Preparation of Foam Dressings Based on Gelatin, Hyaluronic Acid, and Carboxymethyl Chitosan Containing Fibroblast Growth Factor-7 for Dermal Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
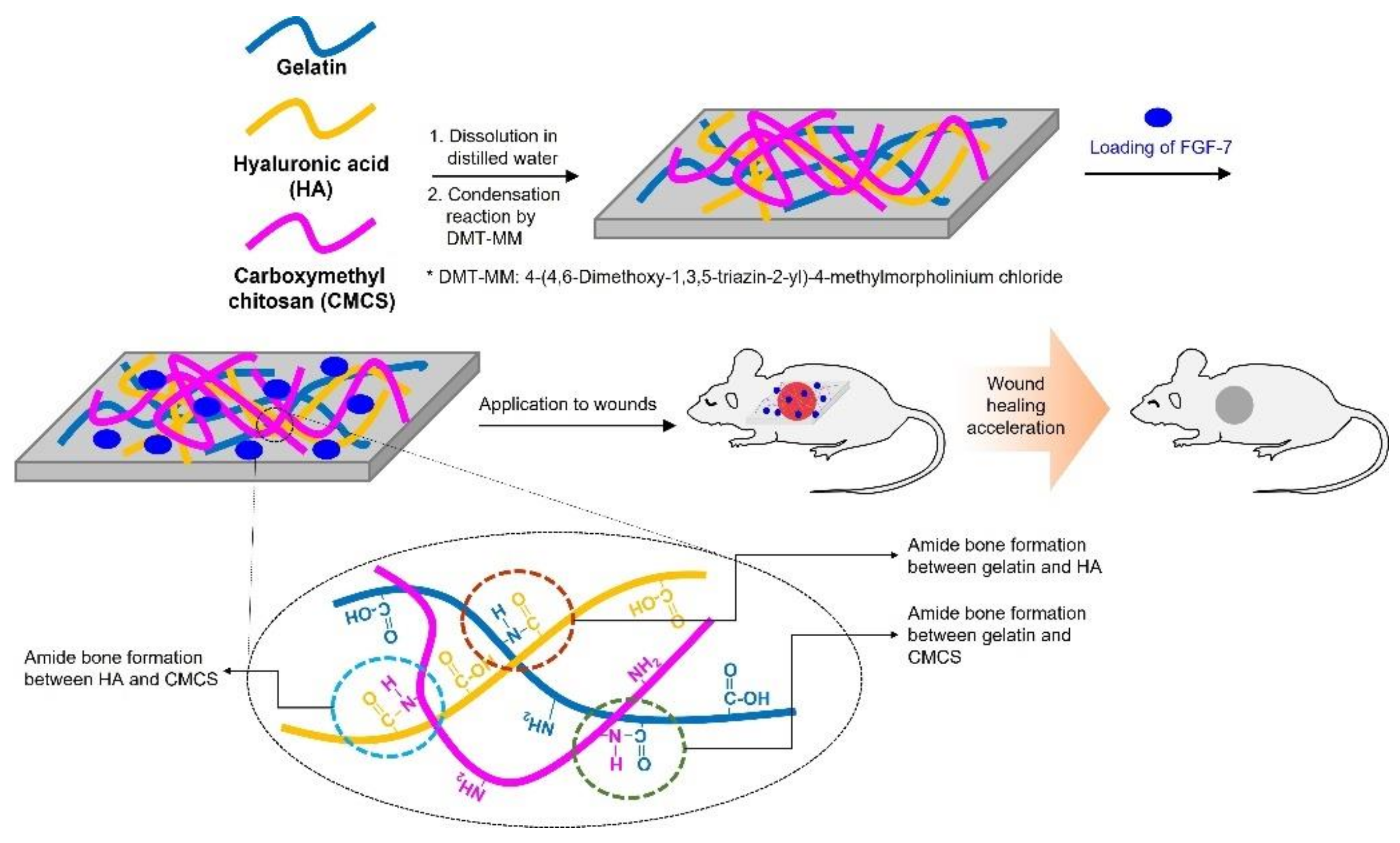
2.2. FDs Comprising Gelatin, CMCS, and HA (GHC-FDs)
2.2.1. Preparation
2.2.2. NIH/3T3 Cell Proliferation and Live and Dead Assays
2.3. Characterization of 2-GHC-FD-1, 2-GHC-FD-4 and 2-GHC-FD-8
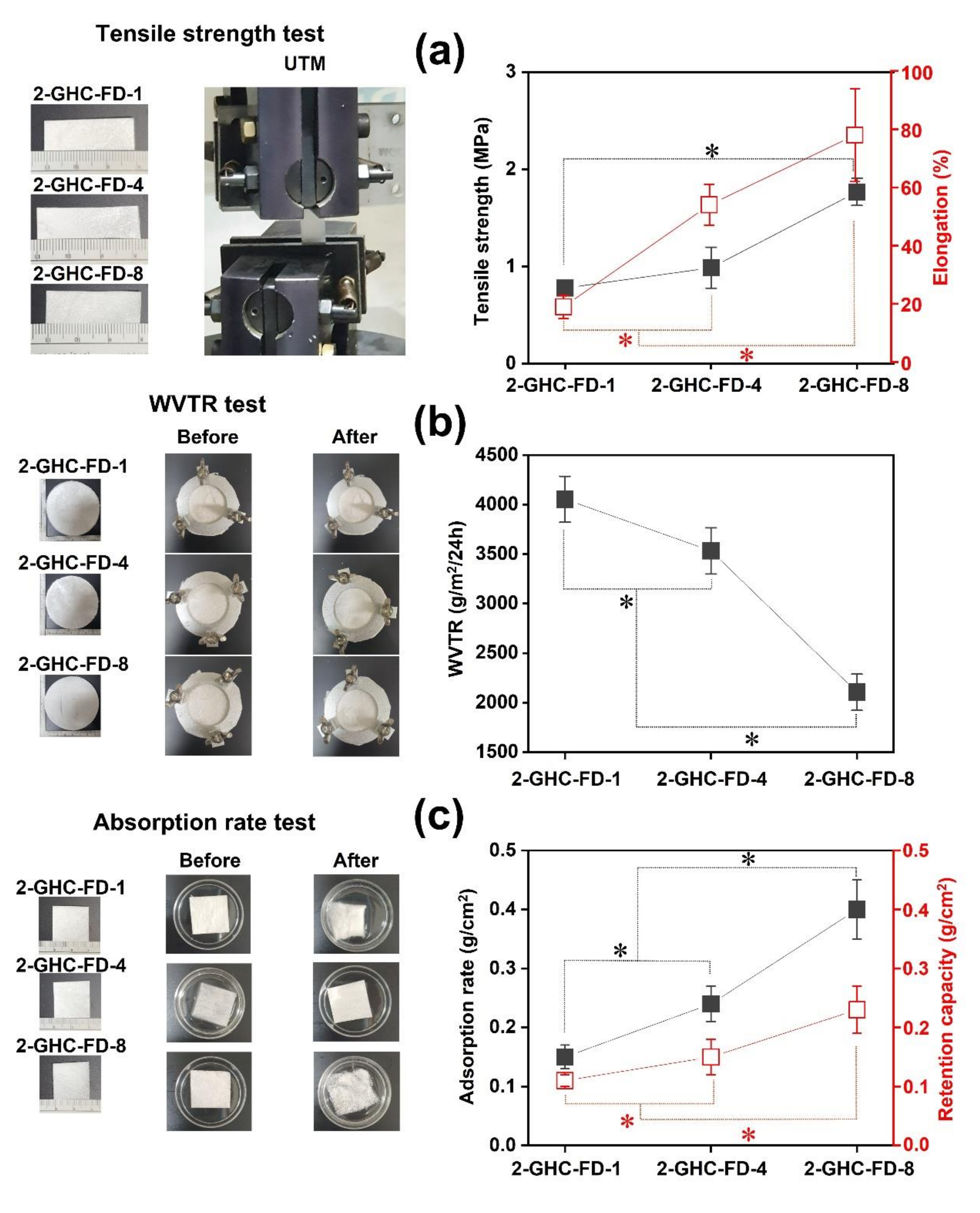
2.3.1. Tensile Strength Measurement
2.3.2. WVTR Test
2.3.3. Absorption Rate Test
2.3.4. Animal Intradermal Reactivity Test
2.4. Preparation of FDs Containing Various Concentrations of FGF-7
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Release Test of FGF-7
2.7. In Vivo Animal Study
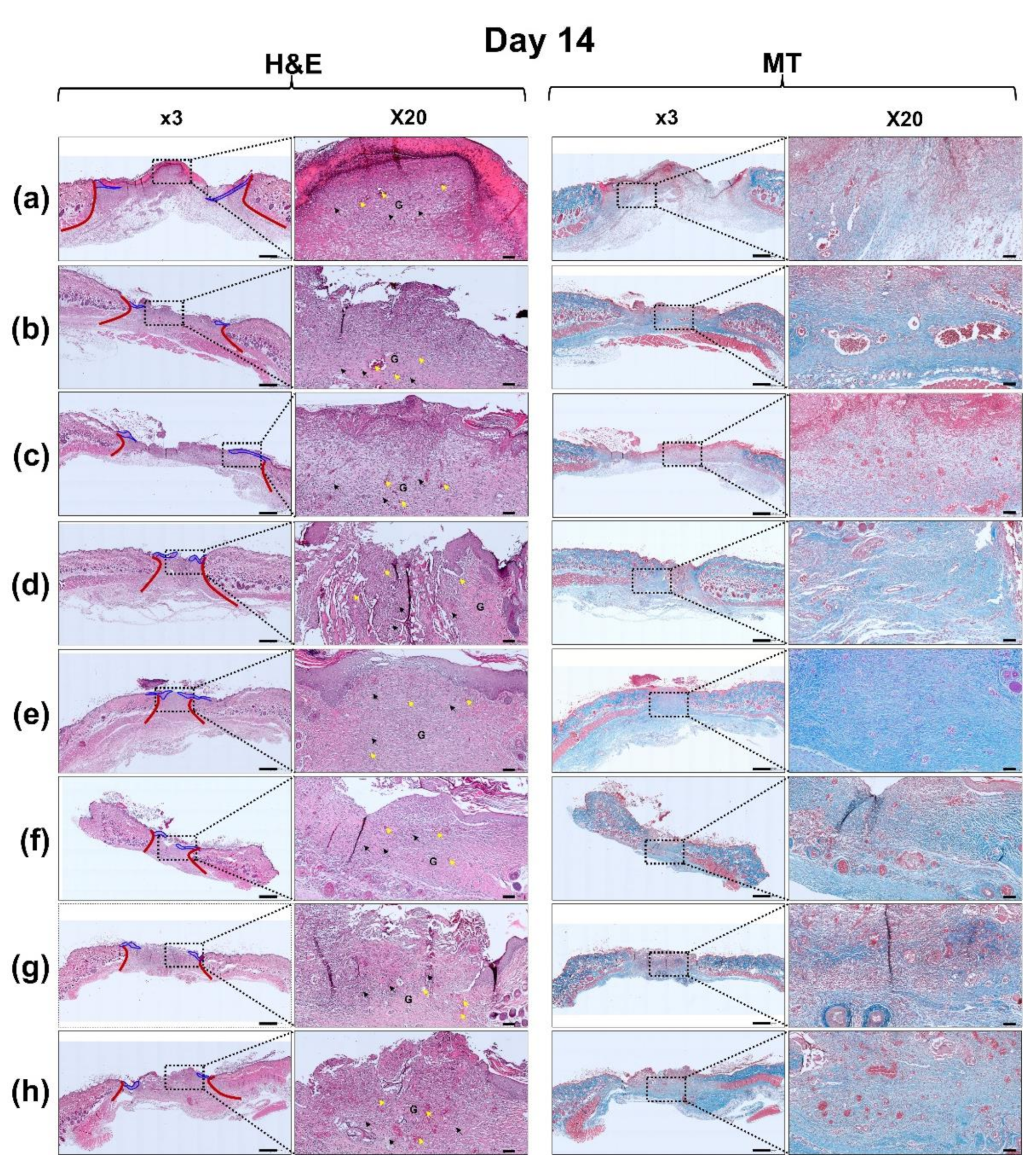
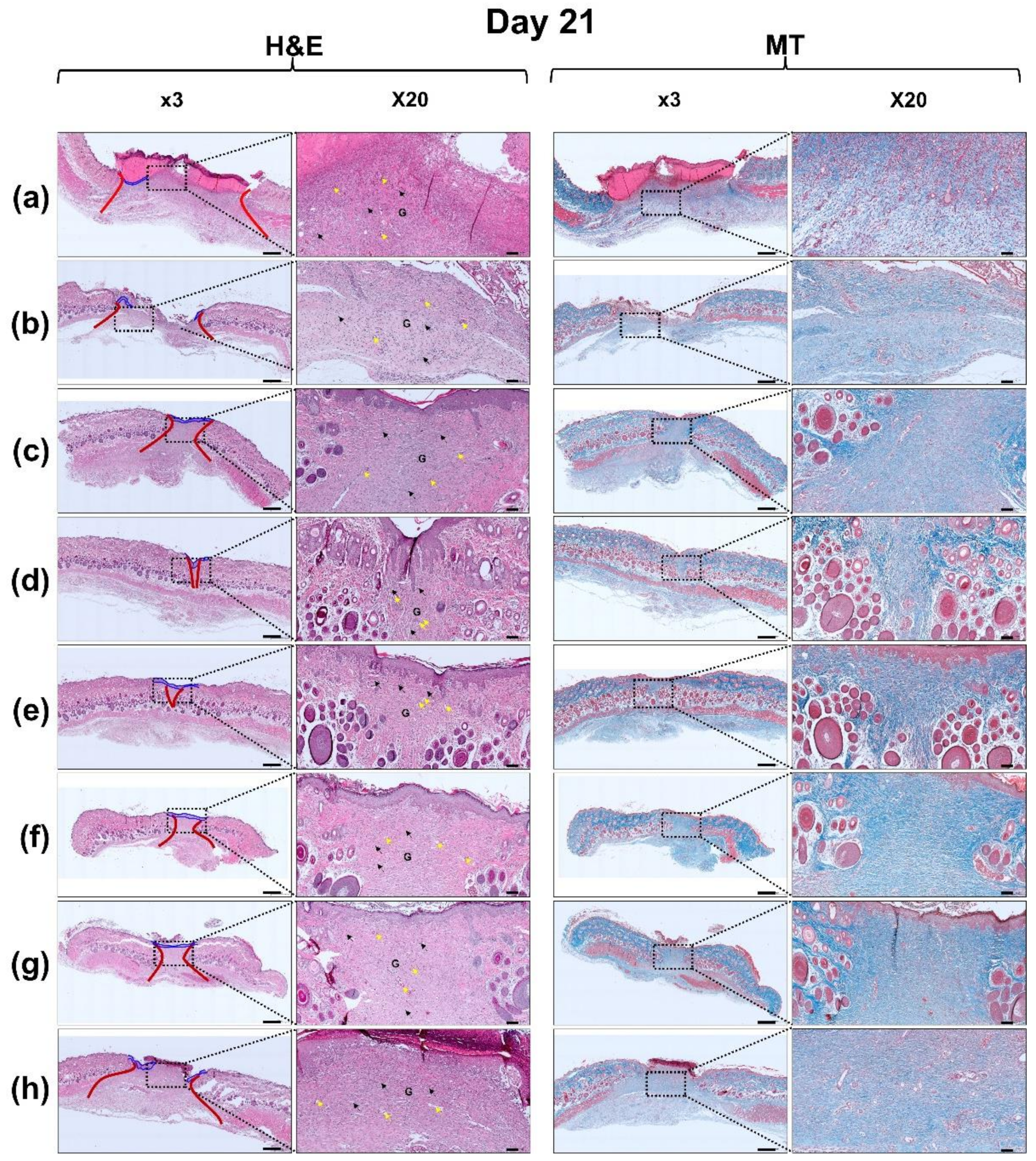
2.8. Hematoxylin and Eosin (H&E) and Masson’s Trichrome (MT) Staining
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Gelatin, CMCS, and HA-blended Foam Dressings (GHC-FDs)
3.1.1. Preparation of FDs
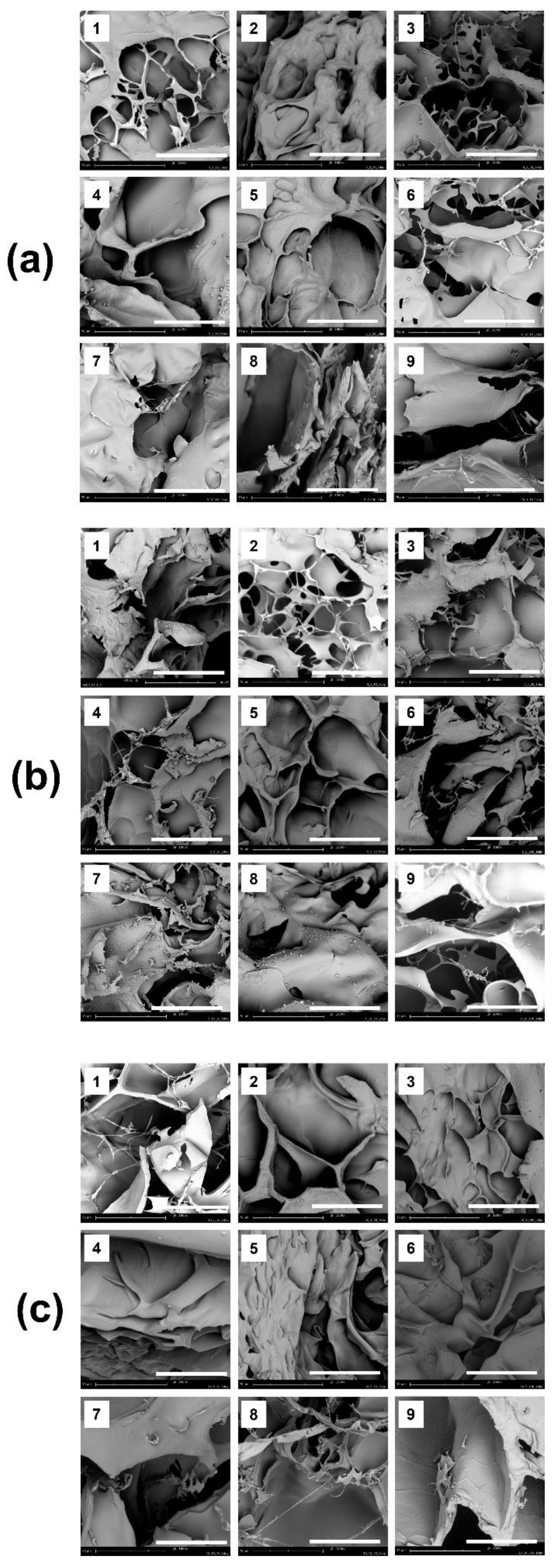
3.1.2. Observation of Surface Morphology
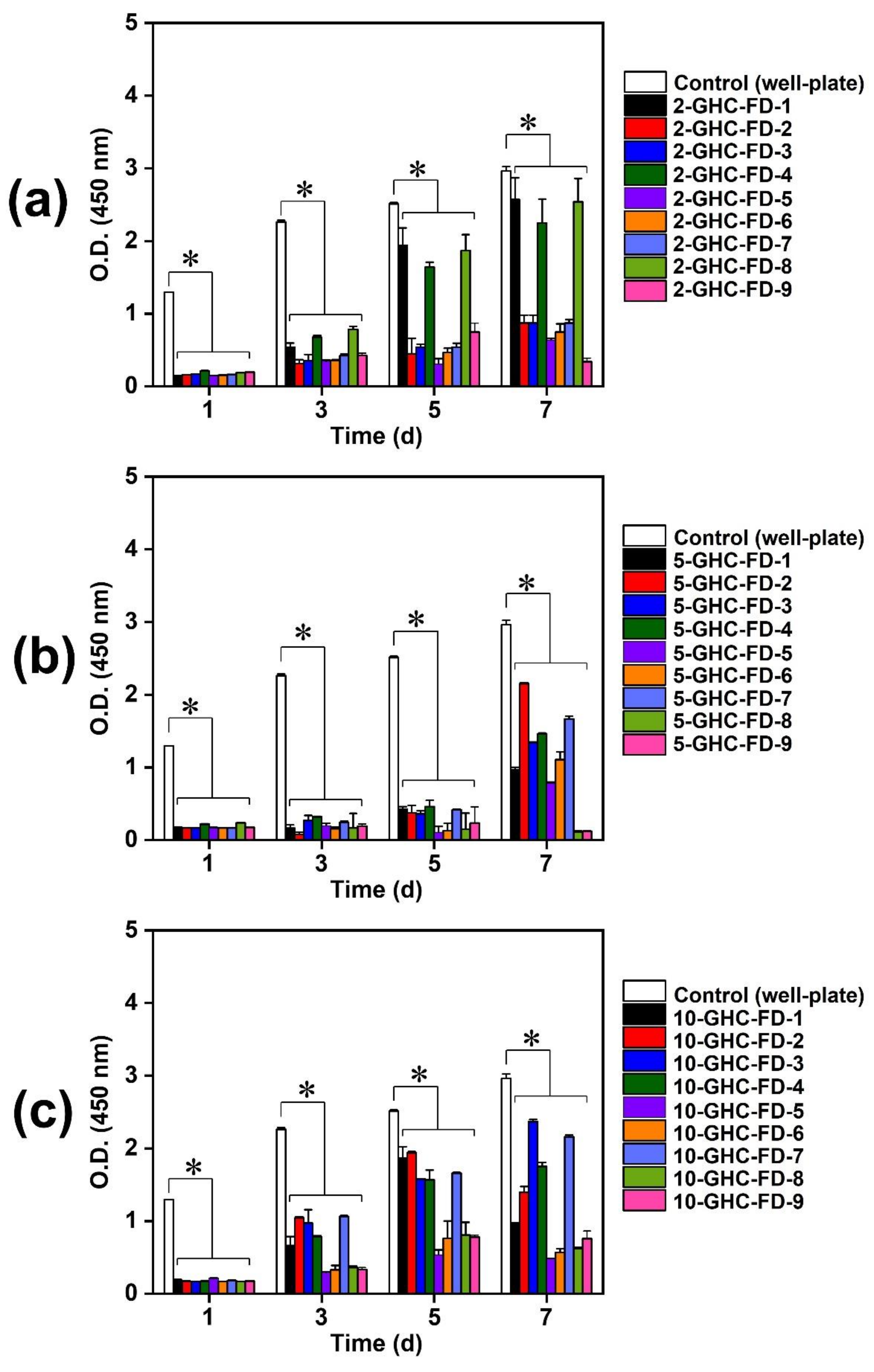
3.1.3. NIH/3T3 Cell Proliferation
3.2. Characterizations of 2-GHC-FD-1, 2-GHC-FD-4, and 2-GHC-FD-8
3.3. Characterization of FGF-7-Loaded 2-GHC-FD-8 (2-GHC-FD-8/F)
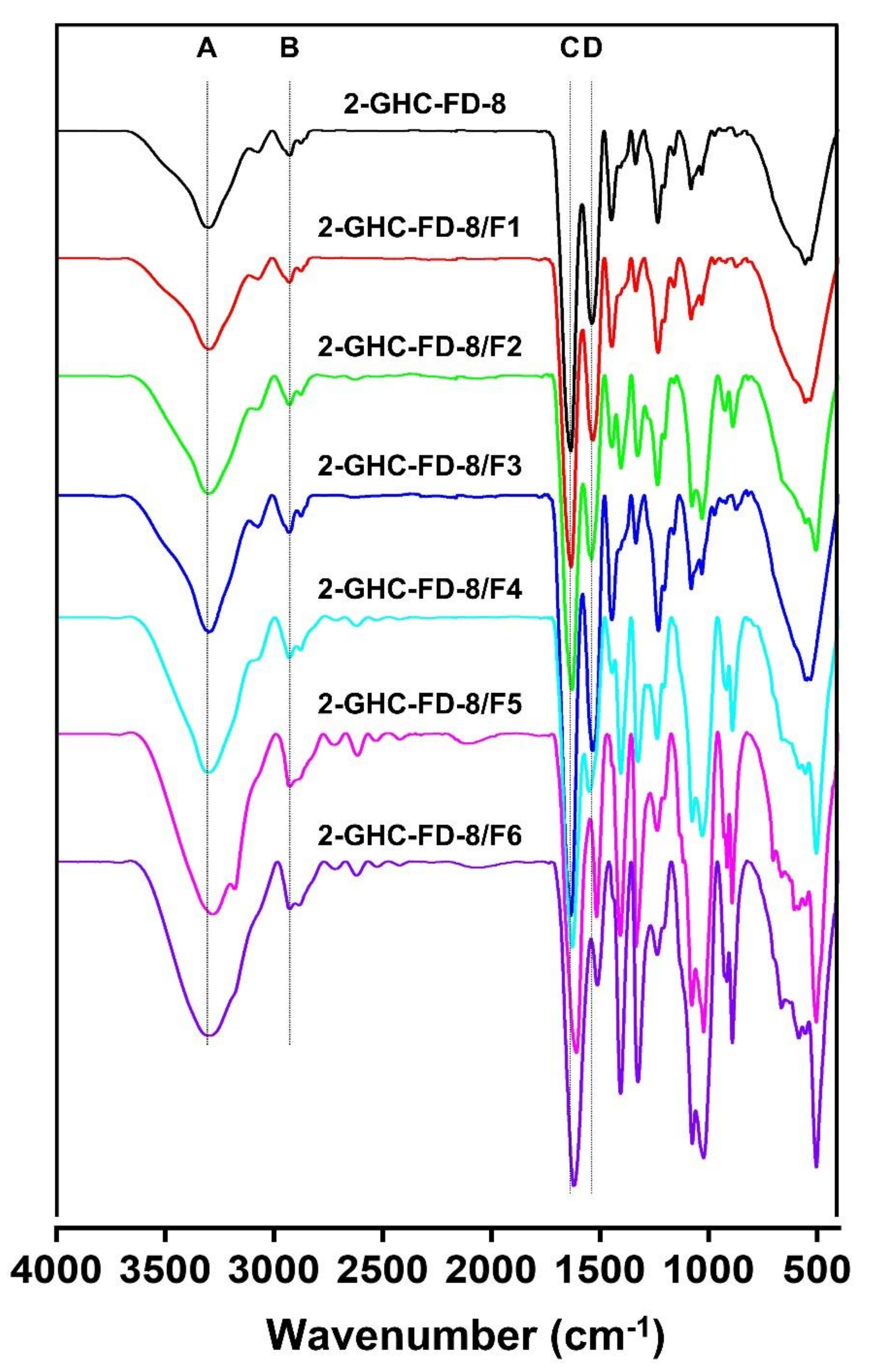
3.3.1. FTIR Spectra
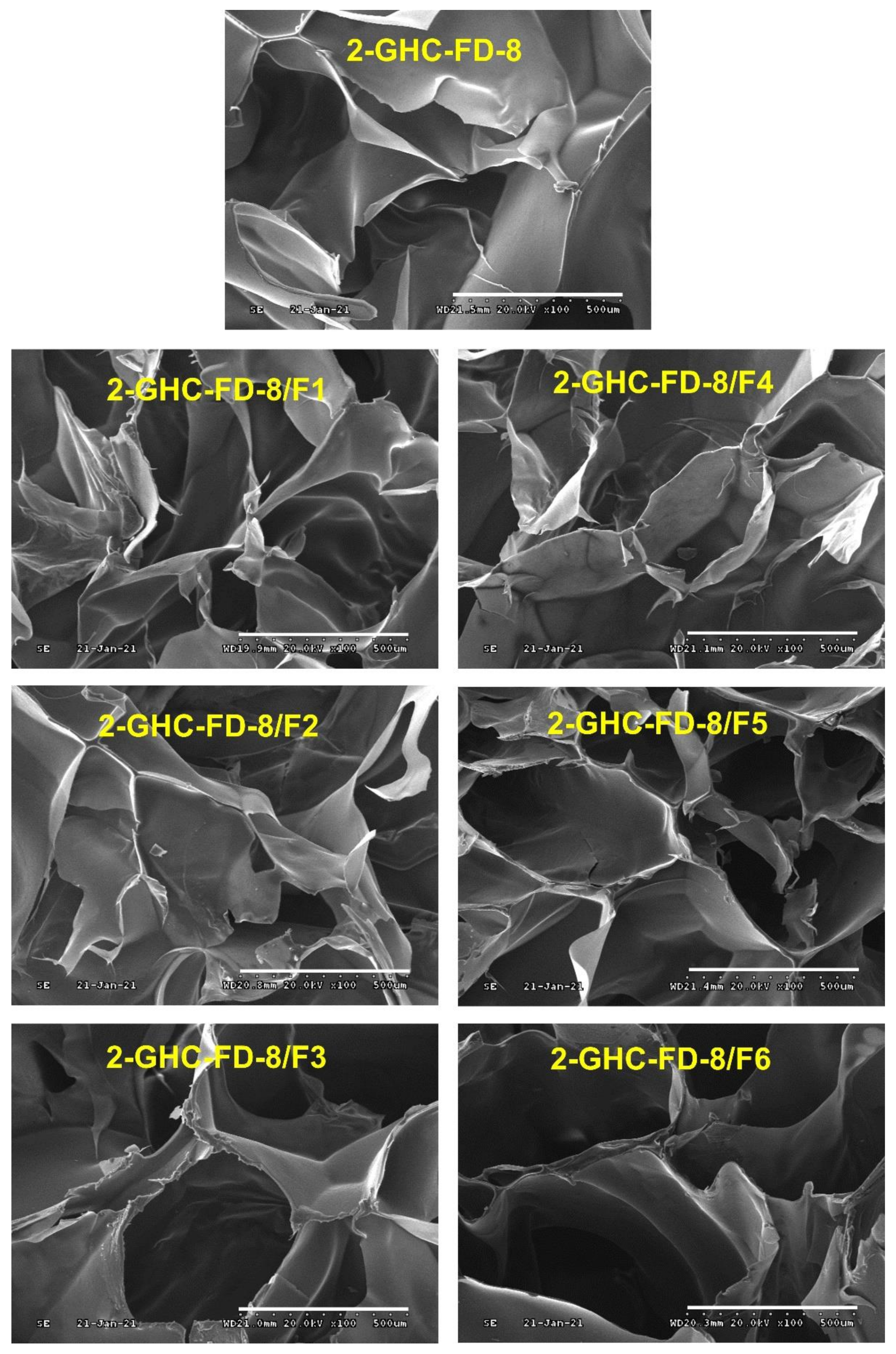
3.3.2. Observation of Surface Morphology and Porosity
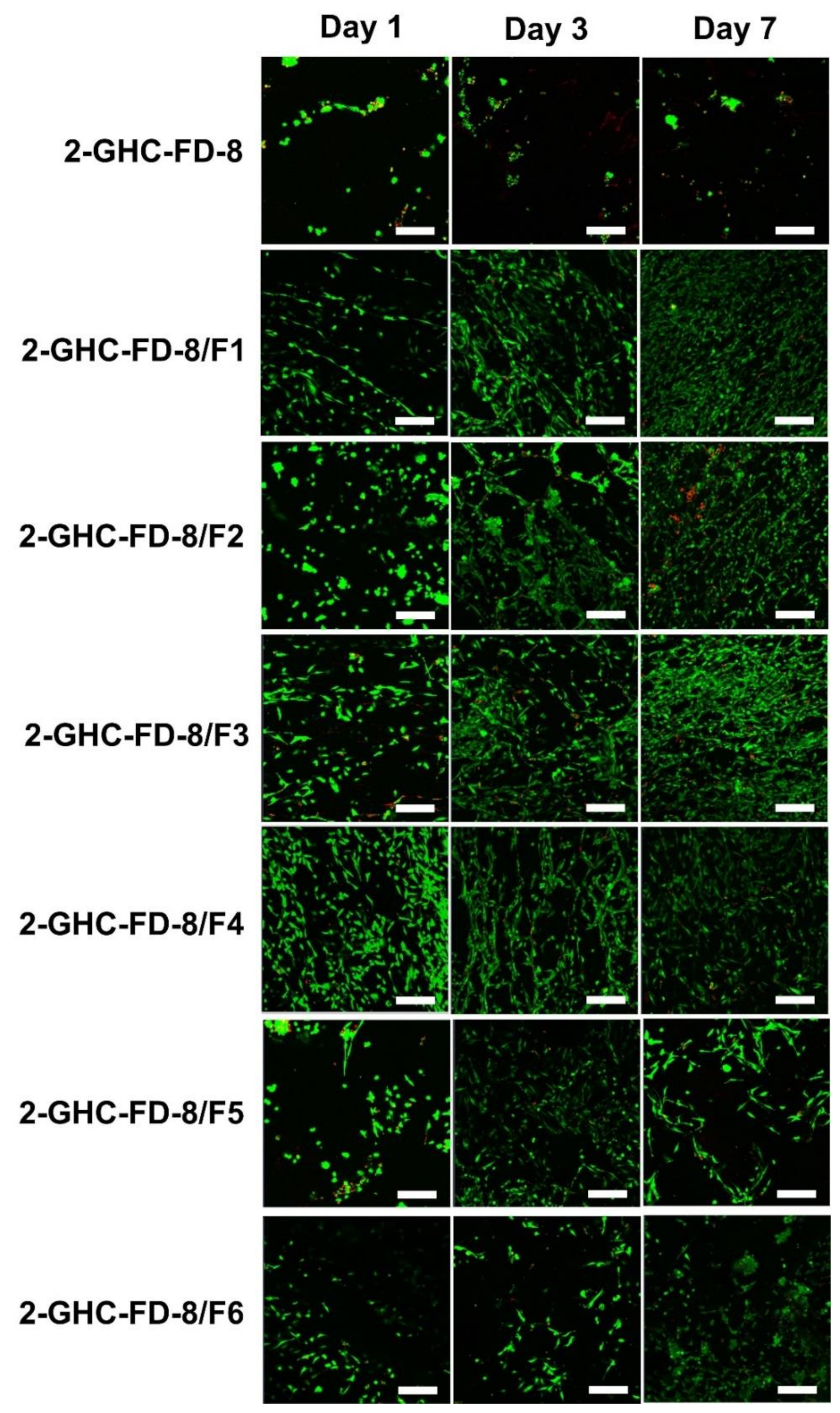
3.3.3. Live and Dead Assay
3.4. Release Behavior of FGF-7
3.5. Macroscopic Observation and Size Calculation of Remaining Wounds
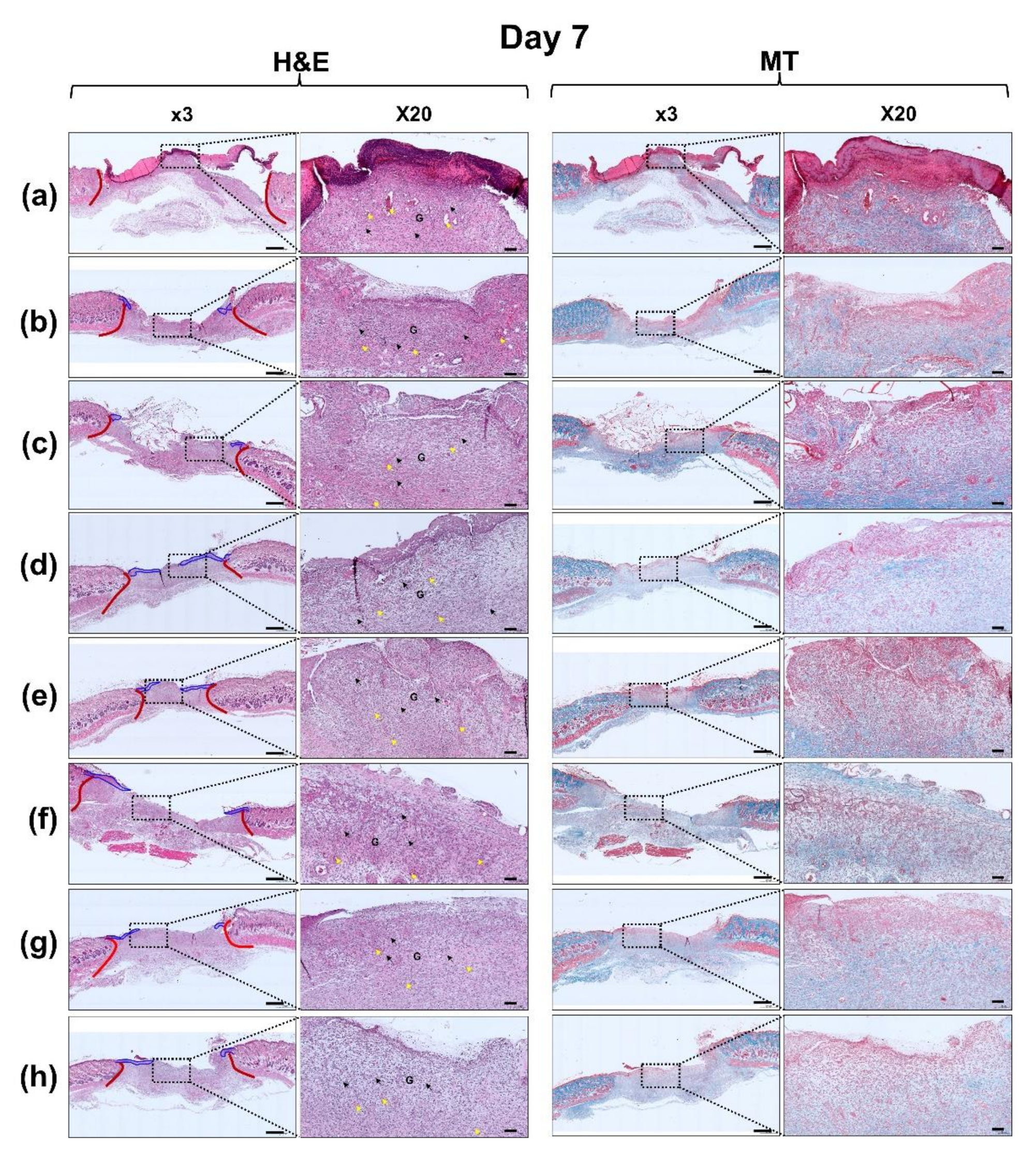
3.6. Histological Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Dhivya, S.; Padma, V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Nielsen, J.; Fogh, K. Clinical utility of foam dressings in wound management: A review. Chronic Wound Care Manag. Res. 2015, 2, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Moura, L.I.F.; Dias, A.M.A.; Carvalho, E.; Sousa, H.C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9, 7093–7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bay, M.Y.; Chen, M.C.; Yu, W.C.; Lin, J.Y. Foam dressing incorporating herbal extract: An all-natural dressing for potential use in wound healing. J. Bioact. Compat. Polym. Biomed. Appl. 2016, 32, 293–308. [Google Scholar] [CrossRef]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 436, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from nature: Biopolymers and biocomposites as smart Wound care materials. Front. Bioeng. Biotech. 2018, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Graca, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid-based wound dressings: A review. Carbohy. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, Y.; Wang, L.; Jiang, Z.; Li, T.; Zhang, W.; Liang, Y. Carboxymeyhyl chitosan/gelatin/hyaluronic acid blended-membranes as epithelia transplanting scaffold for corneal wound healing. Carbohy. Polym. 2018, 192, 240–250. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biolog. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Jo, G.; Lee, B.Y.; Choi, J.W.; Chun, H.J.; Kim, H.S.; Yang, D.H. Injectable glycol chitosan hydrogel containing folic acid-functionalized cyclodextrin-paclitaxel complex for breast cancer therapy. Nanomaterials 2021, 11, 317. [Google Scholar] [CrossRef]
- Moon, Y.J.; Yoon, S.-J.; Koo, J.-H.; Yoon, Y.; Byun, H.J.; Kim, H.S.; Khang, G.; Chun, H.J.; Yang, D.H. β-Cyclodextrin/triclosan complex-grafted maehacrylated glycol chitosan hydrogel by photocrosslinking via visible light irradiation for a tissue bio-adhesive. Int. J. Mol. Sci. 2021, 22, 700. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-cured glycol chitosan hydrogel for ovarian cancer drug delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.H.; You, S.J.; Chun, H.J.; Kim, C.; Lee, W.K.; Lim, Y.M.; Nho, Y.C.; Jang, J.W. In vitro and in vivo biocompatibility of γ-ray crosslinked gelatin-poly(vinyl alcohol) hydrogels. Tissue Eng. Regen. Med. 2009, 6, 414–418. [Google Scholar]
- You, S.J.; Ahn, W.S.; Jang, H.S.; Kang, M.I.; Chun, H.J.; Lim, Y.M.; Nho, Y.C. Preparation and characterization of gelatin-poly(vinyl alcohol) hydrogels for three-dimensional cell culture. J. Ind. Eng. Chem. 2007, 13, 116–120. [Google Scholar]
- Seo, N.M.; Ko, J.H.; Park, Y.H.; Chun, H.J. In vitro biocompatibility of PLGA-HA nano-hybrid scaffold. Tissue Eng. Regen. Med. 2011, 8, 16–22. [Google Scholar]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Chen, B.; Kao, H.K.; Murphy, G.; Orgill, D.; Guo, L. Lack of FGF-7 further delays cutaneous wound healing in diabetic mice. Plast. Reconstr. Surg. 2011, 128, 673–684. [Google Scholar] [CrossRef]
- Finch, P.W.; Rubin, J.S.; Miki, T.; Ron, D.; Aaronson, S.A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science 1989, 245, 752–755. [Google Scholar] [CrossRef] [Green Version]
- Robert, G. Growth factors and chronic wound healing: Past, present, and future. Adv. Skin Wound Care 2004, 17, 24–35. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Hyun, H.; Lee, D.-W.; Yang, D.H. Visible light-cured glycol chitosan hydrogel containing a beta-cyclodextrin-curcumin inclusion complex improves wound healing in vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef] [Green Version]
- Andrgie, A.T.; Darge, H.F.; Mekonnen, T.W.; Birhan, Y.S.; Hanurry, E.Y.; Chou, H.-Y.; Wang, C.-F.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Ibuprofen-loade heparin modified thermosensitive hydrogel for inhibiting excessive inflammation and promoting wound healing. Polymers 2020, 12, 2619. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Park, J.-U.; Choi, M.-H.; Kim, S.; Kim, H.-E.; Jeong, S.-H. Polydeoxyribonucleotide-delivering therapeutic hydrogel for diabetic wound healing. Sci. Rep. 2020, 10, 16811. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.H.; Park, H.S. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nadieh, K.; Aliasghar, B.; Mohammad, T.K.; Masoud, M. Platelet-rich plasma-hyaluronic acid/chondroitin sulfate/carboxymethyl chitosan hydrogel for cartilage regeneration. Biotech. Appl. Biochem. 2021, 1–14. [Google Scholar] [CrossRef]
- Cam, C.; Zhu, S.; Truong, N.F.; Scumpia, P.O.; Segura, T. Systematic evaluation of natural scaffolds in cutaneous wound healing. J. Mater. Chem. B 2015, 3, 7986–7992. [Google Scholar] [CrossRef]
- Nagahama, H.; Divya Rani, V.V.; Shalumon, K.T.; Jayakumar, R.; Nair, S.V.; Koiwa, S.; Furuike, T.; Tamura, H. Preparation, characterization, bioactive and cell attachment studies of α-chitin/gelatin composite membranes. Int. J. Biolog. Macromol. 2009, 44, 333–337. [Google Scholar] [CrossRef]
- Davis, G.E. Affinity of integrins for damaged extracellular matrix: αvβ3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992, 182, 1025–1031. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Li, Q.; Zou, Q.; Du, C. Biomimetic mineralization of carboxymethyl chitosan nanofibers with improved osteogenic activity in vitro and in vivo. Carbohy. Polym. 2018, 195, 225–234. [Google Scholar] [CrossRef]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
- Elsner, J.J.; Zilberman, M. Novel antibiotic-eluting wound dressings: An in vitro study and engineering aspects in the dressing’s design. J. Tissue Viability 2010, 19, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Zahouani, H.; Pailler-Mattei, C.; Sohm, B.; Vargiolu, R.; Cenizo, V.; Debret, R. Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Skin Res. Technol. 2009, 15, 68–76. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.; Zhang, H.; Jackson, J.K.; Lim, C.J.; Chiao, M. Janus films with stretchable and waterproof properties for wound care and drug delivery applications. RSC Adv. 2016, 6, 79900–79909. [Google Scholar] [CrossRef]
- Ekaterina, I.S.; Elena, D.N.; Olga, N.V.; Tatiana, G.V. Experimental wound dressings of degradable PHA for skin defect repair. J. Mater. Sci. Mater. Med. 2016, 27, 165. [Google Scholar] [CrossRef] [Green Version]
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Cai, Q.; Wang, C.; Lu, N.; Wang, S.; Bei, J. Fabrication and biocompatibility of cell scaffolds of poly(L-lactic acid) and poly(L-lactic-co-glycolic acid). Polym. Adv. Technol. 2002, 13, 227–232. [Google Scholar] [CrossRef]
- Frederic, R.; Sandra, H.; Jean-Marie, V.; Valerie, R.; Gaelle, L.R.; Norman, J.M.; Olivier, C.; Jean, F.; Philippe, B. FGF7/KGF triggers cell transformation and invasion on immortalized human prostatic epithelial PNT1A cells. Int. J. Cancer 1999, 82, 237–243. [Google Scholar] [CrossRef]
- Jiao, J.; Peng, C.; Li, C.; Qi, Z.; Zhan, J.; Pan, S. Dual bio-active factors with adhesion function modified electrospun fibrous scaffold for skin wound and infections therapeutics. Sci. Rep. 2021, 11, 11–13. [Google Scholar] [CrossRef]
- Park, J.P.; Lee, Y.M.; Lee, J.K.; Seol, Y.J.; Chung, C.P.; Lee, S.J. Controlled release of platelet-derived growth factor-BB from chondroitin sulfate-chitosan sponge for guided bone regeneration. J. Control Release 2000, 67, 385–394. [Google Scholar] [CrossRef]
- Xizo, Z.; Zheng, X.; An, Y.; Wang, K.; Zhang, J.; He, H. Zwitterionic hydrogel for sustained release of growth factors to enhance wound healing. Biomater. Sci. 2021, 9, 882–891. [Google Scholar]
- Lin, M.J.; Lu, M.C.; Chang, H.Y. Sustained Release of Insulin-Like Growth Factor-1 from Bombyx mori L. Silk Fibroin Delivery for Wound Therapy. Int. J. Mol. Sci. 2021, 22, 6267. [Google Scholar] [CrossRef]
- Doi, H.; Kitajima, Y.; Luo, L.; Yang, C.; Tateishi, S.; Ono, Y.; Urata, Y.; Goto, S.; Mori, R.; Masuzaki, H.; et al. Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci. Rep. 2016, 6, 18844. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.H.; Seo, D.I.; Lee, D.-W.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and evaluation of visible-light cured glycol chitosan hydrogel dressing containing dual growth factors for accelerated wound healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Rufaihah, A.J.; Huang, N.F.; Jamé, S.; Lee, J.C.; Nguyen, H.N.; Byers, B.; De, A.; Okogbaa, J.; Rollins, M.; Reijo-Pera, R.; et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arteriscler. Thromb. Vasc. Biol. 2011, 31, e72–e79. [Google Scholar] [CrossRef] [Green Version]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar] [CrossRef]
- Ulubayram, K.; Cakar, A.N.; Korkusuz, P.; Ertan, C.; Hasirci, N. EGF containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound healing promotion by hyaluronic acid: Effect of molecular weight on gene expression and in vivo wound closure. Phamaceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Yang, C.; Cao, M.; Liu, H.; He, Y.; Xu, J.; Du, Y.; Liu, Y.; Wang, W.; Cui, L.; Hu, J.; et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012, 287, 43094–43107. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mack, J.A.; Maytin, E.V. CD44 inhibits α-SMA gene expression via a novel G-actin/MRTF-mediated pathway that intersects with TGFβR/p38MAPK signaling in murine skin fibroblasts. J. Biol. Chem. 2019, 294, 12779–12794. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Ramez, M.; Gilad, E.; Singleton, P.A.; Man, M.-Q.; Crumrine, D.A.; Elias, P.M.; Feingold, K.R. Hyauronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J. Investig. Dermatol. 2006, 126, 1356–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.G.; Wang, Z.; Liu, W.S.; Park, H.J. The effect of carboxymethyl-chitosan on proliferation and collagen secretion of normal and keloid skin fibroblasts. Biomaterials 2002, 23, 4609–4614. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly(ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohy. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liancu, W.; Glenn, F.P.; Robert, D.G.; Thomas, A.M. Keratinocyte growth factor induces granulation tissue in ischemic dermal wounds. Arch. Surg. 1996, 131, 660. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Tarpley, J.E.; Morris, B.; Van, G.Y.; Morawiecki, A.; Callahan, W.; Goldenberg, M.; Hershenson, S.; Pierce, G.F. Differing targets and effects of keratinocyte growth factor, platelet-derived growth factor-BB, epidermal growth factor, and neu differentiation factor. Am. J. Pathol. 1995, 147, 1261–1277. [Google Scholar]
- Guo, L.; Yu, Q.C.; Fuchs, E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993, 12, 973–986. [Google Scholar] [CrossRef]



| FDs | Gelatin (w/v%) | HA (w/w%) | CMCS (w/w%) | DMT-MM (w/v%) | Hydrogelation | |
|---|---|---|---|---|---|---|
| 1- | GHC-1 | 1 | 1 | 10 | 2 | × |
| GHC-2 | 50 | |||||
| GHC-3 | 100 | |||||
| GHC-4 | 5 | 10 | ||||
| GHC-5 | 50 | |||||
| GHC-6 | 100 | |||||
| GHC-7 | 10 | 10 | ||||
| GHC-8 | 50 | |||||
| GHC-9 | 100 | |||||
| 2- | GHC-1 | 2 | 1 | 10 | 2 | O |
| GHC-2 | 50 | |||||
| GHC-3 | 100 | |||||
| GHC-4 | 5 | 10 | ||||
| GHC-5 | 50 | |||||
| GHC-6 | 100 | |||||
| GHC-7 | 10 | 10 | ||||
| GHC-8 | 50 | |||||
| GHC-9 | 100 | |||||
| 5- | GHC-1 | 5 | 1 | 10 | 2 | O |
| GHC-2 | 50 | |||||
| GHC-3 | 100 | |||||
| GHC-4 | 5 | 10 | ||||
| GHC-5 | 50 | |||||
| GHC-6 | 100 | |||||
| GHC-7 | 10 | 10 | ||||
| GHC-8 | 50 | |||||
| GHC-9 | 100 | |||||
| 10- | GHC-1 | 10 | 1 | 10 | 2 | O |
| GHC-2 | 50 | |||||
| GHC-3 | 100 | |||||
| GHC-4 | 5 | 10 | ||||
| GHC-5 | 50 | |||||
| GHC-6 | 100 | |||||
| GHC-7 | 10 | 10 | ||||
| GHC-8 | 50 | |||||
| GHC-9 | 100 | |||||
| Formation of erythema and callus | |
| No erythema | 0 |
| Slight erythema | 1 |
| Well-defined erythema | 2 |
| Intermediate erythema | 3 |
| Severe erythema (dark red) and callus | 4 |
| Formation of edema | |
| No edema | 0 |
| Slight edema | 1 |
| Well-defined edema | 2 |
| Intermediate edema (about 1 mm) | 3 |
| Severe edema (>1 mm) | 4 |
| Stimulus score at maximum | 8 |
| Samples | Gelatin (w/v%) | HA (w/w%) | CMCS (w/w%) | FGF-7 (×10−11 M) |
|---|---|---|---|---|
| 2-GHC-FD-8/F1 | 2 | 5 | 10 | 2 |
| 2-GHC-FD-8/F2 | 4 | |||
| 2-GHC-FD-8/F3 | 8 | |||
| 2-GHC-FD-8/F4 | 16 | |||
| 2-GHC-FD-8/F5 | 32 | |||
| 2-GHC-FD-8/F6 | 64 |
| Time | Control | 2-GHC-FD-1,4,8 | |||
|---|---|---|---|---|---|
| Polar Solvent | Non-Polar Solvent | Polar Solvent | Non-Polar Solvent | ||
| Erythema/Callus | 24 | 0 | 0 | 0 | 0 |
| 48 | 0 | 0 | 0 | 0 | |
| 72 | 0 | 0 | 0 | 0 | |
| Total score | 0 | 0 | 0 | 0 | |
| Edema | 24 | 0 | 0 | 0 | 0 |
| 48 | 0 | 0 | 0 | 0 | |
| 72 | 0 | 0 | 0 | 0 | |
| Total score | 0 | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Yoon, S.-J.; Lee, D.H.; Pyun, Y.C.; Kim, W.Y.; Lee, J.H.; Khang, G.; Chun, H.J.; Yang, D.H. Preparation of Foam Dressings Based on Gelatin, Hyaluronic Acid, and Carboxymethyl Chitosan Containing Fibroblast Growth Factor-7 for Dermal Regeneration. Polymers 2021, 13, 3279. https://doi.org/10.3390/polym13193279
Jin L, Yoon S-J, Lee DH, Pyun YC, Kim WY, Lee JH, Khang G, Chun HJ, Yang DH. Preparation of Foam Dressings Based on Gelatin, Hyaluronic Acid, and Carboxymethyl Chitosan Containing Fibroblast Growth Factor-7 for Dermal Regeneration. Polymers. 2021; 13(19):3279. https://doi.org/10.3390/polym13193279
Chicago/Turabian StyleJin, Longhao, Sun-Jung Yoon, Dae Hoon Lee, Yun Chang Pyun, Woo Youp Kim, Ju Hwa Lee, Gilson Khang, Heung Jae Chun, and Dae Hyeok Yang. 2021. "Preparation of Foam Dressings Based on Gelatin, Hyaluronic Acid, and Carboxymethyl Chitosan Containing Fibroblast Growth Factor-7 for Dermal Regeneration" Polymers 13, no. 19: 3279. https://doi.org/10.3390/polym13193279








