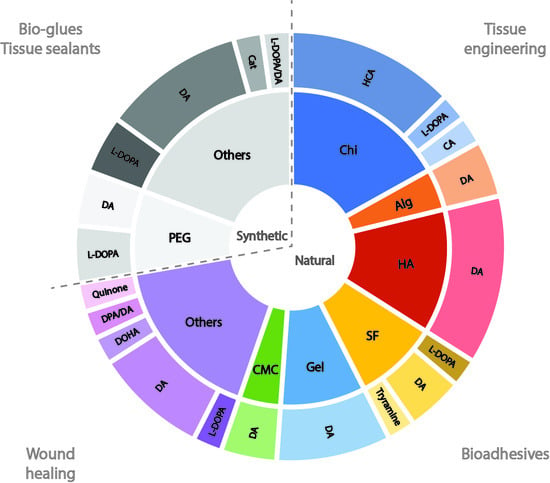
Sourced from animal, plant or bacterial sources, natural polymers display favourable physical and chemical properties for applications in regenerative medicine. In the context of this literature review, two main groups of natural polymers were predominant in the final list of references: (i) polysaccharides, including chitosan, hyaluronic acid, alginate and carboxymethylcellulose; and (ii) proteins, namely, gelatine and silk fibroin. Target areas for application included tissue regeneration, wound healing and bioadhesives.
Chitosan (Chi)
Catechol modification of Chi, a linear polysaccharide obtained from the outer skeleton of shellfish and composed of randomly distributed β-linked D-glucosamine and N-acetyl-D-glucosamine monomers, has been extensively tested for the development of bioadhesives with potential applications in wound healing and tissue regeneration.
Aiming at developing a new generation of tissue adhesives that overcome the drawbacks of the existing clinical options (i.e., fibrin sealants and cyanoacrylates), Zeng et al. [
17] synthesised a thiol-grafted catechol-conjugated Chi derivative (ChiDS) via standard EDC chemistry (reaction with dihydrocaffeic acid and acetyl-L-cysteine for catechol and thiol addition, respectively), with the contents of thiol (215 µmol/g) and catechol groups (75 µmol/g) determined by UV–Vis spectroscopy. Gelation of ChiDS hydrogels was promoted by sodium periodate (NaIO
4) in phosphate-buffered saline (PBS) solution, with results showing that ChiDS solutions at 4% (
w/V) could rapidly form hydrogels (within 1 min of mixing), with the modulation of gelation times achieved by altering the ChiDS concentration and molar ratio of periodate/catechol. Similarly, rheological studies showed that the mechanical properties of hydrogels depend on the concentration of ChiDS and the molar ratio of periodate/catechol, with more catechol groups and higher molar ratios resulting in hydrogels with stronger crosslinking density. Adhesion (lap shear) strength tests were performed in porcine skin, to mimic human tissue, with adhesives showing strengths as high as 50 kPa, which were proportional to the concentration of ChiDS. In vitro assays did not show significant toxicity towards L-929 mouse fibroblast cells, thus suggesting that these hydrogels present optimal functional properties, including fast curing times, mechanical strength, high adhesion and biocompatibility.
Similarly, Ryu et al. [
16] designed injectable and thermosensitive hydrogels, to be used as tissue adhesives and haemostatic materials, by combining Chi with Pluronic (a synthetic polymer composed of triblocks of polyethylene oxide-polypropilene oxide-polyethylene oxide, PEO-PPO-PEO). For this purpose, Chi was functionalised with catechol groups by EDC/NHS-mediated reactions with hydrocaffeic acid (HCA) (DS = 9.9–14.8%), and further crosslinked with terminally thiolated Pluronic F-127 triblock copolymer (PluSH), to produce temperature-sensitive and adhesive sol–gel transition hydrogels. Indeed, although catechol-conjugated Chi (Chi-C) was capable of gelation under alkaline conditions, a simple mixture of catechol-conjugated Chi and PluSH (Chi-C/PluSH) achieved instant gelation at physiological temperature and pH, with hydrogels revealing enhanced strength due to intermolecular crosslinking between the two polymers. Moreover, wet adhesion experiments (performed in a universal testing machine—UTM, using mouse subcutaneous tissue) revealed higher detachment stress values for Chi-C/PluSH (~15.0 kPa) compared to controls lacking catechol (~5.3 kPa) or thiol (~6.6 kPa) groups, thus suggesting a role for catechol in the hydrogel adhesion to tissues and in the interaction with thiol groups (quinone–thiol bond) for the formation of stable hydrogels. Importantly, whereas Pluronic hydrogels were found to quickly erode, Chi-C/PluSH hydrogels exhibited significant stability in vitro (in aqueous solution) and in vivo (upon subcutaneous implantation in mice).
In another study, Ryu et al. [
15] reported the development of an alternative method for the crosslinking of hydrogels that avoids the use of enzymes (e.g., horseradish peroxidase (HRP) or Tyr) or high concentrations of oxidating agents, based on a water-soluble enzyme-mimetic biocatalyst (hematin, Hem). In this regard, hydrogels were obtained within 5 min of mixing hematin-grafted Chi (Chi-g-Hem) with EDC-mediated HCA-functionalised Chi (Chi-C), with crosslinking catalysed by Hem under mild physiological conditions. Wet adhesion experiments (performed in a UTM using mouse subcutaneous tissue) revealed increased adhesive force for Chi-g-Hem/Chi-C hydrogels (~33.6 kPa) compared to those formed by pH-induced catechol oxidation (~20.6 kPa). Similar observations were reported for catechol-containing Chi-g-Hem-catalysed HA-C hydrogels (compared with untreated HA) and biocatalyst-induced polyvinyl alcohol (PVA)-C hydrogels. Importantly, the existence of optimal windows for hydrogel gelation times and polymer concentrations indicated that unreacted catechol residues (i.e., those not involved in the crosslinking network) contributed to the increased adhesion properties of the hydrogels.
Zhou et al. [
22] explored the use of Chi and ε-polylysine (PLL) as adhesives for peripheral nerve regeneration, a field in which the current clinical options, cyanoacrylates and fibrin glue, have shortcomings that limit their application. The authors designed a nerve-adhesive hydrogel formed in situ and composed of a polysaccharide (Chi) and polypeptide (PLL), mimicking the polysaccharide/protein structure of natural epineurium matrices, which crosslinked via Michael-type addition between maleimide (PLL) and thiol (Chi) reactive groups. To improve the adhesivity of the hydrogel, catechol groups were conjugated onto the PLL backbone using standard EDC chemistry. Gelation occurred within 10 s and was not significantly influenced by the introduction of catechol groups. A lower catechol DS (5%) favoured the simultaneous improvement of the cohesive force of the hydrogel (storage modulus elevated to more than 2.4 kPa) and the interfacial adhesive force between the hydrogel and epineurium (>0.185 N of force, eightfold higher than that of fibrin glue). In vivo testing revealed better nerve regeneration performance of the hydrogel compared to current nerve surgery methods, with data at 8 weeks showing the morphology of the repaired nerve fibre coated by the hydrogel close to the morphology of normal nerve, and the axon cross ratio of the regenerated nerves coated using hydrogel (57%) higher than that using the suture technique (35%). Finally, in vitro and in vivo compatibility tests confirmed good nerve biocompatibility and low immunogenicity of hydrogels towards the nerve.
Aiming at developing new formulations for wound dressing and tissue adhesion, Park et al. [
18] described the optimisation of catechol conjugation to CChi via two different functionalisation strategies: carbodiimide (EDC/NHS) chemistry (C-CChi) and enzymatic oxidation mediated by tyrosinase (Tyr) activity (E-CChi). For C-CChi synthesis, the carboxylic group of HCA reacted with free amines on Chi in the presence of EDC, whereas for E-CChi, a two-step protocol was performed, with initial Tyr-mediated conversion of HCA to o-quinone and subsequent reactions of this intermediate with amines of Chi. Grafting of catechol to the Chi backbone was confirmed by NMR analysis, whereas DS was calculated from UV–Vis measurements at 280 nm (8–27%). Structural properties of oxidatively-crosslinked CChi hydrogels were proportional to the degree of oxidation, i.e., constructs with increased degrees of oxidation presented denser microstructures, increased porosity, and smaller pore sizes. When mechanical properties were analysed by lap shear adhesion tests, the biomaterial adhesiveness did not change with increasing catechol DS for C-CChi and E-CChi. However, both formulations displayed higher lap shear strength compared to that of unmodified chitosan (at pH 7 and 8) and other catechol-modified Chi, as well as that reported in the literature for fibrin glue (~20 kPa). In vitro studies with NIH3T3 mouse fibroblast cells demonstrated that C-CChi and E-CChi hydrogels do not impact cell viability; higher cell adhesivity was observed for E-CChi in platelet adhesion studies. As the better-performing formulation, E-CChi was tested in an in vivo wound healing assay, with results revealing better healing performance for E-CChi hydrogels compared to the commercially available Dermabond
® adhesive, as well as similar healing performance, higher epidermis epithelialisation and collagen deposition in the dermis, compared to a standard suture procedure.
Shi et al. [
23] described the development of a hybrid hydrogel system, composed of 3,4-dihydroxyphenyl propionic acid (DPA)-functionalised Chi and dopamine (DA)-modified gamma-polyglutamic acid (γ-PGA), to be used as an adhesive for biomedical applications. Functionalisation of the Chi involved preliminary activation of DPA with NHS and EDC (1:1:1), followed by mixing with a solution of Chi, whereas γ-PGA was activated with NHS and EDC (3:0.1:0.1) before the addition of DA. Hydrogels were then prepared by mixing the two solutions at different volume ratios. In this regard, fast electrostatic-mediated gelation kinetics were observed immediately upon mixing of the two solutions, with G’ ≈ G’’ for t < 10 s and G’ > G’’ for t > 10 s (i.e., the formation of hydrogel with a viscoelastic profile). Adhesion tests on material surfaces (formaldehyde resin, polyurethane, and aluminium) revealed increased adhesion strength with increased catechol content, independent on the substrates, with higher values obtained in formaldehyde resin. Importantly, high adhesion strength was also obtained in lap shear tests performed in biological substrates, with values reported for fresh porcine skin (25 kPa) and arthrodial cartilage (145 kPa) significantly larger than those reported for fibrin glue (10 kPa). Degradation of the bioadhesives, evaluated by measuring the weight change after incubation in PBS (pH 7.4, 37 °C), revealed initial stability of the constructs (20% weight loss after 1 day) followed by quick degradation (98% weight loss) over 1 week. Finally, in vitro biocompatibility studies showed reduced cytotoxicity (>90% cell viability) for L-929 mouse fibroblast cells incubated with hydrogels.
In addition to general tissue adhesives, catechol-modified Chi has been tested for application/delivery in oral and intestinal mucosa. Xu et al. [
13] developed a buccal drug delivery system using a novel catechol-functionalised Chi hydrogel. Covalently bonded catechol groups were attached to the backbone of Chi by reaction with HCA, and crosslinked to the polymer with genipin. The gelation time and the mechanical properties of catechol-functionalised Chi hydrogels were similar to those of Chi-only hydrogels but the catechol groups significantly enhanced mucoadhesion in vitro (7/10 catechol-functionalised Chi hydrogels were still in contact with the porcine mucosal membrane after 6 h, whereas all Chi hydrogels lost contact after 1.5 h). The improved adhesive hydrogels were able to sustain the release of an anaesthetic drug in the mouth over longer periods of time.
Reaction with HCA was also used by Kim et al. [
14] for the introduction of catechol groups onto Chi, generating polymers (Chi-C) with various degrees of catechol conjugation (7.2%, 12% and 20.5%). Modification with Chi, confirmed by surface plasmon resonance spectroscopy, resulted in enhanced mucoadhesion (over fourfold) compared to the unmodified polymer. Moreover, the retention in the intestinal mucosal layer of Chi-C (~10 h) was significantly longer than that of Chi or the widely used anionic mucoadhesive polymer poly(acrylic acid) (PAA, Mw 450 kDa), which were rarely detected 3 h after oral administration in mice. Importantly, the authors demonstrated that the improved gastrointestinal retention of Chi-C resulted from the formation of irreversible catechol-mediated crosslinking with mucin.
Striving to establish an effective treatment for oral mucositis, Ryu et al. [
20] developed a Chi-based oral patch, modified with HCA for enhanced mucoadhesion. Functionalisation of the Chi backbone with catechol moieties (Chi-C) was mediated by EDC (DS = 6.5%, without catechol oxidation), with patches then prepared by freeze-drying of the Chi-C solution, creating porous, sponge-like constructs. Time-sweep measurements of Chi-C showed rapid dissolution of the patches in distilled water, with the addition of saliva quickly generating a high elastic modulus. Moreover, lap shear tests performed in porcine keratinised and non-keratinised mucosa revealed higher detachment forces for Chi-C (10.3 kPa and 20 kPa, respectively), compared to unmodified Chi patches (4.1 kDa and 3.2 kPa, respectively), thus indicating that interpolymer complexation mechanisms with mucosal macromolecules were mediated by catechol. Indeed, further testing confirmed the interaction of mucin and catechol groups to significantly improve the tissue adhesion force and stability of Chi-C patches in the oral cavity. Using triamcinolone acetonide (TA) as a model drug, release studies revealed an initial burst release (up to 4 h) followed by a slower sustained TA release. Importantly, the sustained release of Chi-C-formulated TA (25 μg) accelerated epithelial regeneration in severe oral ulcers, compared to TA-loaded Chi-C (12.5 μg), Chi-C or a commercially available adhesive.
Aiming at developing mucoadhesive biomaterial-based carriers for the treatment of oral cancer, Pornpitchanarong et al. [
21] fabricated dual-polymer nanoparticles (NPs), functionalised with catechol (Cat) moieties and encapsulating doxorubicin (DOX), for strong adhesion and sustained drug release at the tumour site. For the preparation of NPs, Chi and hyaluronic acid (HA) were modified with DA, with Chi functionalisation involving a two-step reaction (i.e., the introduction of succinyl groups followed by EDC/NHS-mediated coupling of DA, to form SChiCat), while the same coupling chemistry was used for the direct modification of HA with DA (Hacat). Cat-NPs were then prepared via the ionic gelation of SChiCat and Hacat, under strong stirring and probe sonication. Dynamic light scattering (DLS) and scanning electron microscopy (SEM) analysis revealed the formation of negatively charged spherical structures (160 to 305 nm), with smaller and more homogeneous NPs obtained upon the mixing of SChiCcat and Hacat at a 2:1 ratio. Non-covalent DOX loading onto the best-performing Cat-NPs was found to be proportional to the drug concentration, with the highest loading efficiency (~70%) for a Cat-NPs:DOX ratio of 1:0.5, which was associated with fast DOX release (over 3 h) followed by slower release over 24 h. In an ex vivo porcine flow-through study, it was demonstrated that >60% of Cat-NPs remained attached to buccal mucosa after rinsing with artificial saliva, compared to control SChi/HA NPs (~50%) and dextran (10%), confirming that catechol functionalisation contributes to stronger mucoadhesive properties. In vitro studies in human gingival fibroblasts (HGF) and NH22 oral squamous carcinoma cells confirmed the biocompatibility of Cat-NPs (>80% cell viability after incubation for 24 h), whereas increased rates of uptake, accumulation and apoptosis were found for DOX-loaded Cat-NPs (compared to free DOX).
Innovating on the field of cancer therapy, Wang et al. [
19] described the development of a new bioinspired manganese dioxide (MnO
2) hybrid hydrogel (BMH) for simultaneous melanoma therapy, tissue regeneration and anti-bacterial activity. For this purpose, 2D anisotropic MnO
2 nanosheets were combined with caffeic acid (CA)-modified chitosan (ChiCA, DS = 12.5%). MnO
2-mediated crosslinked hydrogels exhibited a complex 3D porous structure with embedded black dots corresponding to the MnO
2 nanosheets. Favourable adhesive properties were evident after adhesion of hydrogels to the finger knuckle, as well as in lap shear mechanical tests, which showed increasing hydrogel adhesiveness with increasing polymer or catechol content (up to 3.35 kPa), although not significantly different from that of fibrin glue. BMH hydrogels also demonstrated self-healing capacity when subjected to cycles of intercalating strains and shear thinning behaviour, making them suitable for wound dressing applications. The presence of MnO
2 nanosheets not only led to crosslinking, but allowed (i) catalytic conversion of H
2O
2 into O
2, to mitigate the hypoxic tumour microenvironment, (ii) controlled spatiotemporal release of DOX, and (iii) photo-mediated increases in the local temperature (up to 49 °C), leading to the induction of apoptosis and extensive cell death of A375 melanoma cancer cells. Importantly, testing of BMH hydrogels, carried out in two different disease models, revealed (i) >95% growth inhibition of cancer cells in an in vivo subcutaneous melanoma mouse model, and (ii) accelerated closure and re-epithelialisation of the wound site in a microbial-infected full-thickness murine cutaneous wound model.
Hyaluronic Acid (HA)
HA is an anionic non-sulphated glycosaminoglycan, composed of repeating disaccharide units of D-glucuronic acid and N-acetyl-D-glucosamine. Present in most connective/epithelial tissues, and abundant in human joints, HA molecular weight is proportional to the number of repeating monomers, with values ranging between 4 kDa and 8000 kDa. Several manuscripts described the functionalisation of HA with catechol moieties for application in different biomedical areas requiring adhesivity, including tissue regeneration, wound healing and biosensing.
In a well-designed study, Koivusalu et al. [
28] focused on the development of novel adhesive and implantable biomaterials for the transplantation of distinct cell populations to the cornea, aiming at achieving corneal regeneration. Using established carbodiimide coupling strategies, the authors synthesised DA-grafted HA via the activation of carboxyl groups with EDC (DS = 14%), while a similar approach was used for the conjugation of carbodihydrazide (CDH) to HA or HA-DA (DS = 10%). Hydrogels were produced by the combination of equal volumes of HA-CDH/HA-DA-CDH and aldehyde-modified HA (DS = 10%), using conventional hydrazone crosslinking chemistry. Rheological profiling of DA-functionalised hydrogels and control constructs (without DA) showed G’ > G’’ for the screened frequencies, indicating the formation of stable, viscoelastic-like hydrogels with strong elastic character (G’/G’’~0.003). Although both hydrogels showed similar swelling behaviour, the kinetics were dependent on the surrounding buffering conditions, with those incubated in PBS reaching equilibrium after 1 week, whereas a sustained linear increase was found for those incubated in culture medium. Furthermore, analysis of hydrogel adhesion to porcine corneal tissue (via tack adhesion tests) revealed greater adhesion strength for DA-modified hydrogels. Importantly, tissue-like cellular compartmentalisation was achieved by the encapsulation of hASCs inside the hydrogels and the seeding of limbal epithelial stem cells (LESC) in the hydrogel surface, which was previously modified with thiolated basal membrane proteins. The compartmentalised DA-modified hydrogels not only allowed the maintenance of cell phenotype and functionality, but displayed excellent tissue adhesion upon sutureless implantation in a porcine corneal organ culture model.
Similarly, Shin et al. [
25] successfully synthesised HA–catechol conjugates (HA-Cat), with adhesive and cohesive properties, for minimally invasive cell-mediated liver regeneration. HA-Cat was produced by the conjugation of DA to the HA backbone via EDC/NHS-mediated carbodiimide coupling reactions (DS = 4–10%), with gelation achieved via the oxidative crosslinking of catechol groups using NaIO
4 under alkaline conditions. Compared to a widely used photo-crosslinkable methacrylate HA (HA-ME), fabricated by the transesterification of HA with methacrylate anhydride (DS = 9.8%), HA-Cat presented (i) greater swelling capacity, (ii) faster degradation (i.e., 50% weight loss after incubation for 2–4 h in hyaluronidase-containing buffer), and (iii) lower storage moduli (1321 vs. 3443 Pa), indicative of softer hydrogels, with a stable viscoelastic profile (G′ > G’’) confirmed by measurements in the entire frequency spectrum. Importantly, testing of hydrogel adhesivity to liver tissue, using a UTM, revealed the superior adhesion strength of HA-Cat hydrogel (~1.4 KPa, DS = 8.8%) compared to that of HA-ME hydrogels (~0.19 KPa, DS = 9.8%), which was directly proportional to the degree of catechol substitution. In addition to improving the viability and function of encapsulated hepatocytes and adipose-derived human mesenchymal stroma-like cells (hASCs), and enhancing in vivo attachment to liver and heart tissues, HA-Cat hydrogels enabled the minimally invasive transplantation of hepatocytes to the liver, with constructs supporting the adhesion, proliferation and biological activity of the transplanted hepatocytes.
Aiming at developing innovative hydrogel-based wearable devices for human motion monitoring, with optimal stretchability, conductivity and self-adhesive properties, Lv et al. [
27] designed a tri-component system featuring DA-modified HA (HAC), acrylamide (AM) and borax (as a dynamic cross-linker), as well as the conductive cations lithium and sodium. HAC was synthesised by a reaction of HA with carboxyl-activating agents EDC and NHS, followed by the addition of DA (DS = 34%, without the oxidation of catechol). Optimisation of reaction conditions included fine-tuning the HAC/AM ratio, with values >6% (wt.%) preventing hydrogel formation, because the reduced catechol groups delayed AM polymerisation. Stable hydrogels, supported by catechol, hydroxyl, and amine groups present in the HAC chains and surrounding network, which form interchain and intrachain hydrogen bonds, were produced, with mechanical and electrical tests revealing enhanced stretchability (2870%), swelling ability, and high tensile toughness (42 kPa). Moreover, the catechol groups in HAC enhanced (i) self-adhesiveness, as demonstrated by the strong adhesive strength of the constructs to porcine skin (49 kPa), and (ii) efficient self-healing without stimuli at room temperature, as reflected by quick wound healing times (~1 h). Reduced conductivity was detected for HAC-B-PAM hydrogels (0.018 S/m), whereas increased values were found for HAC–B–PAM hydrogels containing lithium (1.10 S/m).
For the development of HA-based hydrogels with improved adhesive properties and stability, Zhou et al. [
26] devised a strategy for the preparation of DA-functionalised HA hydrogels with enhanced catechol content, based on Schiff’s reaction of DA with aldehyde-modified HA. Compared to carbodiimide-based coupling strategies (average DS ~10%), higher DS was found for the coupling of DA to dialdehyde-modified HA (DAHA) (25% to 45%), without the formation of polydopamine or oxidation of catechol. The kinetics of oxidative crosslinking and gelation were assessed by time sweep rheology, with measurements revealing fast gelation for catechol-functionalised DAHA (CatHA) constructs (G’ = G’’ under 60 s), and G’ > G’’ until complete stabilisation of the hydrogels (at ~8 min). CatHA hydrogels presented a porous interconnected structure (20−350 μm pores) and significant swelling capacity (15–40 times greater than their initial weight), which was inversely proportional to the DS of DA (i.e., smaller pores and lower swelling capacity were associated with a denser structure characteristic of higher DS). Adhesion of CatHA hydrogels to porcine skin, measured via lap shear tests, was found to be directly proportional to the catechol content of the constructs, with higher values obtained for CatHA (~90 kPa), compared to conventional DA-conjugated HA hydrogels (~10 kPa), UV-crosslinked HA hydrogels (~13 kPa) and fibrin glue (2–40 kPa). Importantly, biocompatibility assays performed in L-929 cells revealed no significant cytotoxicity of the constructs (metabolic activity > 80%), which were found to support cell adhesion and proliferation.
Similarly, Zhang et al. [
29] described a novel method for the preparation of catechol (Cat)-functionalised HA, involving the conjugation of Cat functional groups to the HA backbone in organic phases under a nitrogen atmosphere, to increase the DS and prevent the oxidation of catechol. By reacting DA with the carboxyl groups of HA through carbodiimide/N-hydroxybenzotriazole (HOBt) coupling chemistry, the authors reported an average DS of 33% (compared to 7% for synthesis with the conventional method), without the detection of UV peaks characteristic of oxidised catechol. Following simple oxidant-mediated gelation, stable HA-Cat hydrogels were formed in ~20 min, with high DS correlating with faster gelation times. Similarly, wet adhesion testing of HA-Cat or control catechol-free HA hydrogels in chicken skin revealed a higher adhesion strength for HA-Cat hydrogels (compared to those without catechol or commercially available fibrin glue), which was directly proportional to the catechol content. Importantly, extensive cell adhesion and spreading were observed for human mesenchymal stem cells (hMSCs) seeded on the surface of the HA-Cat hydrogels, with cells presenting a typical spindle-like morphology, whereas very few cells adhered and expanded on the surface of control catechol-free hydrogels. Moreover, metabolic assays confirmed that hMSCs encapsulated in HA-Cat hydrogels remain metabolically active, at levels similar to those of well-established catechol-free hydrogels. Finally, studies with BMP-2-laden HA-Cat hydrogels demonstrated the capacity of the constructs to promote osteogenic differentiation of the encapsulated hMSCs, via sustained release of the therapeutic cargo.
Sousa et al. [
24] used a versatile and robust layer-by-layer technique to developed bioinspired films for wound-dressing, combining (via electrostatic interaction) Chi, alginate and HA modified with DA via carbodiimide chemistry (HA-DA, DS = 24%). SEM images revealed that the surface of the DA-containing membranes was more porous than the control unmodified counterparts. Moreover, DA-modified membranes showed higher average thickness values (for the same number of layers), improved mechanical strength and enhanced adhesion (assessed by lap shear strength test) compared to unmodified controls. The adhesion and proliferation of human dermal fibroblasts were also improved by DA-modified membranes, whereas treatments of dermal wounds in rats with DA membranes resulted in the strongest decrease in skin inflammation, compared with control conditions.
Silk Fibroin (SF)
Fibroin is the main component of silk, providing the internal structure and mechanical strength, with sericin forming an outer glue-like coating. Due to its excellent mechanical properties (as result of the formation of microfibrils with strong interlocks) and high biocompatibility, silk has been tested for use in several biomedical applications.
In this regard, Sogawa et al. [
31] reported on the adhesive profile of SF, modified to express dihydroxyphenylalanine (DOPA) via the environmentally friendly enzymatic (Tyr) conversion of tyrosine. The content of DOPA was evaluated by amino acid composition analysis, with results showing an average DS of 1.3 mol%, which was generally constant for all the tested concentrations, and a tyrosine conversion ratio of ~25%. The adhesive strength of DOPA-modified SF (DOPA-SF) towards soft and hard material surfaces, including mica, paper, polypropylene, wood and silk film, were assessed by lap shear tests, with results for the mica substrate showing an improvement in both adhesive strength and fracture upon the introduction of DOPA at any silk concentration and pH level (maximum values of 0.97 MPa and 19.1 kJ/m
3, respectively, at pH 10). Moreover, Fourier-transform infrared (FTIR) measurements demonstrated that the adhesivity of DOPA-SF was not directly related to the formation of secondary β-sheet structures, but to the catechol content. Importantly, DOPA-SF exhibited stronger adhesivity to all tested substrates (compared to unmodified SF), thus confirming the added value of DOPA functionalisation towards the improved adhesion of SF.
Using a similar enzyme-based strategy, Heichel et al. [
32] described an improved approach for the in situ functionalisation of SF with catechol groups, towards its application in wound healing. Using a multi-step protocol, the authors initially modified purified SF by carboxylation, to increase the carboxylate content of SF for reactions with tyramine. Conjugation of tyramine to SF, via COMU or CDI coupling, enabled the enrichment of SF in phenolic side chains (~30% phenolic addition for both methods), which were further oxidised into catechol groups via mushroom Tyr. Rheological time sweeps performed at 37 °C revealed that formation of hydrogels with elastic profiles (G’ > G’’ for all frequencies), with faster gelation kinetics for conjugates containing tyramine or higher SF concentrations. Modification of SF with tyramine did not prevent the formation of secondary beta sheet structures within SF, thus allowing for noncovalent interactions to strengthen the structure. Single lap shear tests measuring the adhesion of SF conjugates to porcine intestines revealed enhanced adhesivity for tyramine–SF conjugates, compared to that of SF without tyramine or fibrin glue, without significant differences between tyramine(CDI)-SF and tyramine(COMU)-SF (due to similar DS). Moreover, the induction of beta-sheet secondary structures (via sonication) further improved adhesivity, thus suggesting that, in addition to enzymatic (covalent) crosslinking, physical (non-covalent) crosslinking of the constructs provides extra strength and adhesiveness. Finally, in vitro biocompatibility assays in CaCO-2 intestinal epithelial cells, assessing the toxicity of SF extracts, cell attachment and proliferation, revealed no significant toxicity from possible leachables.
To balance its hydrophobicity, SF was modified by Burke et al. [
30] via the conjugation of hydrophilic polyethylene glycol (PEG) side chains (5 kDa), prior to reactions with DA for the chemical coupling of catechol groups. Although the efficiency of catechol functionalisation was not affected by the degree of PEG substitution, fine-tuning PEG modification was essential for aqueous solubility. Measurement of the adhesive bond of the modified silks to aluminium shims, using single lap shear testing, revealed that DA-modified SF formed a stronger adhesive bond compared to the unmodified material (>20 kPa improvement). Interestingly, the authors showed that incorporation of as little as 6 wt.% PEG prior to catechol functionalisation resulted in complete aqueous solubility of the catechol conjugates and increased the adhesive strength compared to silk lacking catechol moieties. PEG-SF conjugates also maintained the ability to form β-sheet secondary structures, which can be exploited to reduce swelling. Finally, in vitro biocompatibility tests revealed that the modified and unmodified SF supported the attachment and proliferation of hMSCs for up to 15 days, regardless of PEG and catechol conjugation.
Liu et al. [
33] reported the development of novel SF-based bioadhesive hydrogels. For this purpose, the authors modified purified SF with catechol functional groups through a one-step cross-linking protocol involving NHS/EDC activation and subsequent DA loading (NESFB-DA, DS = 0–15%, without catechol oxidation). For gelation, different amounts of PEG (Mw 20 kDa) were added to NESFB-DA, to obtain pegylated NEFSB-DA hydrogels. The optimal ratios of SF:PEG (5:5), NHS:EDC (2:1), and the amount of DA (5 wt.%) were identified after fine-tuning of the reaction conditions. As revealed in dry adhesive testing, increasing the SF:PEG ratio from 5:1 to 5:5 (
w/w) resulted in over a fourfold increase in the tensile strength of NESFB-DA hydrogels (from 48 kPa to 217 kPa), while decreasing the time required for hydrogel formation (from 116 min to 10 min). Importantly, adhesive tests in wet conditions (using fresh porcine skin) confirmed that pegylated NESFB-DA hydrogels display higher tensile strength compared to those without DA or clinically used fibrin sealants, which highlights the important role of DA in the adhesive properties of the hydrogels. Finally, degradation studies revealed a slow degradation rate for NESFB-DA constructs in PBS buffer (91% remaining weight after 55 days) and a better degradation profile compared to NESFB.
Gelatine
Gelatine is composed of a mixture of polypeptides produced by the hydrolysis of collagen. It is commonly used as a biomaterial for cell culture applications due to its biocompatibility, degradability and similarity to the extracellular matrix (ECM) at chemical and biological levels. In this regard, several adhesive gelatine-based constructs have been described for applications in wound healing and tissue regeneration.
Aiming at developing a new low-cost sealing materials with improved functional properties based on Gelfoam (an FDA-approved non-adhesive gelatine sponge for haemostatic applications), Hong et al. [
36] modified gelatine (Gel) by conjugation with DA via an EDC coupling reaction, resulting in a modified polymer (GelDA) with ~7–8 catechol groups per backbone unit. Fe
3+ complexation of Gel or GelDA yielded hexavalent Fe
3+ complexes with metallo-bioadhesive properties. The adhesive strength of Gel/Fe and GelDA/Fe was measured by lap shear testing on porcine skin under natural, moist conditions, with results revealing better tissue sealing performance for GelDA/Fe over a wide range of FeCl
3 concentrations (50–1000 mM), and especially at lower concentrations (over a twofold improvement in adhesive strength versus Gel/Fe for FeCl
3 concentrations between 50 and 200 mM). Upon subcutaneous implantation in mice, both materials were completely degraded after 23 days, with GelDA/Fe showing a longer half-life (7 days) compared to Gel/Fe (2 days), possibly due to the covalent nature of the crosslinking induced by Fe
3+.
An innovative approach was developed by Xuan et al. [
38] for the treatment of acute traumatic wound healing. Based on material nanosheets, the authors designed a two-layer nanosheet, composed of DA-functionalised antimicrobial peptide (AMP)-modified gelatine and polycaprolactone (PCL), with strong mechanical properties, adhesivity and antimicrobial activity. Gelatine methacrylate (GelMA) was initially modified by conjugation with DA via EDC/NHS coupling chemistry, followed by functionalisation with thiolated AMP (via thiol-ene click chemistry). Composite nanosheets, containing UV-crosslinked GelMA/DA/AMP (GDP), Ca
2+ and PCL, were manufactured by spin coating, producing thin films with final bilayer thickness of 45–95 nm and a rough PCL surface, which was smoothened after combination with GDP. Burst pressure and dynamic mechanical analysis confirmed that GDP/PCL constructs display suitable mechanical properties, including burst pressure superior to blood pressure and tensile modulus closer to that of human soft tissues. The flexible GDP/PCL nanosheets could also firmly attach to porcine tissue, although a gap was found with a more rigid control GDP hydrogel. Moreover, under wet conditions, GDP/PCL nanosheets remained attached to porcine tissue slices, whereas PCL nanosheets detached from the substrate, thus suggesting a role for catechol groups in the GDP on wet adhesion. Due to the incorporation of Ca
2+ (a known activator of coagulation) in the GDP layer, the constructs were also found to promote rapid haemostasis, whereas the presence of AMP contributed decisively to strong antibacterial activity (~100% killing activity after 2 h), compared to GDP/PCL without AMP (<2% killing activity). Finally, studies in an in vivo mouse model of wound healing revealed the successful sealing of bleeding wounds upon applications of GDP/PCL, which was associated with reduced clotting time and blood loss, as well as an absence of strong inflammatory responses or microbial activity.
Wu et al. [
37] designed a double hydrogel/patch system for the treatment of myocardial infarction (MI), for which single approaches have revealed limited therapeutic efficacy. Based on naturally occurring macromolecules, the authors developed a gelatine-based adhesive and conductive hydrogel patch, and an HA-based injectable hydrogel, for coadministration in the infarcted tissue. The injectable hydrogel was designed for in situ crosslinking via Schiff base reaction between oxidised sodium HA (HA-CHO) and hydrazided HA (HHA), whereas the self-adhesive cardiac patch was prepared via the ionic (Fe
3+-mediated) crosslinking of gelatine−DA (GelDA) and DA-functionalised polypyrrole (DA−PPy). The proposed combinatorial approach involved injection of the HACHO/HHA hydrogel into the infarcted cardiac tissue, followed by layering of the GelDA/DA−PPy patch onto the outer surface of the myocardium, without the need for additional sutures. Adhesion tests performed in porcine myocardium and skin revealed significant catechol-mediated adhesion strength for GelDA/DA−PPy-0.6% hydrogels (10 kDa and 16 kDa, respectively), which could withstand twisting, bending and soaking in water, as well as conductivity values matching those of myocardial tissue (2.85 × 10
−4 S/cm). Rheology tests with HA-CHO/HHA hydrogels (5% HA-CHO, 0.4−0.8% HHA, wt.%) revealed fast gelation kinetics and the formation of stable viscoelastic structures (i.e., G′ > G″ for the entire frequency range). Importantly, echocardiographical, histological and angiogenic outcomes were improved in rats, 28 days after surgical administration of the combined therapy, compared to those untreated or receiving single therapy.
Aiming at manufacturing small-diameter vasculature, containing a smooth muscle and endothelium, Cui et al. [
39] developed a smart mussel-inspired bioink, based on catechol-functionalised gelatine methacrylate (GelMA/C), capable of forming elastic hydrogels upon oxidative in situ crosslinking, with controllable mechanical strength, enhanced adhesivity and tuneable physical cues for bioprinting of stratified architectures. GelMA/C was synthesised in a stepwise process, involving carbodiimide coupling reactions of porcine gelatine with methacrylic anhydride, followed by DA grafting via EDC/NHS coupling chemistry (DS = 6–13%). Physico-chemical characterisation of the hydrogels included analyses of rheological properties, swelling and adhesion. In this regard, the authors noted a catechol- and solution-concentration-dependent gelation time, with GelMA/C at >15 wt.% favouring rapid gelation compatible with 3D bioprinting, whereas the high viscosity of bioink solutions at >30 wt.% hindered the extrusion process. G’ > G’’ was found for GelMA/C hydrogels over the entire frequency range, with the swelling of constructs reaching equilibrium or saturation in 3 days, and the adhesion strength of GelMA/C significantly higher than that of the GelMA counterpart. Cell-laden 3D tubular constructs were produced by coaxial printing with a double-needle system, using 15 wt.% GelMA/C (DS = 13%), human coronary artery smooth muscle cells (HCASMCs) and human umbilical vein endothelial cells (HUVECs). Using a cross-printing process, a well-connected vessel network was obtained, with an inner porous structure that allowed continuous perfusion, as well as gas and nutrient exchange. Indeed, in vitro cell assays confirmed the maintenance of viability and proliferation of encapsulated HCASMCs and HUVECs after 7 days in culture, with beneficial permeability parameters measured in the vascularised channels. Importantly, in vivo evaluation of degradability and biocompatibility of the 3D-bioprinted vasculature, using a mice xenograft model of transplantation, revealed slow (but incomplete) degradation of the subcutaneously implanted constructs over 16 weeks, and an inflammatory response typical of foreign body responses.
Carboxymethylcellulose (CMC)
CMC is an anionic water-soluble cellulose derivative, composed of repeated glucopyranose monomers modified by the conjugation of carboxymethyl groups, which is widely used as a thickener, lubricant and pharmaceutical excipient. A couple of studies addressing improved adhesiveness of CMC were found to comply with the search/scoring criteria.
Fu et al. [
44] designed a hybrid system, based on CMC, to be used as an underwater adhesion hydrogel. For this purpose, oxidised CMC (OCMC) was prepared using the periodate oxidation method, and subsequently modified by combination with DA via carbodiimide (EDC/NHS) crosslinker chemistry (average DS = 15%). OCMC-DA/PAM hydrogels were obtained upon the conjugation of acrylamide monomers (AM) to OCMC-DA via a Schiff base reaction, followed by the chemically induced polymerisation of AM. Structural/morphological and functional analysis of hydrogels included mechanical, rheological and adhesivity tests, as well as assessments of swelling behaviour and cytotoxicity. In this regard, the authors reported the formation of hydrogels with porous 3D structure, G’ > G’’ (at 0.1–50 Hz) and storage modulus increasing proportionally to the concentration of OCMC-DA. The adhesion strength of OCMC-DA6/PAM5 (one of the synthesised hydrogels) reached ~86 kPa (compared to 5 kPa for PAM5) and reduced to 43 kPa after immersion in water for 9 days. Moreover, the maximal adhesion strength was obtained when the G’ and G’’ of hydrogel were very close. Finally, no significant toxicity was found when NIH-3T3 mouse embryonic fibroblasts were incubated with extracts prepared from different OCMC-DA/PAM hydrogels.
Aiming at preparing mussel-inspired adhesive hydrogels for wound closure applications, Zhong et al. [
45] prepared novel CMC-based constructs amenable to in situ enzymatic crosslinking. Following EDC/NHS-catalysed CMC functionalisation with catechol moieties, CMC-DA hydrogels were fabricated via horseradish peroxidase (HRP)-mediated crosslinking in the presence of hydrogen peroxide (H
2O
2). Extensive testing was performed to investigate the impact of HRP/H
2O
2/CMC-DA concentration and DS on gelation/degradation kinetics, swelling and mechanical properties. Spectroscopic quantification of catechol aromatic rings revealed a DS between 4.5% and 13.5%, which was proportional to the amount of reacting DA. The hydrogel crosslinking rate was affected differently by the HRP/H
2O
2/CMC-DA concentrations, including (i) decreasing the gelation time with increased HRP/CMC-DA concentration, and (ii) decreasing the gelation time with increased H
2O
2 concentration up to 5 mM. On the mechanics of in situ gelation and degradation, time sweep rheology measurements revealed a more elastic profile for hydrogels with higher catechol content, as result of a denser 3D structure, which was associated with slower cellulase-mediated degradation kinetics (40–60% weight loss over 1 week). Greater adhesivity was also detected for CMC-DA hydrogels with higher DS (up to 28.5 kPa), which was superior to that of commercially available fibrin glue (2–6-fold increase). Finally, normal cell morphology and viability >89% were detected for L-929 murine fibroblasts cells cultured on the surface of CMC-DA hydrogels.
Other Natural Polymers
For several other naturally occurring polymers, including polysaccharides such as chondroitin sulphate, dextran, xanthan gum and lignin, a single manuscript complied with the search and scoring criteria.
Shin et al. [
42] designed a new hydrogel for cartilage repair based on the functionalisation of chondroitin sulphate (CS) with DA (CS-DA) using carbodiimide (EDC/NHS) chemistry. Conjugates with a DS around 20.9% showed (i) the least degradability when exposed to chondroitinase, (ii) the highest elastic modulus (~11 kPa), and (iii) the strongest adhesion to porcine cartilage (20−60 times greater than control methacrylated CS (CS-ME) hydrogels), thus suggesting a key role for catechol groups in hydrogel performance. CS-DA also showed catechol-related biocompatibility, by enabling high cell viability of encapsulated hASCs (>97% for all CS-DA groups on day 7) and presenting low immunogenicity, as per ELISA quantification of the pro-inflammatory cytokine tumour necrosis factor alpha (TNF-α) secreted by RAW264.7 macrophages. In vivo testing of the CS-DA hydrogels was performed in a dorsal subcutaneous rabbit model upon combination of the composites with printed PCL scaffolds (to provide stronger structure). In this regard, better cartilage formation and the higher expression of chondrogenic markers Sox9 and Col2A1 was found for CS-DA-PCL combined with autologous diced rabbit cartilage, compared to those without diced cartilage. Following implantation in an auricular cartilage tissue transplantation model, CS-DA-PCL hydrogels containing ear cartilage attached tightly to the wound edge, while similar CS-ME-PCL hydrogels detached from the defect, thus confirming the role of catechol moieties in the adhesion of CS-CA hydrogels to tissue.
Aiming at improving the adhesive profile of PEG:dextran hydrogels for use as surgical sealants, which are significantly affected by material swelling around soft tissues, Shazly et al. [
43] modified star PEG amine and dextran aldehyde polymers by the covalent conjugation of L-DOPA to linear dextran aldehyde using dynamic imine (amine–aldehyde) chemistry. Functional analysis of hydrogels, formed by spontaneous reaction upon mixing of the two components, included mechanical testing (ex vivo burst pressure assays in rat intestinal wounds), analysis of swelling and compressive modulus, as well as evaluations of local immune responses upon subcutaneous implantation in mice. In this regard, results clearly demonstrated overall improved performance for DOPA-modified PEG:dextran hydrogels (at 3 mM DOPA/M of aldehyde), including (i) lower swelling (50.3% less), (ii) higher stiffness (>280% increase), and (iii) stronger adhesive wound closure strength (>50%) compared to control unmodified hydrogels. However, although higher concentrations of DOPA (up to 11 mM/M of aldehyde) were similarly effective in reducing swelling, the adhesive strength and biocompatibility of the hydrogels were significantly reduced. Indeed, thicker fibrous layers were found surrounding conjugates containing 8 mM and especially 11 mM DOPA (compared to minimal fibrosis for unmodified PEG:dextran implants).
Aiming at addressing the poor healing of postoperative anastomotic leakage (PAL), a severe complication of gastrointestinal surgery, Huang et al. [
41] developed a novel hydrogel formulation composed of xanthan gum (Xan) modified with DA via EDC/NHS coupling (DS = 4% to 16%), to obtain a functionalised Xan hydrogel (DA-g-Xan) with improved adhesive properties. The combination of moderately adhesive Xan with DA was found to be sufficient for the induction of hydrogel crosslinking, due to the abundant intermolecular hydrogen bond interactions. Indeed, gelation could be easily observed upon inversion and shaking of the hydrogels, with DA (in 5 wt.% DA-g-Xan) providing additional structural integrity to resist centrifugation forces, whereas 5 wt.% Xan was destroyed by centrifugation. These results were corroborated by (i) rheological analysis, revealing that G’ increases with increasing polymer concentration, with DA grafting further improving G’ for each polymer concentration, and (ii) lap shear strength tests, showing a synergistic effect between Xan and DA adhesiveness towards the overall adhesive strength of DA-g-Xan, with values surpassing those of the commercially available fibrin glue. Interestingly, DA-g-Xan hydrogels presented self-healing properties but quick degradation in vitro (i.e., almost no biomaterial remaining after incubation for 96 h at 37 °C in PBS). On what concerns the influence of the biomaterial on cell activity, an anti-inflammatory profile was detected upon the incubation of RAW264.7 macrophages with DA-g-Xan (20 µg/mL) for 24 h, which included the overexpression of IL-4, IL-10 and CD206. Importantly, DA-g-Xan hydrogels improved the healing of colonic anastomosis in a rat model of disease, thus proving to be an approach with clinical potential.
Inspired by the mechanisms of adhesion in mussels, Gan et al. [
46] designed tough, adhesive and antibacterial hydrogels based on plant-derived polymers. To achieve the necessary redox balance, the authors built a system for the continuous generation of catechol groups driven by reductive phenolic/methoxy groups present in lignin, which are capable of reducing silver ions (Ag
+) to metallic silver nanoparticles (Ag-NPs) while oxidising to the corresponding o-quinone. Photogenerated electrons produced by Ag-NPs are then responsible for the conversion of o-quinone to catechol. In this regard, hydrogels with optimal mechanical properties were produced by the combination of Ag-lignin NPs with pectin and polyacrylic acid (PAA), which creates, upon gelation at room temperature (mediated by ammonium persulfate), an internal network of covalent and non-covalent bonds endowing high hydrogel toughness. The continuous and dynamic generation of catechol moieties by Ag-lignin NPs in the inner redox hydrogel network allowed for repeatable adhesiveness; the pectin/PAA mesh provided significant stretching capacity (>26 times its initial length) and quick recovery after deformation. Strong long-lasting adhesiveness was also measured for hydrogels in various surfaces, including glass, titanium, polytetrafluoroethylene and porcine skin (38, 50, 65, and 27.5 kPa, respectively), with further experiments suggesting a role for carboxyl groups of PAA and catechol groups of Ag-lignin in the molecular mechanisms of adhesivity. Importantly, in vitro and in vivo studies in a rabbit model clearly revealed strong antibacterial activity of the developed hydrogels, thus highlighting their application potential in wound healing.