Modification of Electrospun Regenerate Cellulose Nanofiber Membrane via Atom Transfer Radical Polymerization (ATRP) Approach as Advanced Carrier for Laccase Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
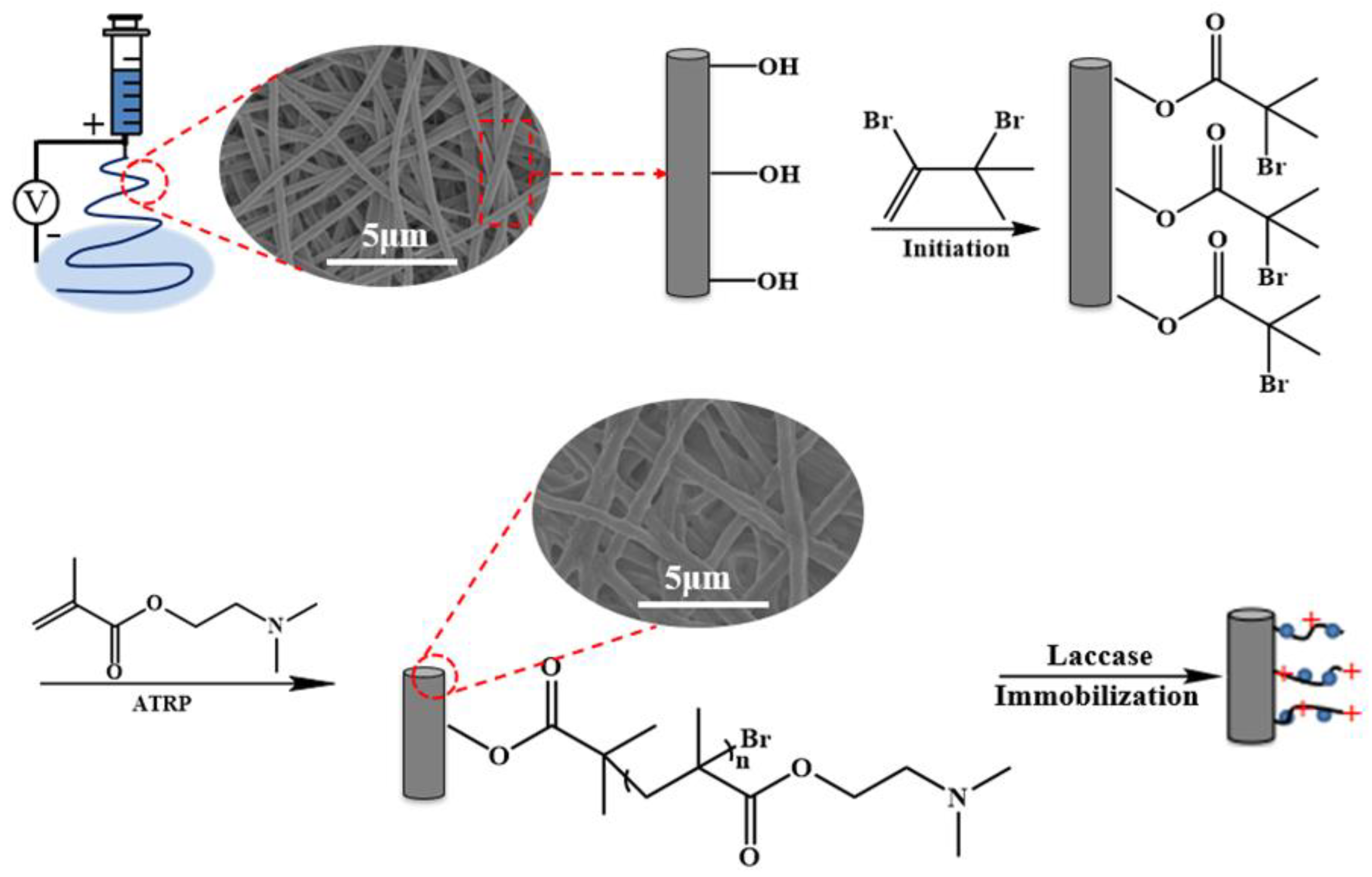
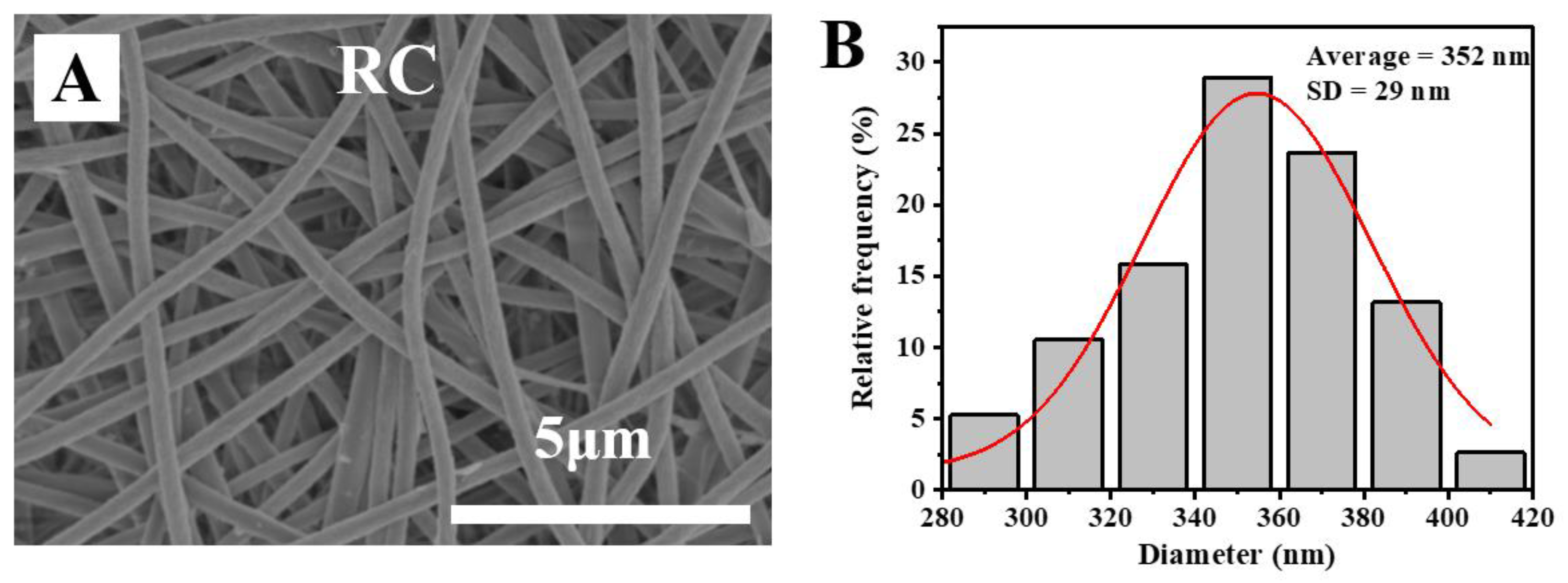
2.2. Preparation of Electrospun RC Nanofiber Membrane
2.3. Initiation of RC Nanofiber Membrane
2.4. Surface-Grafting of Poly (DMAEMA) via ATRP
2.5. Characterization of Different Membranes
2.6. Immobilization of Laccase on Nanofiber Membranes
3. Results
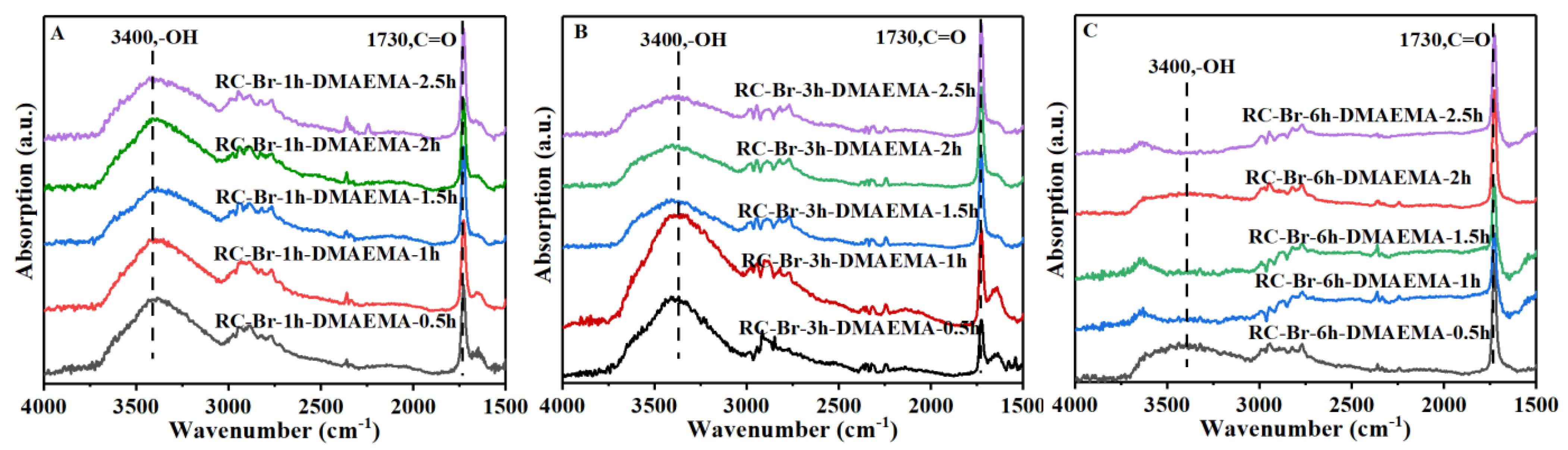
3.1. Surface Modification of RC Nanofiber Membrane via the ATRP Reaction
3.1.1. Initiation of RC Nanofiber Membrane
3.1.2. Surface-Grafting of Poly (DMAEMA) via ATRP
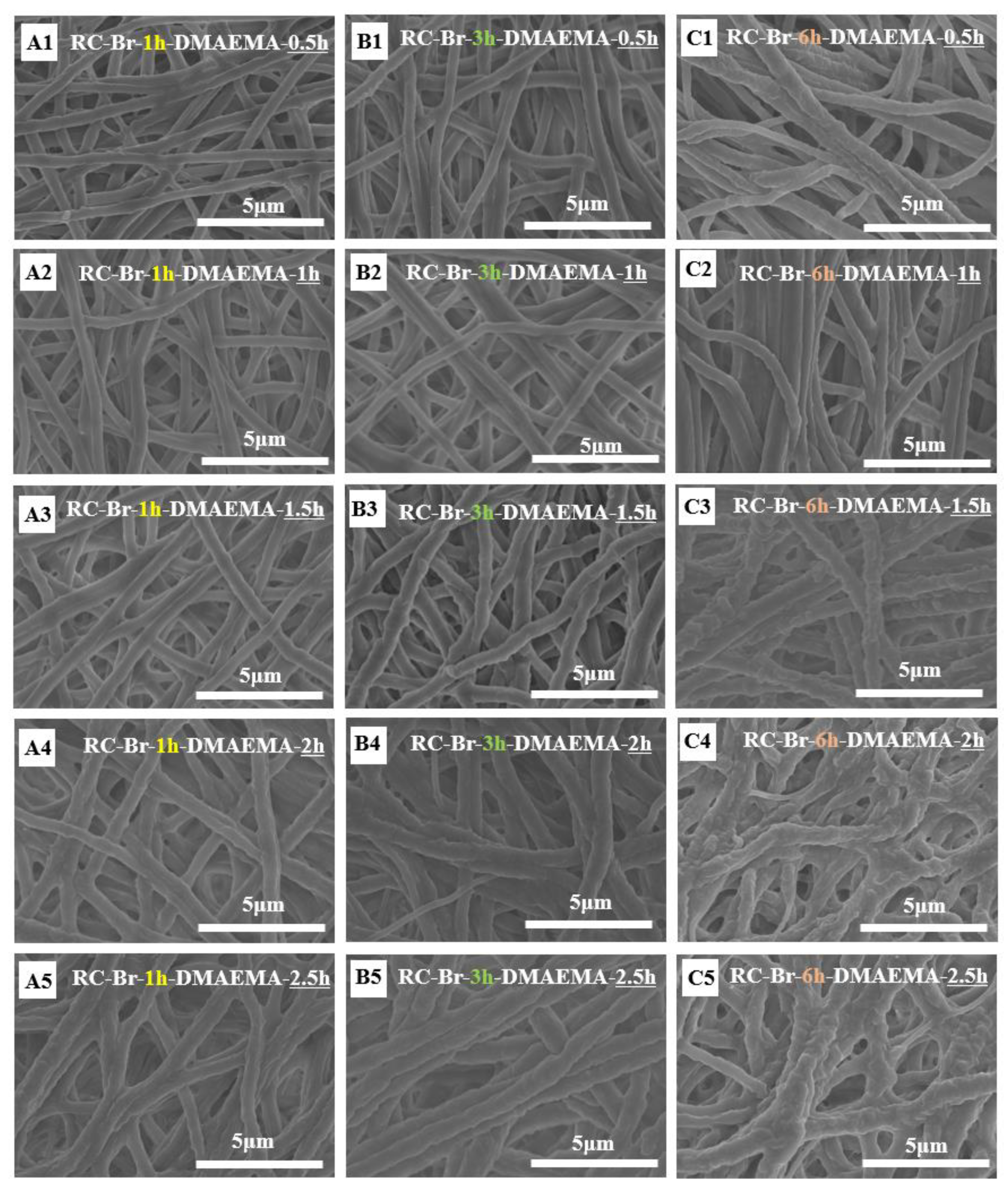
3.2. Morphological Characterization of Poly (DMAEMA)-Grafted RC Membranes
3.3. The Effects of ATRP Reaction Conditions on Enzyme Immobilization
3.3.1. The Effects of Initiation Degree on Laccase Immobilization
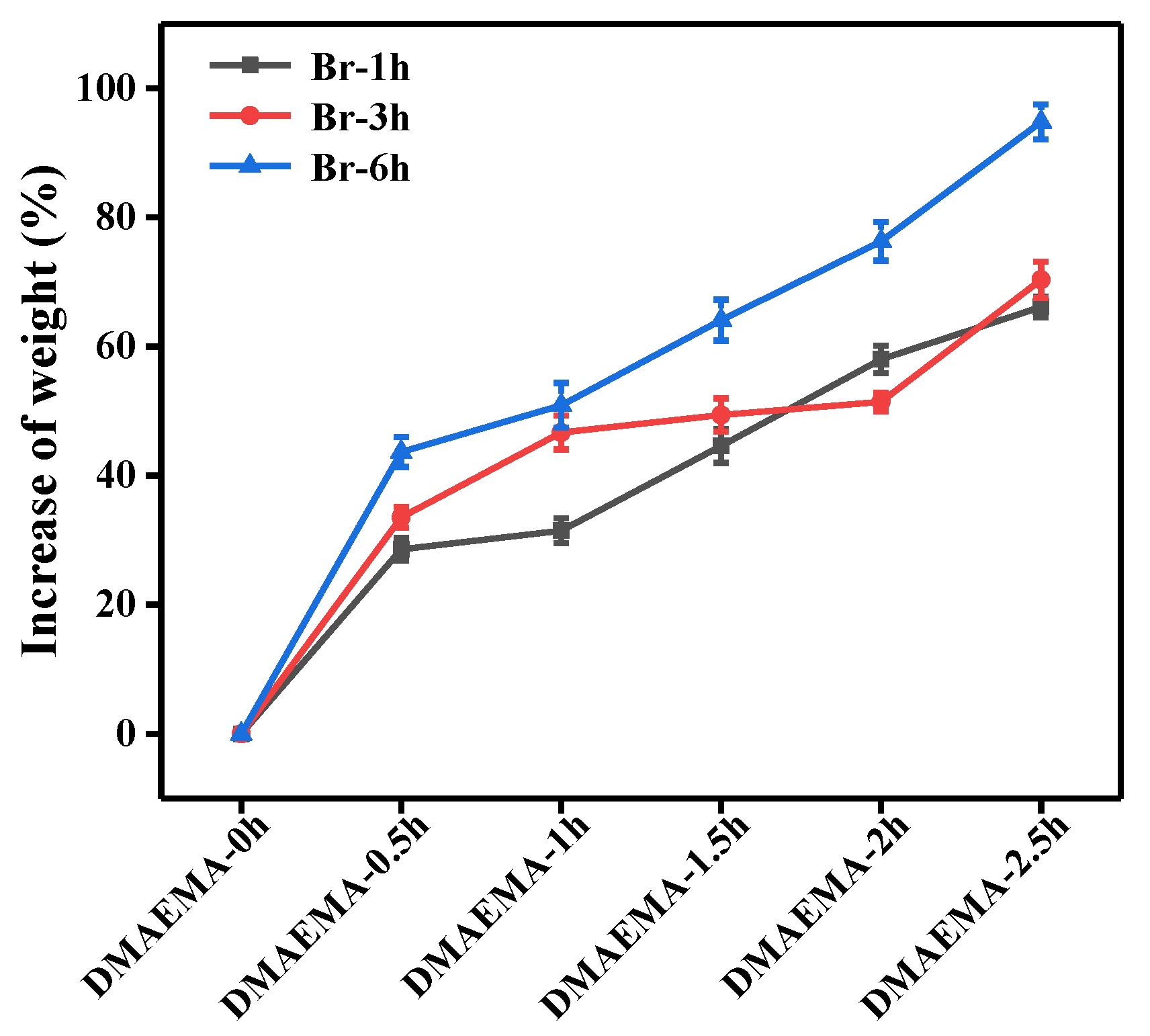
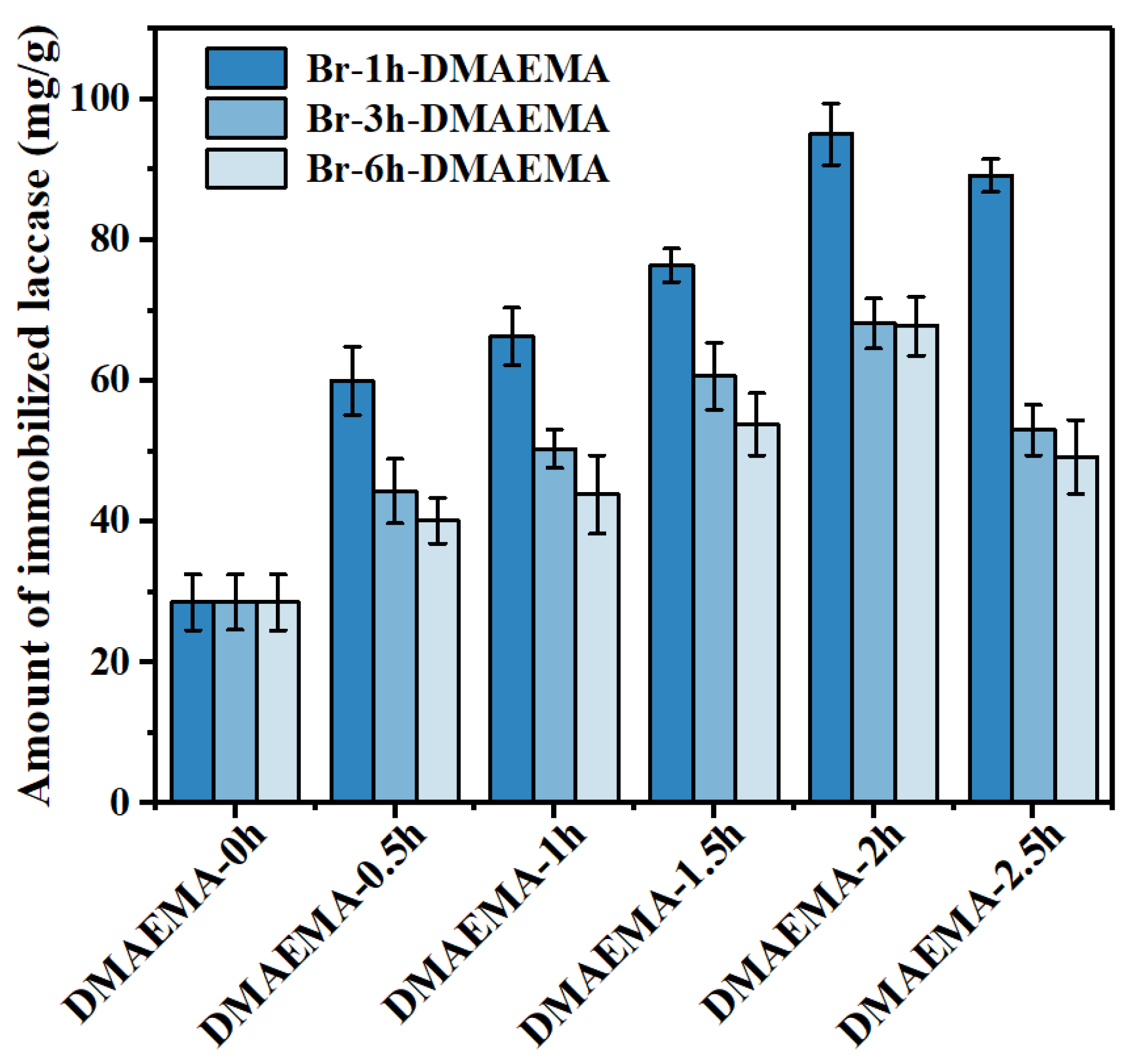
3.3.2. The Effects of Polymer Grafting Amount on Laccase Immobilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Mazurenko, I.; de Poulpiquet, A.; Lojou, E. Recent developments in high surface area bioelectrodes for enzymatic fuel cells. Curr. Opin. Electrochem. 2017, 5, 74–84. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.Q.; Jing, T.; Zhou, J.Y.; Tian, J.Z.; Zheng, Y.J.; Wang, C.; Zhao, Z.Y.; Lin, J.; Liu, H.; Zhao, C.Q.; et al. Laccase immobilized polyaniline/magnetic graphene composite electrode for detecting hydroquinone. Int. J. Biol. Macromol. 2020, 149, 1130–1138. [Google Scholar] [CrossRef]
- Mazzei, R.; Drioli, E.; Giorno, L. Enzyme membrane reactor with heterogenized beta-glucosidase to obtain phytotherapic compound: Optimization study. J. Membr. Sci. 2012, 390, 121–129. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Liu, Z.M.; Huang, X.J.; Xu, Z.K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Castillo, G.F.D.; Koenig, M.; Muller, M.; Eichhorn, K.J.; Stamm, M.; Uhlmann, P.; Dahlin, A. Enzyme Immobilization in Polyelectrolyte Brushes: High Loading and Enhanced Activity Compared to Monolayers. Langmuir 2019, 35, 3479–3489. [Google Scholar] [CrossRef]
- Wu, D.S.; Feng, Q.; Xu, T.; Wei, A.F.; Fong, H. Electrospun blend nanofiber membrane consisting of polyurethane, amidoxime polyarcylonitrile, and beta-cyclodextrin as high-performance carrier/support for efficient and reusable immobilization of laccase. Chem. Eng. J. 2018, 331, 517–526. [Google Scholar] [CrossRef]
- Vinambres, M.; Filice, M.; Marciello, M. Modulation of the Catalytic Properties of Lipase B from Candida antarctica by Immobilization on Tailor-Made Magnetic Iron Oxide Nanoparticles: The Key Role of Nanocarrier Surface Engineering. Polymers 2018, 10, 615. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.C.; Yen, C.I.; Wu, C.C.; Dutta, S.; Chou, L.Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal-Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Zhang, B.; Liu, L.; Xie, Y.D.; Zhang, H.Q.; Liu, J.D. Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 2010, 12, 259–263. [Google Scholar] [CrossRef]
- Vineh, M.B.; Saboury, A.A.; Poostchi, A.A.; Mamani, L. Physical Adsorption of Horseradish Peroxidase on Reduced Graphene Oxide Nanosheets Functionalized by Amine: A Good System for Biodegradation of High Phenol Concentration in Wastewater. Int. J. Environ. Res. 2018, 12, 45–57. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.X.; Kang, S.H.; Wang, H.M.; Li, H.Y.; Zhang, Y.X.; Wang, G.Z.; Zhao, H.J. Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils. J. Hazard. Mater. 2015, 289, 210–218. [Google Scholar] [CrossRef]
- Pang, R.; Li, M.Z.; Zhang, C.D. Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: Diffusional limitation investigation. Talanta 2015, 131, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.H.Y.; Jang, J.; Wu, K.C.W. Cellulase immobilized mesoporous silica nanocatalysts for efficient cellulose-to-glucose conversion. Green Chem. 2011, 13, 2844–2850. [Google Scholar] [CrossRef]
- Taqieddin, E.; Amiji, M. Enzyme immobilization in novel alginate-chitosan core-shell microcapsules. Biomaterials 2004, 25, 1937–1945. [Google Scholar] [CrossRef]
- Nobre, T.M.; Silva, H.D.E.; Furriel, R.P.M.; Leone, F.A.; Miranda, P.B.; Zaniquelli, M.E.D. Molecular view of the interaction between iota-carrageenan and a phospholipid film and its role in enzyme immobilization. J. Phys. Chem. B 2009, 113, 7491–7497. [Google Scholar] [CrossRef]
- Feng, Q.; Hou, D.Y.; Zhao, Y.; Xu, T.; Menkhaus, T.J.; Fong, H. Electrospun regenerated cellulose nanofibrous membranes surface-grafted with polymer chains/brushes via the atom transfer radical polymerization method for catalase immobilization. ACS Appl. Mater. Interfaces 2014, 6, 20958–20967. [Google Scholar] [CrossRef]
- Soti, P.L.; Weiser, D.; Vigh, T.; Nagy, Z.K.; Poppe, L.; Marosi, G. Electrospun polylactic acid and polyvinyl alcohol fibers as efficient and stable nanomaterials for immobilization of lipases. Bioprocess. Biosyst. Eng. 2016, 39, 449–459. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Dogac, Y.I.; Deveci, I.; Mercimek, B.; Teke, M. A comparative study for lipase immobilization onto alginate based composite electrospun nanofibers with effective and enhanced stability. Int. J. Biol. Macromol. 2017, 96, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, Y.C.; Liang, Z.P.; Sun, H.L.; Zheng, F.; Zhu, Z.T.; Zhao, Y.; Fong, H. Three-dimensional monolithic porous structures assembled from fragmented electrospun nanofiber mats/membranes: Methods, properties, and applications. Prog. Mater. Sci. 2020, 112, 35. [Google Scholar] [CrossRef]
- Daronch, N.A.; Kelbert, M.; Pereira, C.S.; de Araujo, P.H.H.; de Oliveira, D. Elucidating the choice for a precise matrix for laccase immobilization: A review. Chem. Eng. J. 2020, 397, 15. [Google Scholar] [CrossRef]
- Chen, H.Y.; Cheng, K.C.; Hsu, R.J.; Hsieh, C.W.; Wang, H.T.; Ting, Y.W. Enzymatic degradation of ginkgolic acid by laccase immobilized on novel electrospun nanofiber mat. J. Sci. Food Agric. 2020, 100, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Mate, D.M.; Alcalde, M. Laccase: A multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Zhang, W.X.; Cai, Y.P. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 403, 15. [Google Scholar] [CrossRef]
- Li, N.; Xia, Q.Y.; Li, Y.; Hou, X.B.; Niu, M.H.; Ping, Q.W.; Xiao, H.N. Immobilizing Laccase on Modified Cellulose/CF Beads to Degrade Chlorinated Biphenyl in Wastewater. Polymers 2018, 10, 798. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Q.Q.; Zhang, J.N.; Li, G.H.; Wei, Q.F. Preparation of Amidoxime-modified Polyacrylonitrile Nanofibers Immobilized with Laccase for Dye Degradation. Fiber. Polym. 2014, 15, 30–34. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Shibraen, M.; Abdel-Fattah, Y.R.; Elzain, A.A. Functionalization of Electrospun Poly (Acrylonitrile-co-Styrene/Pyrrole) Copolymer Nanofibers for Using as a High-performance Carrier for Laccase Immobilization. Fiber. Polym. 2019, 20, 2268–2279. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, D.S.; Huan, S.; Li, M.; Li, X. Study on the Preparation of the AOPAN/MMT Composite Nanofibers and Their Application for Laccase Immobilization. J. Eng. Fiber Fabr. 2016, 11, 45–54. [Google Scholar] [CrossRef]
- Li, X.; Li, D.W.; Lv, P.F.; Hu, J.Y.; Feng, Q.; Wei, Q.F. Immobilization of laccase onto modified PU/RC nanofiber via atom transfer radical polymerization method and application in removal of bisphenol A. Eng. Life Sci. 2019, 19, 815–824. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Z.; Ding, Y.C.; Xu, W.H.; Wu, W.D.; Zhu, Z.T.; Fong, H. Ultralight electrospun cellulose sponge with super-high capacity on absorption of organic compounds. Carbohydr. Polym. 2018, 179, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Du, H.S.; Liu, W.M.; Zhang, M.L.; Si, C.L.; Zhang, X.Y.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L.N. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Prautzsch, V.L.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Electrospun Regenerated Cellulose Nanofiber Membranes Surface-Grafted with Water-Insoluble Poly (HEMA) or Water-Soluble Poly (AAS) Chains via the ATRP Method for Ultrafiltration of Water. ACS Appl. Mater. Interfaces 2017, 9, 4272–4278. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Lee, S.H.; Dreyer, D.R.; An, J.H.; Velamakanni, A.; Piner, R.D.; Park, S.; Zhu, Y.W.; Kim, S.O.; Bielawski, C.W.; Ruoff, R.S. Polymer Brushes via Controlled, Surface-Initiated Atom Transfer Radical Polymerization (ATRP) from Graphene Oxide. Macromol. Rapid Commun. 2010, 31, 281–288. [Google Scholar] [CrossRef]
- Singh, N.; Husson, S.M.; Zdyrko, B.; Luzinov, I. Surface modification of microporous PVDF membranes by ATRP. J. Membr. Sci. 2005, 262, 81–90. [Google Scholar] [CrossRef]
- Xiang, T.; Yue, W.W.; Wang, R.; Liang, S.; Sun, S.D.; Zhao, C.S. Surface hydrophilic modification of polyethersulfone membranes by surface-initiated ATRP with enhanced blood compatibility. Colloid Surf. B Biointerfaces 2013, 110, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Khanmohammadi, F.; Molina, M.A.; Blanco, R.M.; Azizi, S.N.; Marquez-Alvarez, C.; Diaz, I. SBA-15 with short channels for laccase immobilization. Microporous Mesoporous Mat. 2020, 309, 6. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, W.Z.; Liu, Y.; Zhang, K.G.; Chen, Y.; Yin, X.S. Laccase immobilized on chitosan-coated Fe3O4 nanoparticles as reusable biocatalyst for degradation of chlorophenol. J. Mol. Struct. 2020, 1220, 7. [Google Scholar] [CrossRef]
- Zhang, J.B.; Ding, S.Y.; Ge, Y.Y.; Li, Z.L. Enhanced removal of crystal violet in water using a facile-fabricated and environmental-friendly laccase immobilized composite membrane. Process Biochem. 2020, 98, 122–130. [Google Scholar] [CrossRef]
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Correa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; Polizeli, M.; Bracht, A.; et al. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef]
- Shi, L.; Ma, F.Y.; Han, Y.L.; Zhang, X.Y.; Yu, H.B. Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. J. Hazard. Mater. 2014, 279, 203–211. [Google Scholar] [CrossRef]
- Zofair, S.F.F.; Arsalan, A.; Khan, M.A.; Alhumaydhi, F.A.; Younus, H. Immobilization of laccase on Sepharose-linked antibody support for decolourization of phenol red. Int. J. Biol. Macromol. 2020, 161, 78–87. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Karagoz, B.; Arica, M.Y. Cyclic-carbonate functionalized polymer brushes on polymeric microspheres: Immobilized laccase for degradation of endocrine disturbing compounds. J. Ind. Eng. Chem. 2018, 60, 407–417. [Google Scholar] [CrossRef]
- Jiang, X.B.; Yu, Y.T.; Li, X.M.; Kong, X.Z. High yield preparation of uniform polyurea microspheres through precipitation polymerization and their application as laccase immobilization support. Chem. Eng. J. 2017, 328, 1043–1050. [Google Scholar] [CrossRef]
- Maryskova, M.; Ardao, I.; Garcia-Gonzalez, C.A.; Martinova, L.; Rotkova, J.; Sevcu, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzym. Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jee, S.C.; Sung, J.S.; Kadam, A.A. Anti-proliferative applications of laccase immobilized on super-magnetic chitosan-functionalized halloysite nanotubes. Int. J. Biol. Macromol. 2018, 118, 228–237. [Google Scholar] [CrossRef] [PubMed]


| Carriers | Method | Enzyme Loading | Reference |
|---|---|---|---|
| Amino-functionalized SBA-15 silica | Physical adsorption | 57 mg/g | [43] |
| Halloysite nanotubes (HNTs) with Fe3O4 nanoparticles and chitosan | Physical adsorption | 100.12 mg/g | [44] |
| Geopolymer | Physical adsorption | 28.0 mg/g | [45] |
| PU/RC-poly (HEMA) nanofiber membrane | Ion coordination | 84.21 mg/g | [33] |
| Monoaminoethyl-N-aminoethyl (MANAE–agarose) | Ionic adsorption | 18 ± 0.5 mg/g | [46] |
| Concanavalin A-activated Fe3O4 nanoparticles | Ionic adsorption | 29.4 mg/g | [47] |
| Sepharose-linked antibody | Covalent bonding | 33 mg/g | [48] |
| Amidoxime polyacrylonitrile/montmorillonite (AOPAN/MMT) composite nanofibers | Covalent bonding | 89.26 mg/g | [32] |
| Polystyrene-divinylbenzene-poly (glycidyl methacrylate) [PS-co-DVBg-P(CCMA)]-PGMA | Covalent bonding | 47.8 mg/g | [49] |
| Polyurea microspheres | Covalent bonding | 20.63 mg/g | [50] |
| polyamide 6/chitosan (PA6/CHIT) nanofibers modified by (i) bovine serum albumin (BSA) (ii) hexamethylenediamine (HMD) | Covalent bonding | (i) 64.1 ± 7.9 mg/g (ii) 72.9 ± 14.6 mg/g | [51] |
| Supermagnetized (Fe3O4) and chitosan (CS) functionalized halloysite nanotubes (HNTs) (Fe3O4-HNTs-CS) | Covalent bonding | 90 mg/g | [52] |
| RC-poly (DMAEMA) nanofiber membrane | Physical adsorption | 95.04 ± 4.35 mg/g | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Shi, J.; Feng, A.; Wang, Z. Modification of Electrospun Regenerate Cellulose Nanofiber Membrane via Atom Transfer Radical Polymerization (ATRP) Approach as Advanced Carrier for Laccase Immobilization. Polymers 2021, 13, 182. https://doi.org/10.3390/polym13020182
Zeng S, Shi J, Feng A, Wang Z. Modification of Electrospun Regenerate Cellulose Nanofiber Membrane via Atom Transfer Radical Polymerization (ATRP) Approach as Advanced Carrier for Laccase Immobilization. Polymers. 2021; 13(2):182. https://doi.org/10.3390/polym13020182
Chicago/Turabian StyleZeng, Shuo, Jinwei Shi, Anchao Feng, and Zhao Wang. 2021. "Modification of Electrospun Regenerate Cellulose Nanofiber Membrane via Atom Transfer Radical Polymerization (ATRP) Approach as Advanced Carrier for Laccase Immobilization" Polymers 13, no. 2: 182. https://doi.org/10.3390/polym13020182
APA StyleZeng, S., Shi, J., Feng, A., & Wang, Z. (2021). Modification of Electrospun Regenerate Cellulose Nanofiber Membrane via Atom Transfer Radical Polymerization (ATRP) Approach as Advanced Carrier for Laccase Immobilization. Polymers, 13(2), 182. https://doi.org/10.3390/polym13020182





