Simultaneous Electrospinning and Electrospraying for the Preparation of a Precursor Membrane Containing Hydrothermally Generated Biochar Particles to Produce the Value-Added Product of Carbon Nanofibrous Felt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
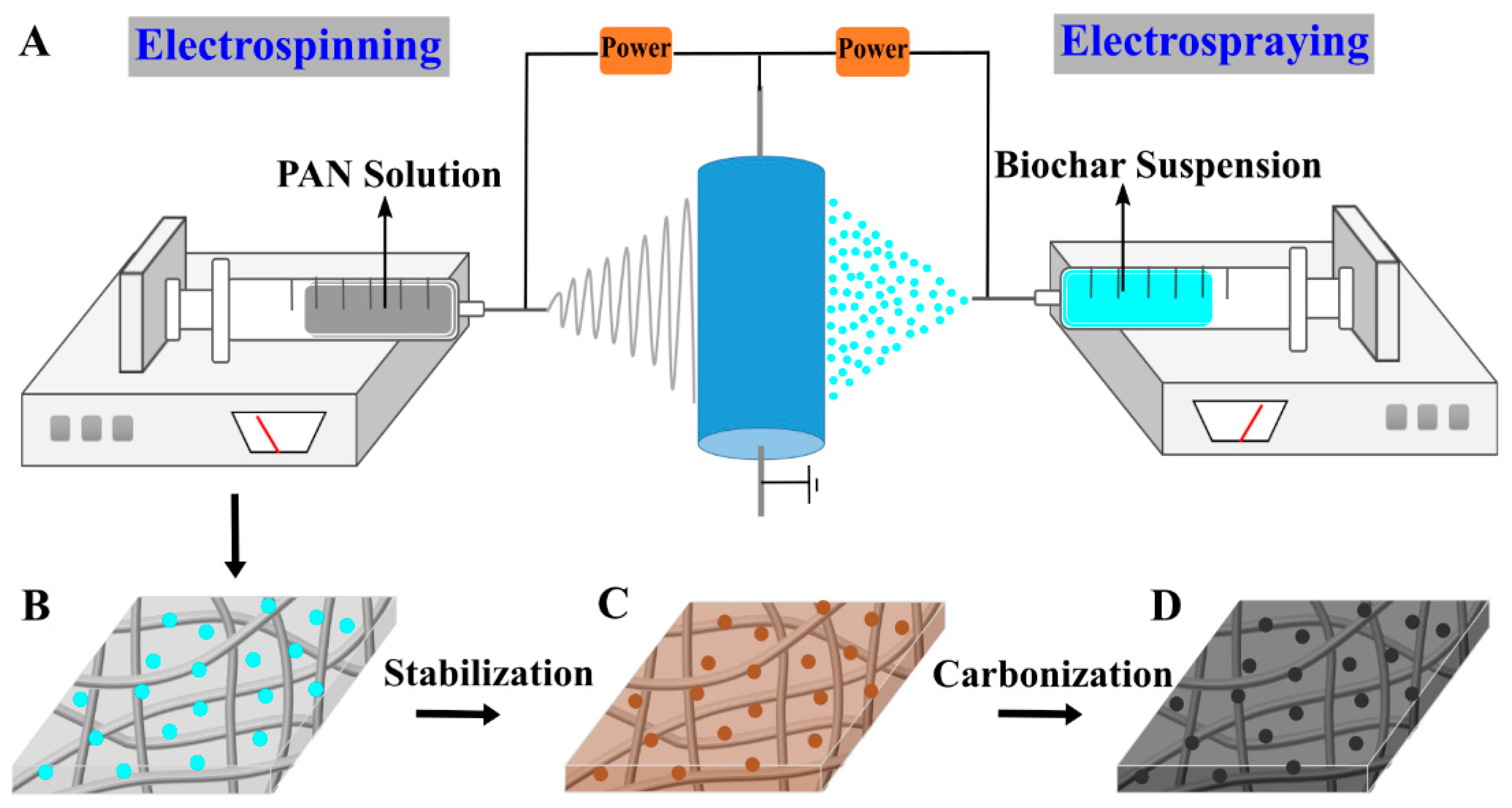
2.2. Apparatus Description and Methods
2.3. Characterization
3. Results and Discussion
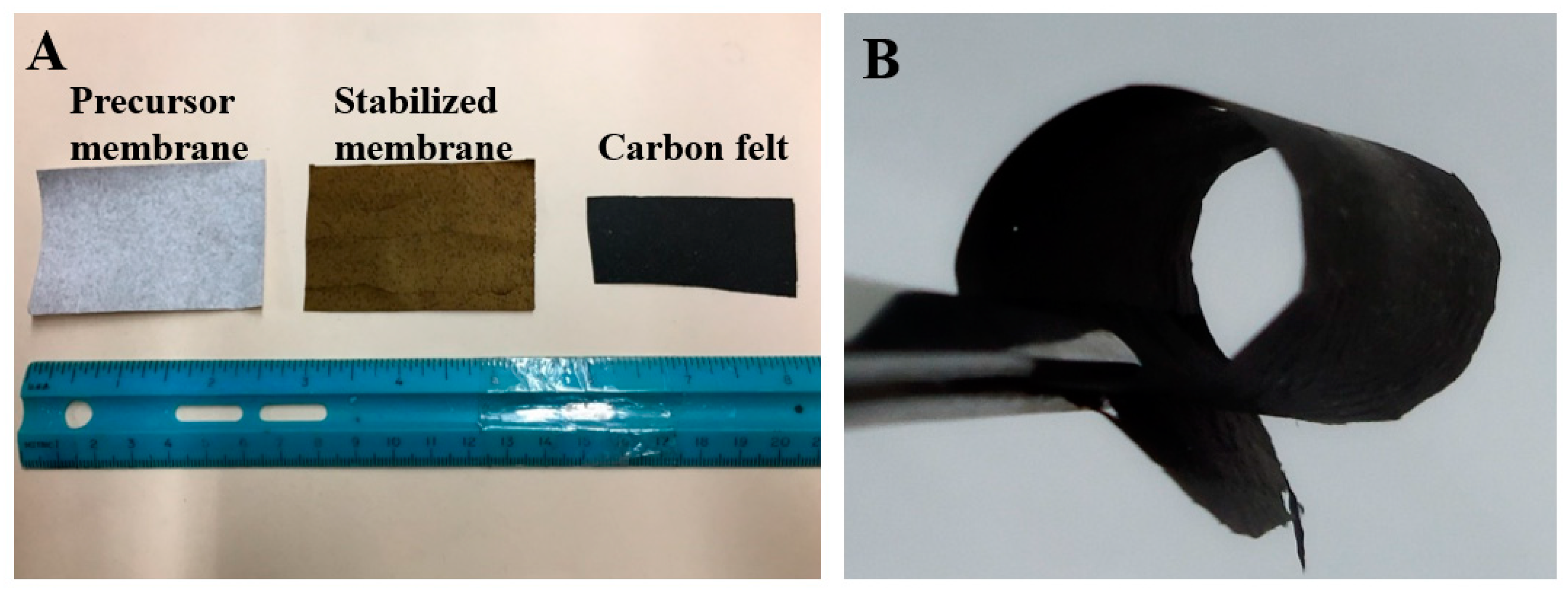
3.1. Fabrication of Biochar/PAN Carbon Nanofibrous Felt
3.2. Morphology and Structure of Biochar/PAN Carbon Nanofibrous Felt
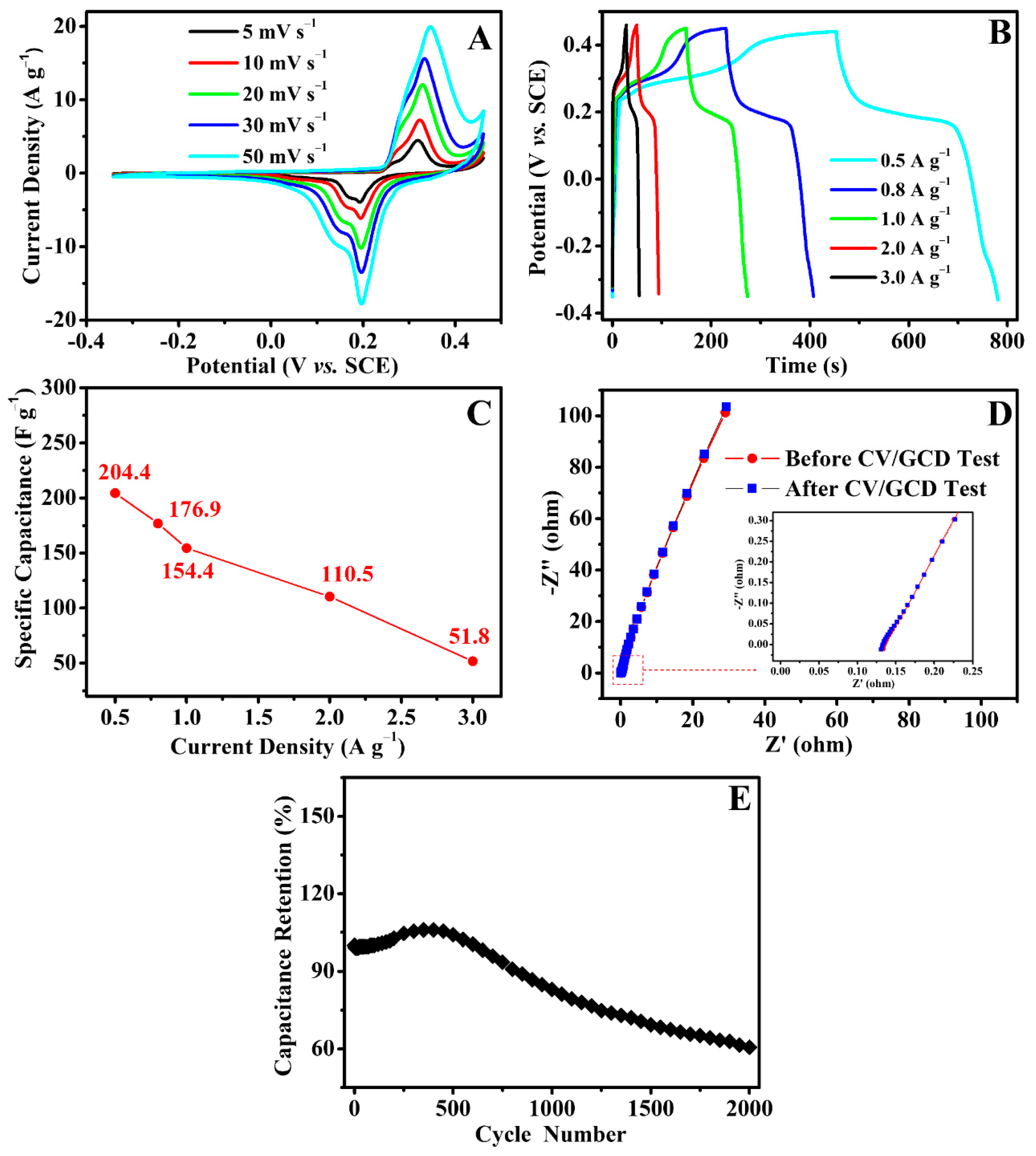
3.3. Electrochemical Properties of Biochar/PAN Carbon Nanofibrous Felt
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhushani, J.A.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solu-tions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef] [Green Version]
- Cloupeau, M.; Prunet-Foch, B. Electrohydrodynamic spraying functioning modes: A critical review. J. Aerosol. Sci. 1994, 25, 1021–1036. [Google Scholar] [CrossRef]
- Wang, F.; Cai, J.; Yu, J.; Li, C.; Yang, Z. Simultaneous electrospinning and electrospraying: Fabrication of a carbon nano-fibre/MnO/reduced graphene oxide thin film as a high-performance anode for lithium-ion batteries. ChemElectroChem 2018, 5, 51–61. [Google Scholar] [CrossRef]
- Hafner, S.; Guthrey, H.; Lee, S.-H.; Ban, C. Synchronized electrospinning and electrospraying technique for manufacturing of all-solid-state lithium-ion batteries. J. Power Sources 2019, 431, 17–24. [Google Scholar] [CrossRef]
- Wang, W.; Liang, Y.; Kang, Y.; Liu, L.; Xu, Z.; Tian, X.; Mai, W.; Fu, H.; Lv, H.; Teng, K.; et al. Carbon-coated SnO2@carbon nanofibers produced by electrospinning-electrospraying method for anode materials of lithium-ion batteries. Mater. Chem. Phys. 2019, 223, 762–770. [Google Scholar] [CrossRef]
- Tang, J.; Wu, C.; Chen, S.; Qiao, Z.; Fan, H. Combining of electrospinning and electrospraying to prepare biomimetic neural scaffold with synergistic cues of topography and electro-transduction. ACS Appl. Bio Mater. 2020, 8, 5148–5159. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. A supercritical CO2 assisted electrohydrodynamic process used to produce microparti-cles and microfibers of a model polymer. J. CO2 Util. 2019, 33, 532–540. [Google Scholar] [CrossRef]
- Chen, T.L.; Elabd, Y.A. Hybrid-capacitors with polyaniline/carbon electrodes fabricated via simultaneous electrospin-ning/electrospraying. Electrochim. Acta 2017, 229, 65–72. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhu, Y.J.; Han, F.D.; Luo, C.; Wang, C.S. 3D Si/C fiber paper electrodes fabricated using a combined electrospray/electrospinning technique for li-ion batteries. Adv. Energy Mater. 2015, 5, 1400753. [Google Scholar] [CrossRef]
- Nan, W.; Shende, A.R.; Shannon, J.; Shende, R.V. Insight into Catalytic Hydrothermal Liquefaction of Cardboard for Biofuels Production. Energy Fuels 2016, 30, 4933–4944. [Google Scholar] [CrossRef]
- Verheijen, F.G.; Graber, E.R.; Ameloot, N.; Bastos, A.C.; Sohi, S.; Knicker, H. Biochars in soils: New insights and emerging re-search needs. Eur. J. Soil Sci. 2014, 65, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Zhang, Y.; Li, A.; Lei, Z. Facile synthesis of high-surface area mesoporous biochar for energy storage via in-situ template strategy. Mater. Lett. 2018, 230, 183–186. [Google Scholar]
- Wu, J.; Dou, L.; Hu, L.; Wang, X.; Qian, G. Layered double hydroxide functioned as a novel template for the synthesis of gra-phene-oxide-like biochar and enhanced electrochemical performances. Energy Fuels 2020, 34, 16220–16227. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, Y.; Zou, Y.; Shi, M.; Deng, Q.; Zhao, N.; Wang, J.; You, C.; Yang, R.; Xu, Y. Sulfonated polyaniline coated bam-boo-derived biochar/sulfur cathode for Li-S batteries with excellent dual conductivity and polysulfides affinity. Electrochim. Acta 2019, 310, 45–57. [Google Scholar] [CrossRef]
- Wang, J.; Fan, L.; Yao, T.; Gan, J.; Zhi, X.; Hou, N.; Gan, T.; Zhao, Y.; Li, Y. A High-Performance Direct Carbon Fuel Cell with Reed Rod Biochar as Fuel. J. Electrochem. Soc. 2019, 166, F175–F179. [Google Scholar] [CrossRef]
- Qin, C.; Wang, H.; Yuan, X.; Xiong, T.; Zhang, J. Understanding structure–performance correlation of biochar materials in en-vironmental remediation and electrochemical devices. Chem. Eng. J. 2019, 382, 122977. [Google Scholar] [CrossRef]
- Qi, F.; Lamb, D.; Naidu, R.; Bolan, N.S.; Yan, Y.; Yong, S.O.; Rahman, M.M.; Choppala, G. Cadmium solubility and bioavailabil-ity in soils amended with acidic and neutral biochar. Sci. Total Environ. 2018, 611, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, D.; Liang, H.; Yin, J.; Xia, Y.; Zuo, K.; Zeng, Y.-P. Ultra-thick wood biochar monoliths with hierarchically porous structure from cotton rose for electrochemical capacitor electrodes. Electrochim. Acta 2020, 352, 136452. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Cuña, A.; Da Silva, E.L.; Amaral-Labat, G.; Silva, G.F.B.L.; Bouafif, H.; Koubaa, A. The conversion of wood residues, using pilot-scale technologies, into porous activated biochars for supercapacitors. J. Porous Mater. 2019, 27, 537–548. [Google Scholar] [CrossRef]
- Ali, A.; Raza, R.; Shakir, M.I.; Iftikhar, A.; Alvi, F.; Ullah, M.K.; Hamid, A.; Kim, J.-S. Promising electrochemical study of titanate based anodes in direct carbon fuel cell using walnut and almond shells biochar fuel. J. Power Sources 2019, 434, 126679. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]
- De La Mora, J.F.; Loscertales, I.G. The current emitted by highly conducting Taylor cones. J. Fluid Mech. 1994, 260, 155–184. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.; Li, Y.; Wang, H.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef]
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.-F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141. [Google Scholar] [CrossRef]
- Jahromi, Z.; Mirzaei, E.; Savardashtaki, A.; Afzali, M.; Afzali, Z. A rapid and selective electrochemical sensor based on elec-trospun carbon nanofibers for tramadol detection. Microchem. J. 2020, 157, 104942. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.D.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, T.; Liang, Z.; Amar, V.S.; Huang, R.; Maddipudi, B.K.; Shende, R.V.; Fong, H. Simultaneous Electrospinning and Electrospraying for the Preparation of a Precursor Membrane Containing Hydrothermally Generated Biochar Particles to Produce the Value-Added Product of Carbon Nanofibrous Felt. Polymers 2021, 13, 676. https://doi.org/10.3390/polym13050676
Li X, Xu T, Liang Z, Amar VS, Huang R, Maddipudi BK, Shende RV, Fong H. Simultaneous Electrospinning and Electrospraying for the Preparation of a Precursor Membrane Containing Hydrothermally Generated Biochar Particles to Produce the Value-Added Product of Carbon Nanofibrous Felt. Polymers. 2021; 13(5):676. https://doi.org/10.3390/polym13050676
Chicago/Turabian StyleLi, Xianfu, Tao Xu, Zhipeng Liang, Vinod S. Amar, Runzhou Huang, Bharath K. Maddipudi, Rajesh V. Shende, and Hao Fong. 2021. "Simultaneous Electrospinning and Electrospraying for the Preparation of a Precursor Membrane Containing Hydrothermally Generated Biochar Particles to Produce the Value-Added Product of Carbon Nanofibrous Felt" Polymers 13, no. 5: 676. https://doi.org/10.3390/polym13050676








