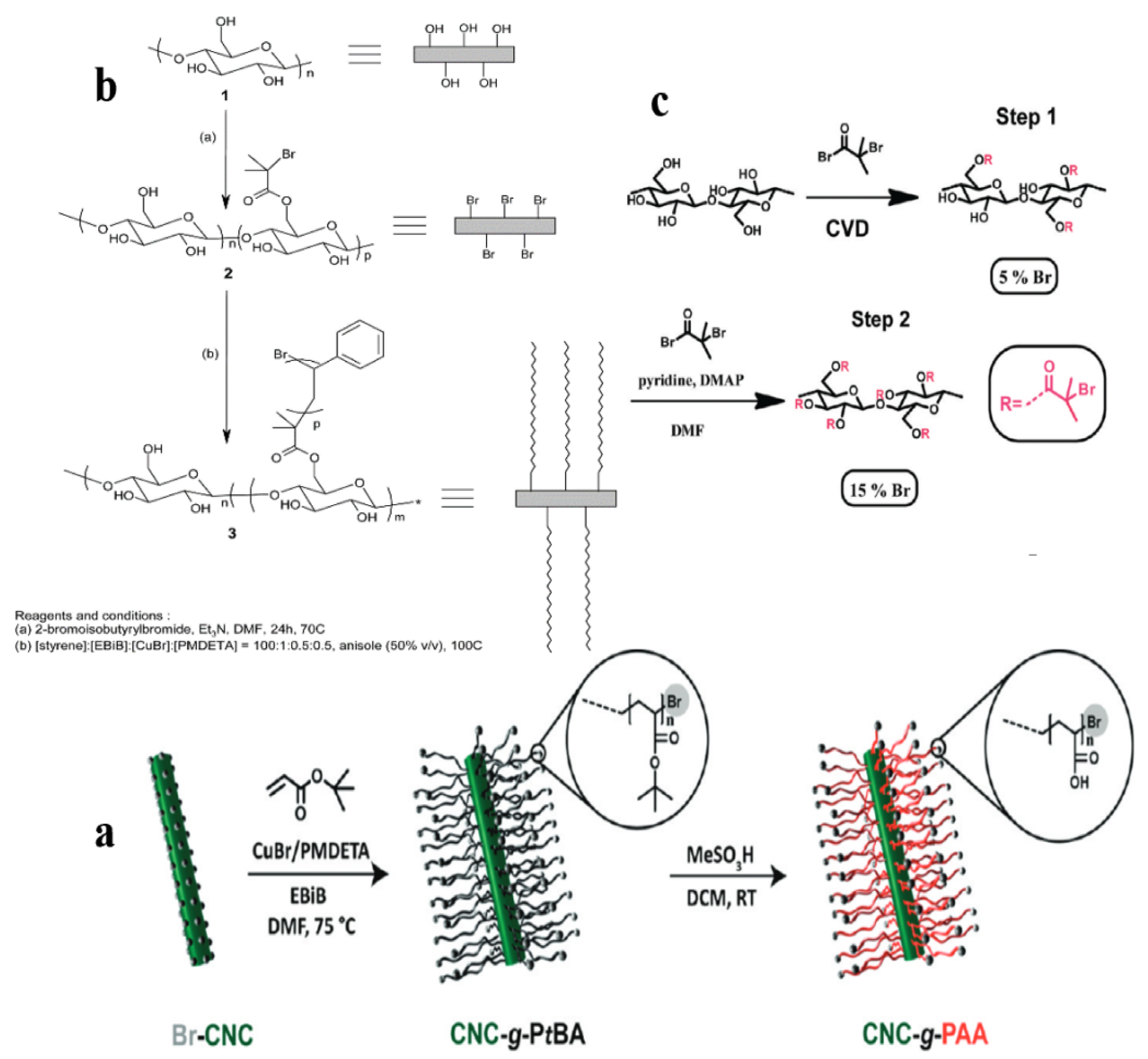
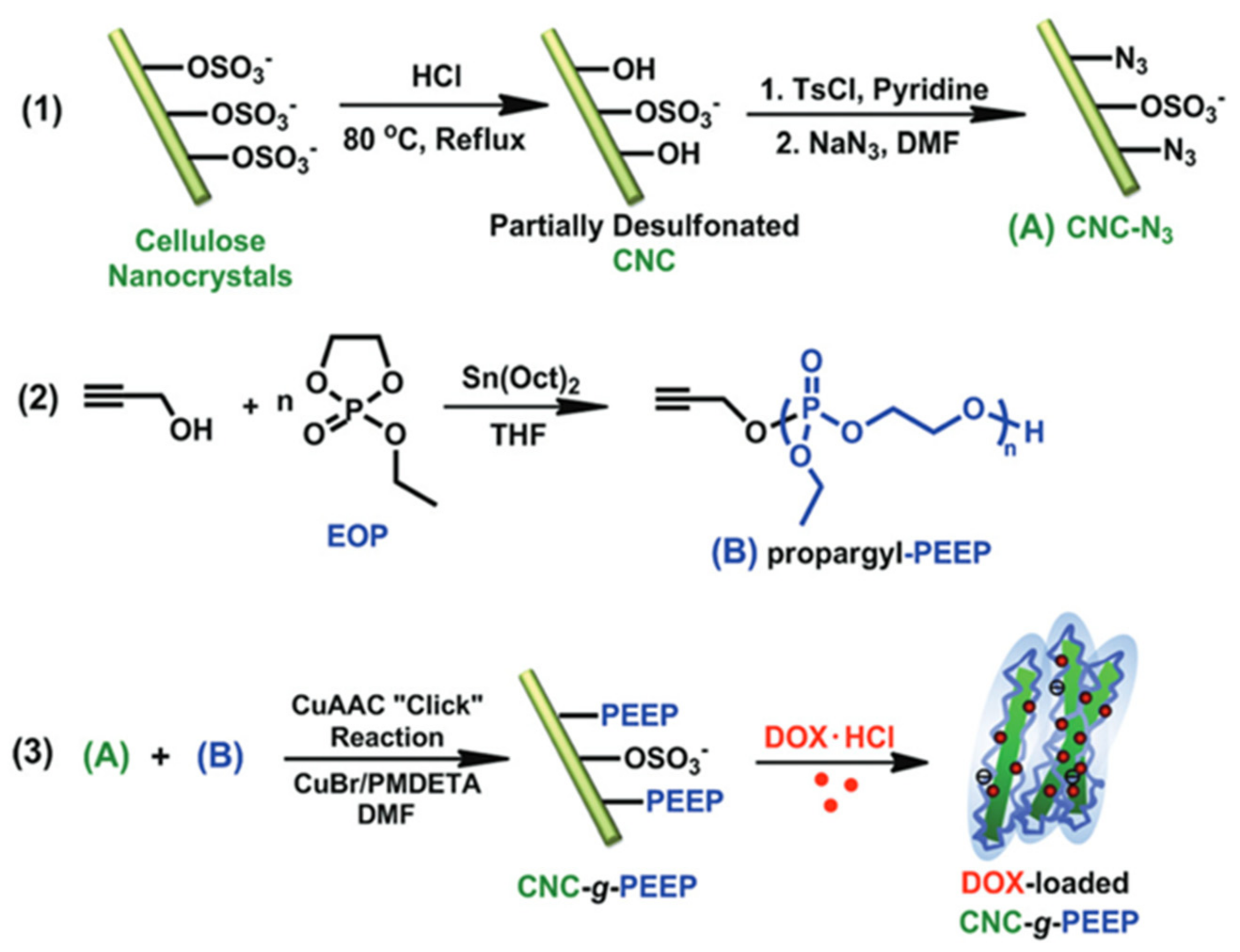
3.2. Macromolecules Chemically “Grafted from” CNCs
3.3. Polymers Chemically “Grafted” on CNCs
3.3.1. “Grafting from”
3.3.2. “Grafting to”
| Types of Modification | Method | Findings | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Small molecule functionalized CNCs | Grafted with lactic acid (CNC-g-LA) | High surface graft density, reduce the graft length to preserve the original size and morphology of CNCs | Green, simple, one-step; good dispersion in chloroform | Bad dispersion in water, ethanol, acetone, THF, toluene | Wu et al. [62] |
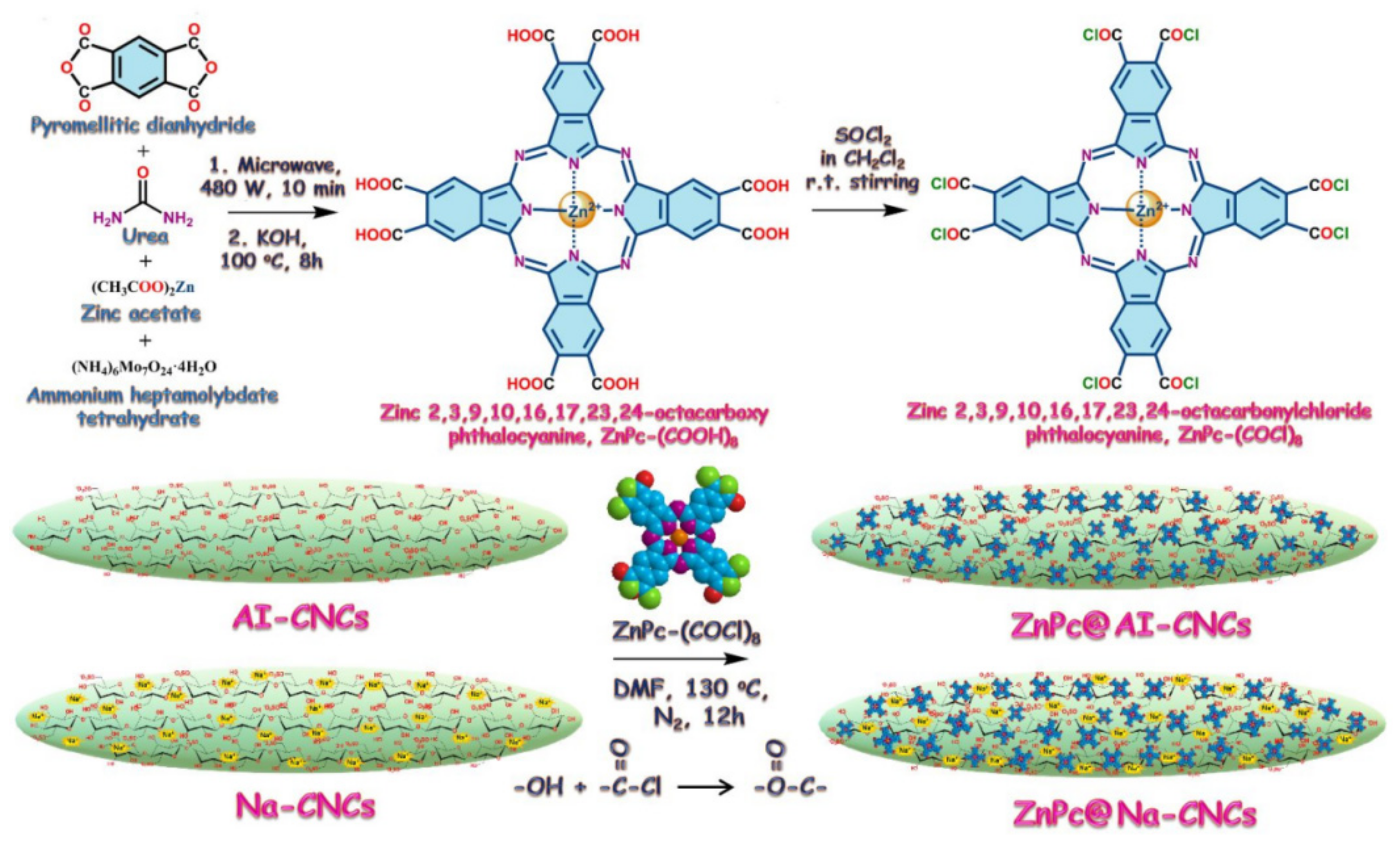
| Zinc phthalocyanine conjugate functionalization | Exhibited absorption and emission maxima at 690 and 715 nm | Photoluminescence-based technologies | Cost, activation of linker | Alam et al. [66] | |
| Aldehyde functionalization | DOTA-NH2 linked to the aldehyde | Makes the OH-groups on the CNC surface accessible for other subsequent functionalization | Low reactivity | Imlimthan et al. [74] | |
| Macromolecule Functionalized CNCs | Polydopamine functionalization | Contact angle shift from 21 to 95.6° | Spontaneous clicking reaction | Low hydrophobicity | Shang et al. [65] |
| CNCs grafted biomacromolecule | Click reaction between azide functionalized CNC and acetylene functionalized protein | Biocompatibility, low cytotoxicity, and nanometer size | Strict reagent selection due to protein’s nature | Karaaslan et al. [85] | |
| ATRP polymerization functionalization followed by mineralization of silica | Graft PDMAEMA brush with CNCs, form SiO2 network in the polymer brush layer to achieve template | Forms microporous and mesoporous silica nanorods | Severe aggregates during the solvent exchange for CNC | Morits et al. [104] | |
| Azo-benzodiazepines functionalization | Hydrophobic | Massive potential in optical information storage, optical switching, and linear optics. | Difficulties in purification and redispersion | Xu et al. [102] | |
| Aldehyde functionalization with polyetherimide | Forms arms of regular four-, five-, or six-branched stars | Creates hierarchical structures | Few reactivity sites | Lin et al. [111] |
3.4. Drawbacks and Future
4. Conclusions and Outlooks
- (1)
- How to modify CNCs in a more environmentally friendly way while maintaining the surface properties of the crystal, since most modifications use expensive and non-sustainable reagents.
- (2)
- How to modify CNCs more efficiently while maintaining the surface properties of the crystal, since most modifications need multiple steps (including solvent exchange and initiator attachment) and due to the low yields of modified CNCs.
- (3)
- How to retain its original size and crystal structure after multiple times of drying and cleansing.
- (4)
- How to keep uniform products after using large volumes of organic solvents in which CNCs are not colloidally stable.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ross, P.; Weinhouse, H.; Aloni, Y.; Michaeli, D.; Weinberger-Ohana, P.; Mayer, R.; Braun, S.; De Vroom, E.; Van Der Marel, G.A.; Van Boom, J.H.; et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 1987, 325, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W. Dynamics, chemistry, and preservation of organic matter in soils. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780080983004. [Google Scholar]
- Islam, M.N.; Rahman, F. Production and modification of nanofibrillated cellulose composites and potential applications. In Green Composites for Automotive Applications; Elsevier Science & Technology: Amsterdam, The Netherlands, 2018; ISBN 9780081021774. [Google Scholar]
- Shankaran, D.R. Cellulose nanocrystals for health care applications. In Applications of Nanomaterials; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Yun, S.; Ounaies, Z. Discovery of cellulose as a smart material. Macromolecules 2006, 39, 4202–4206. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Chen, L.; Sisler, J.; Tam, K.C. Cellulose nanocrystal (CNC)-inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.; Wang, X.; Gao, Q.; Li, X. Surface modification of cellulose nanocrystal using succinic anhydride and its effects on poly(butylene succinate) based composites. Cellulose 2019, 26, 3167–3181. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Radzik, P.; Szefer, E.; Mičušík, M.; Omastová, M.; Pielichowski, K. Surface modification of cellulose nanocrystals with succinic anhydride. Polymers 2019, 11, 866. [Google Scholar] [CrossRef] [Green Version]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. Recent studies on modified cellulose/nanocellulose epoxy composites: A systematic review. Carbohydr. Polym. 2021, 255, 117366. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, S.; Lavoine, N.; Lucia, L.A. Structural reconstruction strategies for the design of cellulose nanomaterials and aligned wood cellulose-based functional materials—A review. Carbohydr. Polym. 2020, 247, 116722. [Google Scholar] [CrossRef]
- Ansari, F.; Berglund, L.A. Toward Semistructural Cellulose Nanocomposites: The Need for Scalable Processing and Interface Tailoring. Biomacromolecules 2018, 19, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.; Liu, Y. Preparation of millimeter-long cellulose I nanofibers with diameters of 30–80 nm from bamboo fibers. Carbohydr. Polym. 2011, 86, 453–461. [Google Scholar] [CrossRef]
- Brito, B.S.L.; Pereira, F.V.; Putaux, J.-L.; Jean, B. Preparation, morphology and structure of cellulose nanocrystals from bamboo fibers. Cellulose 2012, 19, 1527–1536. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Gleisner, R.; Kuster, T.A.; Baxa, U.; McNeil, S.E. Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 2012, 19, 1631–1643. [Google Scholar] [CrossRef]
- Yu, L.; Lin, J.; Tian, F.; Li, X.; Bian, F.; Wang, J. Cellulose nanofibrils generated from jute fibers with tunable polymorphs and crystallinity. J. Mater. Chem. A 2014, 2, 6402. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Jiang, F.; Han, S.; Hsieh, Y.-L. Controlled defibrillation of rice straw cellulose and self-assembly of cellulose nanofibrils into highly crystalline fibrous materials. RSC Adv. 2013, 3, 12366. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef] [Green Version]
- Le Gars, M.; Douard, L.; Belgacem, N.; Bras, J. Cellulose nanocrystals: From classical hydrolysis to the use of deep eutectic solvents. In Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis; IntechOpen: London, UK, 2020. [Google Scholar]
- Bian, H.; Chen, L.; Dai, H.; Zhu, J.Y. Integrated production of lignin containing cellulose nanocrystals (LCNC) and nanofibrils (LCNF) using an easily recyclable di-carboxylic acid. Carbohydr. Polym. 2017, 167. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yu, G.; Yu, Q.; Li, B.; Mu, X. A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr. Polym. 2014, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Jin, T.; He, H.; Liu, L. Preparation of nanocellulose and its potential in reinforced composites: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 919–946. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Wang, B.; Yao, J.; Wang, H. TEMPO-oxidized bacterial cellulose nanofibers-supported gold nanoparticles with superior catalytic properties. Carbohydr. Polym. 2017, 160, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Sattler, S.; Reza, M.; Bismarck, A.; Kontturi, E. Cellulose nanocrystals by acid vapour: Towards more effortless isolation of cellulose nanocrystals. Faraday Discuss. 2017, 202, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 1, 2010–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Pang, B.; Tian, L.; Schäfer, T.; Gutmann, T.; Liu, H.; Volkert, C.A.; Buntkowsky, G.; Zhang, K. Efficient, Self-Terminating Isolation of Cellulose Nanocrystals through Periodate Oxidation in Pickering Emulsions. ChemSusChem 2018, 11, 3581–3585. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, Q.; Ye, D.; Chang, C. Tunicate cellulose nanocrystals reinforced nanocomposite hydrogels comprised by hybrid cross-linked networks. Carbohydr. Polym. 2017, 169, 139–148. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Chen, Y.; Liu, J.; Ren, Z. Facile one-step extraction and oxidative carboxylation of cellulose nanocrystals through hydrothermal reaction by using mixed inorganic acids. Cellulose 2017, 24, 3243–3254. [Google Scholar] [CrossRef]
- Pääkkönen, T.; Spiliopoulos, P.; Nonappa; Kontturi, K.S.; Penttilä, P.; Viljanen, M.; Svedström, K.; Kontturi, E. Sustainable High Yield Route to Cellulose Nanocrystals from Bacterial Cellulose. ACS Sustain. Chem. Eng. 2019, 7, 14384–14388. [Google Scholar] [CrossRef] [Green Version]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhu, J.Y.; Reiner, R.S.; Verrill, S.P.; Baxa, U.; McNeil, S.E. Approaching zero cellulose loss in cellulose nanocrystal (CNC) production: Recovery and characterization of cellulosic solid residues (CSR) and CNC. Cellulose 2012, 19, 2033–2047. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Khoshkava, V.; Kamal, M.R. Effect of drying conditions on cellulose nanocrystal (CNC) agglomerate porosity and dispersibility in polymer nanocomposites. Powder Technol. 2014, 261, 288–298. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.M.; Vianna, A.; De Carvalho, N.; Silva, D.D.J. Cellulose Nanocrystal Production Focusing on Cellulosic Material Pre-Treatment and acid hydrolysis. Artig. Técnico/Tech. Artic. 2019, 80, 59–66. [Google Scholar]
- Fang, Z.; Zhu, H.; Preston, C.; Han, X.; Li, Y.; Lee, S.; Chai, X.; Chen, G.; Hu, L. Highly transparent and writable wood all-cellulose hybrid nanostructured paper. J. Mater. Chem. C 2013, 1, 6191–6197. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly($ε$-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y. Lo Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Bahloul, A.; Kassab, Z.; Aziz, F.; Hannache, H.; Bouhfid, R.; Qaiss, A.E.K.; Oumam, M.; El Achaby, M. Characteristics of cellulose microfibers and nanocrystals isolated from doum tree (Chamaerops humilis var. argentea). Cellulose 2021, 28, 4089–4103. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, M.; Hou, Q.; Liu, R.; Wu, T.; Si, C. Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 2016, 23, 439–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wang, X.; Ni, S.; Rosqvist, E.; Smått, J.H.; Peltonen, J.; Hou, Q.; Qin, M.; Willför, S.; et al. From Biomass to Nanomaterials: A Green Procedure for Preparation of Holistic Bamboo Multifunctional Nanocomposites Based on Formic Acid Rapid Fractionation. ACS Sustain. Chem. Eng. 2019, 7, 6592–6600. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.; Retsina, T.; Iakovlev, M.; van Heiningen, A.; Deng, Y.; Shatkin, J.A.; Mulyadi, A. American process: Production of low cost nanocellulose for renewable, advanced materials applications. In Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 224. [Google Scholar]
- Wijaya, C.J.; Ismadji, S.; Aparamarta, H.W.; Gunawan, S. Hydrophobic modification of cellulose nanocrystals from bamboo shoots using rarasaponins. ACS Omega 2020, 5, 20967–20975. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.M.; Mekonnen, T. Hydrophobic esterification of cellulose nanocrystals for epoxy reinforcement. Polymer 2018, 155, 64–74. [Google Scholar] [CrossRef]
- Chu, Y.; Song, R.; Zhang, L.; Dai, H.; Wu, W. Water-dispersible, biocompatible and fluorescent poly(ethylene glycol)-grafted cellulose nanocrystals. Int. J. Biol. Macromol. 2020, 153, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Gong, Y.B.; Ma, S.; Wang, Y.H.; Gan, L.; Huang, J. Antistatic Structural Color and Photoluminescent Membranes from Co-assembling Cellulose Nanocrystals and Carbon Nanomaterials for Anti-counterfeiting. Chin. J. Polym. Sci. 2020, 38, 1061–1071. [Google Scholar] [CrossRef]
- Adriana; Jalal, R.; Yuniati. Antistatic effect of glycerol monostearate on volume resistivity and mechanical properties of nanocomposite polystyrene-nanocrystal cellulose. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 1977. [Google Scholar]
- Cui, Y.; Huang, H.; Liu, M.; Chen, J.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Facile preparation of luminescent cellulose nanocrystals with aggregation-induced emission feature through Ce(IV) redox polymerization. Carbohydr. Polym. 2019, 223, 115102. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.J.; Meng, F.H.; Liu, J.L. Preparation, modification and functional application of cellulose nanocrystals. Xiandai Huagong/Modern Chem. Ind. 2019, 39, 58–62. [Google Scholar]
- Abushammala, H.; Mao, J. A review of the surface modification of cellulose and nanocellulose using aliphatic and aromatic mono- And di-isocyanates. Molecules 2019, 24, 2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Mai, Y.; He, F.; Yu, L.; Zhang, L.; Tang, H.; Yang, G. Bio-based green composites with high performance from poly(lactic acid) and surface-modified microcrystalline cellulose. J. Mater. Chem. 2012, 22, 15732. [Google Scholar] [CrossRef]
- Wu, H.; Nagarajan, S.; Shu, J.; Zhang, T.; Zhou, L.; Duan, Y.; Zhang, J. Green and facile surface modification of cellulose nanocrystal as the route to produce poly(lactic acid) nanocomposites with improved properties. Carbohydr. Polym. 2018, 197, 204–214. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Mariano, M.; Rabelo, S.C.; Gouveia, R.F.; Lona, L.M.F. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro- to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Hu, Z.; Berry, R.M.; Pelton, R.; Cranston, E.D. One-Pot Water-Based Hydrophobic Surface Modification of Cellulose Nanocrystals Using Plant Polyphenols. ACS Sustain. Chem. Eng. 2017, 5, 5018–5026. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, C.; Hu, Y.; Jia, P.; Hu, L.; Zhou, Y. Bio-inspired hydrophobic modification of cellulose nanocrystals with castor oil. Carbohydr. Polym. 2018, 191, 168–175. [Google Scholar] [CrossRef]
- Alam, K.; Kumar, P.; Gusarov, S.; Kobryn, A.E.; Aarat, P.; Zeng, S.; Goswami, A.; Thundat, T.; Shankar, K. Synthesis and characterization of zinc phthalocyanine-cellulose nanocrystal (CNC) conjugates: Towards highly functional CNCs Synthesis and Characterization of Zinc Phthalocyanine—Cellulose Nanocrystal (CNC) Conjugates: Towards Highly Functional CN. ACS Appl. Mater. Interfaces 2020, 12, 39. [Google Scholar] [CrossRef]
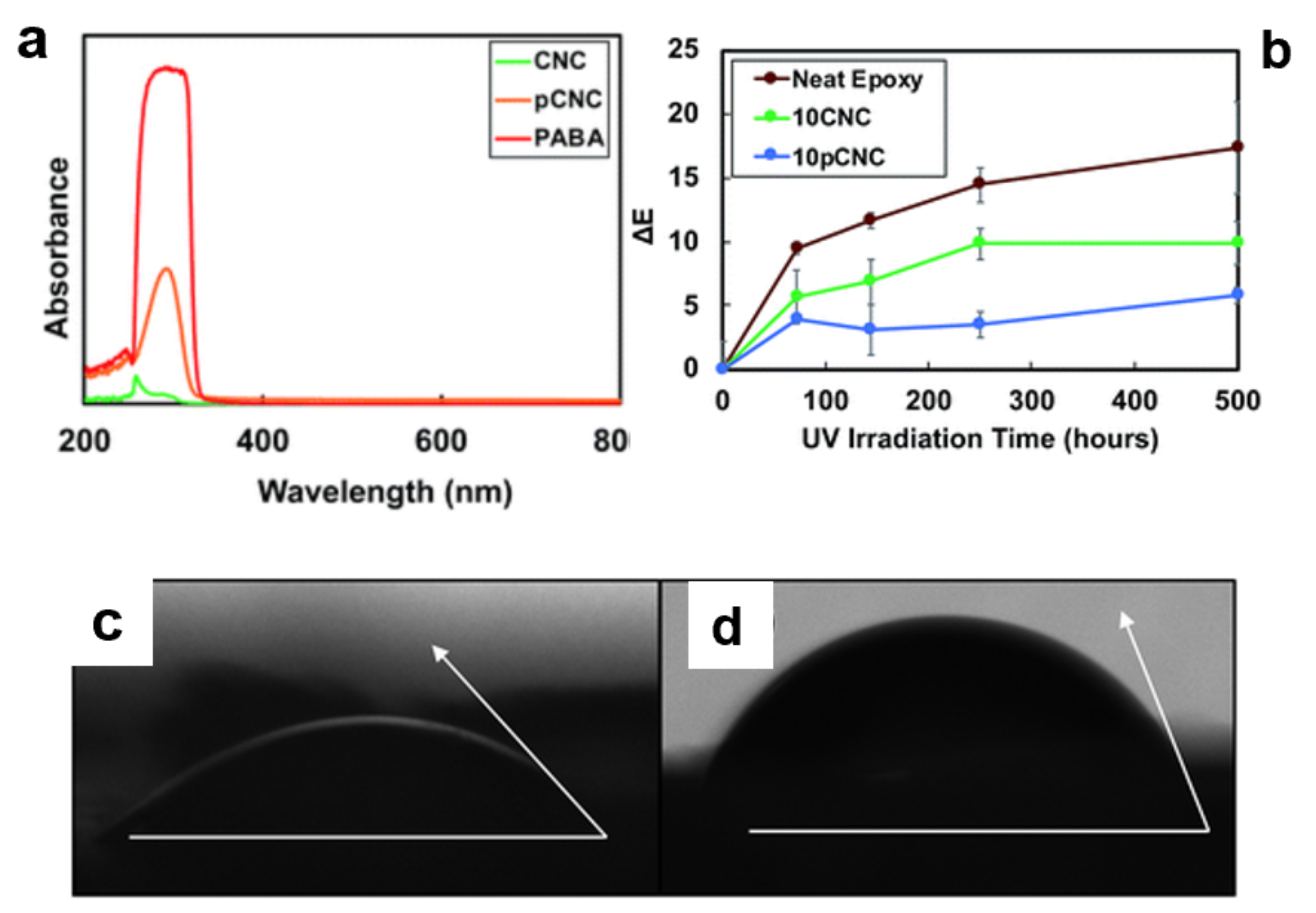
- Panchal, P.; Mekonnen, T.H. Tailored cellulose nanocrystals as a functional ultraviolet absorbing nanofiller of epoxy polymers. Nanoscale Adv. 2019, 1, 2612–2623. [Google Scholar] [CrossRef] [Green Version]
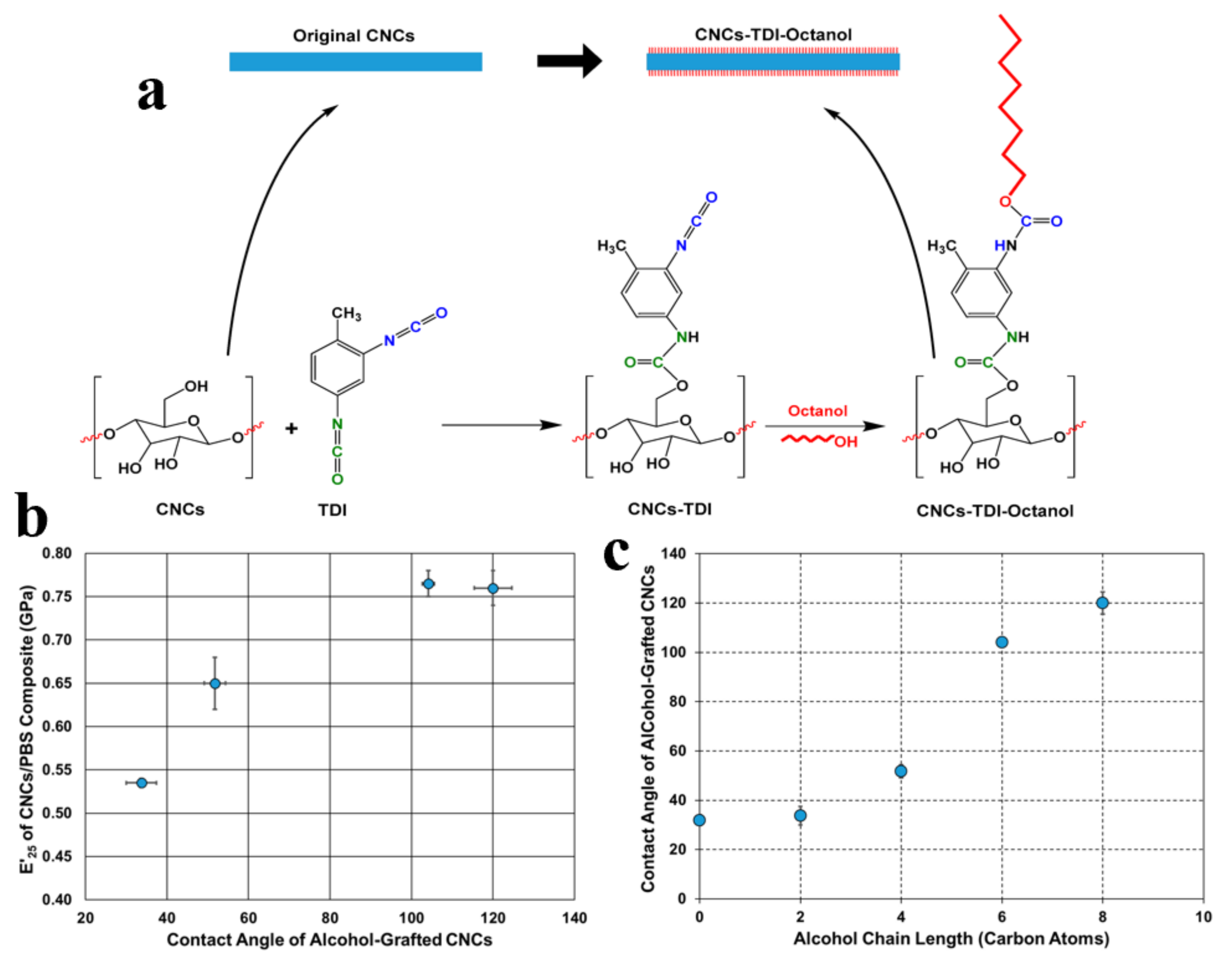
- Abushammala, H. Nano-Brushes of alcohols grafted onto cellulose nanocrystals for reinforcing poly(Butylene succinate): Impact of Alcohol chain length on interfacial adhesion. Polymers 2020, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Fatona, A.; Berry, R.M.; Brook, M.A.; Moran-Mirabal, J.M. Versatile Surface Modification of Cellulose Fibers and Cellulose Nanocrystals through Modular Triazinyl Chemistry. Chem. Mater. 2018, 30, 2424–2435. [Google Scholar] [CrossRef]
- Rosilo, H.; McKee, J.R.; Kontturi, E.; Koho, T.; Hytönen, V.P.; Ikkala, O.; Kostiainen, M.A. Cationic polymer brush-modified cellulose nanocrystals for high-affinity virus binding. Nanoscale 2014, 6, 11871–11881. [Google Scholar] [CrossRef]
- Ly, M.; Mekonnen, T.H. Cationic surfactant modified cellulose nanocrystals for corrosion protective nanocomposite surface coatings. J. Ind. Eng. Chem. 2020, 83, 409–420. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, N. Surface Chemistry of Nanocellulose. In Nanocellulose; Wiley: Hoboken, NJ, USA, 2019; pp. 115–153. [Google Scholar] [CrossRef]
- Chen, H.; Hou, A.; Zheng, C.; Tang, J.; Xie, K.; Gao, A. Light- and humidity-responsive chiral nematic photonic crystal films based on cellulose nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 24505–24511. [Google Scholar] [CrossRef] [PubMed]
- Imlimthan, S.; Otaru, S.; Keinänen, O.; Correia, A.; Lintinen, K.; Santos, H.A.; Airaksinen, A.J.; Kostiainen, M.A.; Sarparanta, M. Radiolabeled Molecular Imaging Probes for the in Vivo Evaluation of Cellulose Nanocrystals for Biomedical Applications. Biomacromolecules 2019, 20, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.R.G.; Bergström, L. Labelling of N-hydroxysuccinimide-modified rhodamine B on cellulose nanofibrils by the amidation reaction. RSC Adv. 2014, 4, 60757–60761. [Google Scholar] [CrossRef]
- Mukumoto, K.; Li, Y.; Nese, A.; Sheiko, S.S.; Matyjaszewski, K. Synthesis and characterization of molecular bottlebrushes prepared by iron-based ATRP. Macromolecules 2012, 45, 9243–9249. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Stenzel, M.H. Surface modified cellulose nanomaterials: A source of non-spherical nanoparticles for drug delivery. Mater. Horizons 2020, 7, 1727–1758. [Google Scholar] [CrossRef]
- Madhusudana Rao, K.; Kumar, A.; Han, S.S. Polysaccharide based bionanocomposite hydrogels reinforced with cellulose nanocrystals: Drug release and biocompatibility analyses. Int. J. Biol. Macromol. 2017, 101, 165–171. [Google Scholar] [CrossRef]
- Pauli, J.; Licha, K.; Berkemeyer, J.; Grabolle, M.; Spieles, M.; Wegner, N.; Welker, P.; Resch-Genger, U. New fluorescent labels with tunable hydrophilicity for the rational design of bright optical probes for molecular imaging. Bioconjug. Chem. 2013, 24, 1174–1185. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.W.; Hung, Y.; Hsiao, J.K.; Yao, M.; Chung, T.H.; Lin, Y.S.; Wu, S.H.; Hsu, S.C.; Liu, H.M.; Mou, C.Y.; et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007, 7, 149–154. [Google Scholar] [CrossRef]
- Li, L.; Tao, H.; Wu, B.; Zhu, G.; Li, K.; Lin, N. Triazole End-Grafting on Cellulose Nanocrystals for Water-Redispersion Improvement and Reactive Enhancement to Nanocomposites. ACS Sustain. Chem. Eng. 2018, 6, 14888–14900. [Google Scholar] [CrossRef]
- Orelma, H.; Johansson, L.S.; Filpponen, I.; Rojas, O.J.; Laine, J. Generic method for attaching biomolecules via avidin-biotin complexes immobilized on films of regenerated and nanofibrillar cellulose. Biomacromolecules 2012, 13, 2802–2810. [Google Scholar] [CrossRef]
- Tao, H.; Lavoine, N.; Jiang, F.; Tang, J.; Lin, N. Reducing end modification on cellulose nanocrystals: Strategy, characterization, applications and challenges. Nanoscale Horizons 2020, 5, 607–627. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, M.A.; Gao, G.; Kadla, J.F. Nanocrystalline cellulose/β-casein conjugated nanoparticles prepared by click chemistry. Cellulose 2013, 20, 2655–2665. [Google Scholar] [CrossRef]
- Uth, C.; Zielonka, S.; Hörner, S.; Rasche, N.; Plog, A.; Orelma, H.; Avrutina, O.; Zhang, K.; Kolmar, H. Eine chemoenzymatische Kupplungsstrategie zur Immobilisierung von Proteinen auf kristalliner Nanocellulose. Angew. Chemie 2014, 126, 12826–12832. [Google Scholar] [CrossRef]
- Bucio, E.; Burillo, G. Radiation-induced grafting of sensitive polymers. J. Radioanal. Nucl. Chem. 2009, 280, 239–243. [Google Scholar] [CrossRef]
- Minko, S. Grafting on solid surfaces: Grafting to and grafting from methods. In Polymer Surfaces and Interfaces: Characterization, Modification and Applications; Springer: Berlin, Germany, 2008; ISBN 9783540738640. [Google Scholar]
- Kim, M.; Schmitt, S.K.; Choi, J.W.; Krutty, J.D.; Gopalan, P. From self-assembled monolayers to coatings: Advances in the synthesis and nanobio applications of polymer brushes. Polymers 2015, 7, 1346–1378. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Lahasky, S.H.; Sheiko, S.S.; Zhang, D. Core-shell molecular bottlebrushes with helical polypeptide backbone: Synthesis, characterization, and solution conformations. Macromolecules 2011, 44, 1491–1499. [Google Scholar] [CrossRef]
- Hegazy, E.S.A.; El-Assy, N.B.; Rabie, A.G.M.; Ishigaki, I.; Okamoto, J. Kinetic study of preirradiation grafting of acrylic acid onto poly(tetrafluoroethylene—Perfluorovinyl ether) copolymer. J. Polym. Sci. 1984, 22, 597–604. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; López-Saucedo, F.; López-Barriguete, J.E.; Varca, G.H.C.; Bucio, E. Radiation Grafting for the Functionalization and Development of Smart Polymeric Materials. Top. Curr. Chem. 2016, 374, 63. [Google Scholar] [CrossRef]
- Cheng, G.; Böker, A.; Zhang, M.; Krausch, G.; Müller, A.H.E. Amphiphilic cylindrical core-shell brushes via a “grafting from” process using ATRP. Macromolecules 2001, 34, 6883–6888. [Google Scholar] [CrossRef]
- Fujiki, K.; Tsubokawa, N.; Sone, Y. Radical grafting from carbon black graft polymerization of vinyl monomers initiated by azo groups introduced onto carbon black surface. Polym. J. 1990, 22, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Xu, Y.; Yao, L.; Peng, H.; Cheng, S. Synthesis of amphihilic semifluorinated cylindrical brushes PBIEM-g-(PAA-b-PFA) VIA ATRP. Acta Polym. Sin. 2009, 9, 1146–1153. [Google Scholar] [CrossRef]
- Beers, K.L.; Gaynor, S.G.; Matyjaszewski, K.; Sheiko, S.S.; Moeller, M. Synthesis of densely grafted copolymers by atom transfer radical polymerization. Macromolecules 1998, 31, 9413–9415. [Google Scholar] [CrossRef]
- Yin, Y.; Tian, X.; Jiang, X.; Wang, H.; Gao, W. Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr. Polym. 2016, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Schmitt, V.; Sèbe, G.; Héroguez, V. Convenient Synthesis of Hybrid Polymer Materials by AGET-ATRP Polymerization of Pickering Emulsions Stabilized by Cellulose Nanocrystals Grafted with Reactive Moieties. Biomacromolecules 2019, 20, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through Surface-Initiated Atom Transfer Radical Polymerization (SI-ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Tam, K.C.; Sèbe, G. A comparative study on grafting polymers from cellulose nanocrystals via surface-initiated atom transfer radical polymerization (ATRP) and activator re-generated by electron transfer ATRP. Carbohydr. Polym. 2019, 205, 322–329. [Google Scholar] [CrossRef]
- Majoinen, J.; Walther, A.; McKee, J.R.; Kontturi, E.; Aseyev, V.; Malho, J.M.; Ruokolainen, J.; Ikkala, O. Polyelectrolyte brushes grafted from cellulose nanocrystals using Cu-mediated surface-initiated controlled radical polymerization. Biomacromolecules 2011, 12, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Peng, S.; Zhou, G.; Xu, X. Highly Hydrophobic, Homogeneous Suspension and Resin by Graft Copolymerization Modification of Cellulose Nanocrystal (CNC). J. Compos. Sci. 2020, 4, 186. [Google Scholar] [CrossRef]
- Park, C.H.; Jeon, S.; Park, S.H.; Shin, M.G.; Park, M.S.; Lee, S.Y.; Lee, J.H. Cellulose nanocrystal-assembled reverse osmosis membranes with high rejection performance and excellent antifouling g. J. Mater. Chem. A 2019, 7, 3992–4001. [Google Scholar] [CrossRef]
- Morits, M.; Hynninen, V.; Nonappa; Niederberger, A.; Ikkala, O.; Gröschel, A.H.; Müllner, M. Polymer brush guided templating on well-defined rod-like cellulose nanocrystals. Polym. Chem. 2018, 9, 1650–1657. [Google Scholar] [CrossRef]
- Lopez, R.; Mendoza, R.; Mathew, A.P.; Valencia, L. Plasma surface-modi fi cation of cellulose nanocrystals: A green alternative towards mechanical reinforcement of ABS. RSC Adv. 2019, 9, 17417–17424. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of cellulose nanocrystals for advanced applications. J. Colloid Interface Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef]
- Wu, W.; Huang, F.; Pan, S.; Mu, W.; Meng, X.; Yang, H.; Xu, Z.; Ragauskas, A.J.; Deng, Y. Thermo-responsive and fluorescent cellulose nanocrystals grafted with polymer brushes. J. Mater. Chem. A 2015, 3, 1995–2005. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting Polymers from Cellulose Nanocrystals: Synthesis, Properties, and Applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; He, J.; Zhang, M.; Tam, K.C.; Ni, P. A new pathway towards polymer modified cellulose nanocrystals via a “grafting onto” process for drug delivery. Polym. Chem. 2015, 6, 4206–4209. [Google Scholar] [CrossRef]
- Lin, F.; Cousin, F.; Putaux, J.L.; Jean, B. Temperature-Controlled Star-Shaped Cellulose Nanocrystal Assemblies Resulting from Asymmetric Polymer Grafting. ACS Macro Lett. 2019, 8, 345–351. [Google Scholar] [CrossRef]
- Hemraz, U.D.; Lu, A.; Sunasee, R.; Boluk, Y. Structure of poly(N-isopropylacrylamide) brushes and steric stability of their grafted cellulose nanocrystal dispersions. J. Colloid Interface Sci. 2014, 430, 157–165. [Google Scholar] [CrossRef]
- He, L.; Zhang, L.; Ye, Y.; Liang, H. Solvent-induced self-assembly of polymer-tethered nanorods. J. Phys. Chem. B 2010, 114, 7189–7200. [Google Scholar] [CrossRef] [PubMed]
- Risteen, B.; Delepierre, G.; Srinivasarao, M.; Weder, C.; Russo, P.; Reichmanis, E.; Zoppe, J. Thermally Switchable Liquid Crystals Based on Cellulose Nanocrystals with Patchy Polymer Grafts. Small 2018, 14, e1802060. [Google Scholar] [CrossRef] [PubMed]
















| Sample | Bamboo [16] | Eucalyptus [16] | Sisal [16] | Curauá [16] | Lemon Seeds [46] | Tomato Peel [47] | Doum Tree [48] | Sugar Palm [49] | Cotton Fiber [50] |
|---|---|---|---|---|---|---|---|---|---|
| Hydrolysis conditions | |||||||||
| Acid-fiber ratio (mL/g) | 10/1 | 9/1 | 15/1 | 15/1 | 20/1 | 8.75/1 | 20/1 | 11/1 | |
| Temperature (°C) | 60 | 50 | 50 | 50 | 45 | 45 | 50 | 45 | 50 |
| Hydrolysis time (min) | 12 | 50–65 | 30 | 45 | 90 | 30 | 30 | 45 | 45 |
| H2SO4 concentration (wt%) | 65 | 65 | 65 | 65 | 64 | 64 | 64 | 60 | 63.9 |
| Sonication time (min) | 4 | 1 | 7 | 7 | 30 | 5 | 5 | 30 | - |
| Characteristics of CNCs | |||||||||
| Yield (%) | 30 | 17 | 9 | - | 27.61 | 15.7 | 29 | 41.7 | |
| Sulfur content (% S) a | 1.04 | 0.96 | 0.53 | 0.95 | - | 0.48 | |||
| Total anionic sites (mmol kg−1) a | 324 | 275 | 166 | 297 | - | - | - | - | - |
| Crystallinity index (%) | - | - | - | - | 69.67 | 80.8 | 90 | 85.9 | 91.26 |
| Zeta potential (mV) | −59 ± 2 | −48 ± 7 | −49 ± 2 | −52 ± 1 | −40.27 | −52.4 ± 1.4 | −61.50 ± 1.65 | ||
| Length (nm) b | 100 ± 28 | 100 ± 33 | 119 ± 45 | 129 ± 32 | 145 ± 20.7 | 135 ± 50 | 450 | 130 ± 30.23 | 140.9 |
| Width (nm) b | 8 ± 3 | 7 ± 1 | 6 ± 1 | 5 ± 1 | 18.5 ± 6.5 | 7.2 ± 1.8 | 5.3 | 9 ± 1.96 | - |
| Height (nm) c | 4.5 ± 0.9 | 4.5 ± 1.0 | 3.3 ± 1.0 | 4.7 ± 1.0 | - | 3.3 ± 1.2 | - | - | |
| Aspect ratio | 22 | 22 | 36 | 27 | 8 | 21 | 85 | 15 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Luo, Q.; Zhou, G.; Xu, X. Recent Advances on Cellulose Nanocrystals and Their Derivatives. Polymers 2021, 13, 3247. https://doi.org/10.3390/polym13193247
Peng S, Luo Q, Zhou G, Xu X. Recent Advances on Cellulose Nanocrystals and Their Derivatives. Polymers. 2021; 13(19):3247. https://doi.org/10.3390/polym13193247
Chicago/Turabian StylePeng, Shuting, Qiguan Luo, Guofu Zhou, and Xuezhu Xu. 2021. "Recent Advances on Cellulose Nanocrystals and Their Derivatives" Polymers 13, no. 19: 3247. https://doi.org/10.3390/polym13193247
APA StylePeng, S., Luo, Q., Zhou, G., & Xu, X. (2021). Recent Advances on Cellulose Nanocrystals and Their Derivatives. Polymers, 13(19), 3247. https://doi.org/10.3390/polym13193247






