Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review
Abstract
:1. Introduction
2. 1D Materials
2.1. Titanate (TNTs) and Halloysite Nanotubes (HNTs)
2.2. Carbon Nanotubes (CNTs)
- (a)
- SWCNT (Single-Walled Carbon NanoTubes) consist of a single graphitic sheet wound on itself.
- (b)
- MWCNT (Multi-Walled Carbon NanoTubes) are formed by several sheets coaxially wound one on top of the other.
2.2.1. Poly(vinyl alcohol) (PVA)
2.2.2. Chitosan (CS)
2.2.3. Aromatic Polymers
3. 2D Materials
3.1. Layered Double Hydroxides (LDHs)
3.1.1. Poly(vinyl alcohol) (PVA) and Chitosan (CS)
3.1.2. Polysulfone (PSU)
3.1.3. Poly(phenylene oxide) (PPO)
3.1.4. Poly(vinylidene fluoride) (PVDF)
3.2. MXenes
3.3. Graphene Oxide (GO) and Graphene
3.3.1. Poly(vinyl alcohol) (PVA)
3.3.2. Polysulfone (PSU)
3.3.3. Poly(phenylene oxide) (PPO)
3.3.4. Polybenzimidazole (PBI)
3.3.5. Other Aromatic Polymers
3.3.6. Other Aliphatic Polymers
3.4. Carbon and Boron Nitride (BN)
4. 3D Materials
4.1. Silica and Silicates
4.1.1. Poly(vinylidene fluoride) (PVDF) and Poly(vinyl alcohol) (PVA)
4.1.2. Aromatic Polymers
4.2. Metal Oxides and Derivatives
4.2.1. Aluminum Oxides
4.2.2. Zirconium Oxides
4.2.3. Titanium Dioxide and Titanates
4.3. Other Inorganics
4.4. Metal Organic Frameworks (MOFs)
4.5. Carbon Dots (CDs)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varcoe, J.R.; Slade, R.C.T. Prospects for Alkaline Anion-Exchange Membranes in Low Temperature Fuel Cells. Fuel Cells 2004, 5, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Maurya, S.; Shin, S.-H.; Kim, Y.; Moon, S.-H. A review on recent developments of anion exchange membranes for fuel cells and redox flow batteries. RSC Adv. 2015, 5, 37206–37230. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Antolini, E. Composite materials for polymer electrolyte membrane microbial fuel cells. Biosens. Bioelectron. 2015, 69, 54–70. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. J. Ind. Eng. Chem. 2018, 70, 70–86. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2019, 100, 101177. [Google Scholar] [CrossRef]
- Knauth, P.; Pasquini, L.; Narducci, R.; Sgreccia, E.; Becerra-Arciniegas, R.-A.; Di Vona, M. Effective ion mobility in anion exchange ionomers: Relations with hydration, porosity, tortuosity, and percolation. J. Membr. Sci. 2020, 617, 118622. [Google Scholar] [CrossRef]
- Omasta, T.J.; Park, A.M.; LaManna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S.; et al. Beyond catalysis and membranes: Visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs. Energy Environ. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Omasta, T.J.; Peng, X.; Wang, L.; Varcoe, J.R.; Pivovar, B.S.; Mustain, W.E. Quantifying and elucidating the effect of CO2 on the thermodynamics, kinetics and charge transport of AEMFCs. Energy Environ. Sci. 2019, 12, 2806–2819. [Google Scholar] [CrossRef] [Green Version]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2020, 113, 101345. [Google Scholar] [CrossRef]
- Lim, K.; Wong, C.; Wong, W.; Loh, K.; Selambakkannu, S.; Othman, N.; Yang, H. Radiation-Grafted Anion-Exchange Membrane for Fuel Cell and Electrolyzer Applications: A Mini Review. Membranes 2021, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Kamarudin, S. Titanium dioxide nanotubes (TNT) in energy and environmental applications: An overview. Renew. Sustain. Energy Rev. 2017, 76, 212–225. [Google Scholar] [CrossRef]
- Premchand, Y.D.; Djenizian, T.; Vacandio, F.; Knauth, P. Fabrication of self-organized TiO2 nanotubes from columnar titanium thin films sputtered on semiconductor surfaces. Electrochem. Commun. 2006, 8, 1840–1844. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef] [Green Version]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Elumalai, V.; Sangeetha, D. Preparation of anion exchangeable titanate nanotubes and their effect on anion exchange membrane fuel cell. Mater. Des. 2018, 154, 63–72. [Google Scholar] [CrossRef]
- Elumalai, V.; Sangeetha, D. Synergic effect of ionic liquid grafted titanate nanotubes on the performance of anion exchange membrane fuel cell. J. Power Sources 2018, 412, 586–596. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Z.; Buregeya, I.; Pan, X.; Li, N.; Chen, S.; Liu, Y. Composite Anion Exchange Membranes from Quaternized Poly(arylene ether ketone) and Quaternized Titanate Nanotubes with Enhanced Ion Conductivity. Fuel Cells 2019, 19, 663–674. [Google Scholar] [CrossRef]
- Shi, B.; Li, Y.; Zhang, H.; Wu, W.; Ding, R.; Dang, J.; Wang, J. Tuning the performance of anion exchange membranes by embedding multifunctional nanotubes into a polymer matrix. J. Membr. Sci. 2016, 498, 242–253. [Google Scholar] [CrossRef]
- Pan, W.-H.; Lue, S.J.; Chang, C.-M.; Liu, Y.-L. Alkali doped polyvinyl alcohol/multi-walled carbon nano-tube electrolyte for direct methanol alkaline fuel cell. J. Membr. Sci. 2011, 376, 225–232. [Google Scholar] [CrossRef]
- Lue, S.J.; Pan, W.-H.; Chang, C.-M.; Liu, Y.-L. High-performance direct methanol alkaline fuel cells using potassium hydroxide-impregnated polyvinyl alcohol/carbon nano-tube electrolytes. J. Power Sources 2012, 202, 1–10. [Google Scholar] [CrossRef]
- Lo, C.-F.; Wu, J.-F.; Li, H.-Y.; Hung, W.-S.; Shih, C.-M.; Hu, C.-C.; Liu, Y.-L.; Lue, S.J. Novel polyvinyl alcohol nanocomposites containing carbon nano-tubes with Fe3O4 pendants for alkaline fuel cell applications. J. Membr. Sci. 2013, 444, 41–49. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lin, J.-S.; Pan, W.-H.; Shih, C.-M.; Liu, Y.-L.; Lue, S.J. Alkaline direct ethanol fuel cell performance using alkali-impregnated polyvinyl alcohol/functionalized carbon nano-tube solid electrolytes. J. Power Sources 2016, 303, 267–277. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, M.; He, X.; Qiao, J. Poly(vinyl alcohol)/Poly(diallyldimethylammonium chloride) anion-exchange membrane modified with multiwalled carbon nanotubes for alkaline fuel cells. J. Mater. 2019, 5, 286–295. [Google Scholar] [CrossRef]
- Zhou, T.; He, X.; Lu, Z. Studies on a novel anion-exchange membrane based on chitosan and ionized organic compounds with multiwalled carbon nanotubes for alkaline fuel cells. J. Appl. Polym. Sci. 2018, 135, 46323. [Google Scholar] [CrossRef]
- Jang, S.C.; Chuang, F.S.; Tsen, W.C.; Kuo, T.W. Quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. J. Appl. Polym. Sci. 2019, 136, 47778. [Google Scholar] [CrossRef]
- Jang, S.-C.; Tsen, W.-C.; Chuang, F.-S.; Gong, C. Simultaneously enhanced hydroxide conductivity and mechanical properties of quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. Int. J. Hydrogen Energy 2019, 44, 18134–18144. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Quaternized Poly(Styrene Ethylene Butylene Poly Styrene)/Multiwalled Carbon Nanotube Composites for Alkaline Fuel Cell Applications. J. Nanosci. Nanotechnol. 2013, 13, 5522–5533. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, L.; Liang, S.; Dong, Q.; Jin, B.; Bai, R. Preparation and characterization of composite membranes with ionic liquid polymer-functionalized multiwalled carbon nanotubes for alkaline fuel cells. RSC Adv. 2013, 3, 13477–13485. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, B.; Wu, H.; Cao, L.; He, X.; Li, Y.; Li, J.; Xu, M.; Jiang, Z. Preparation of anion exchange membrane with enhanced conductivity and alkaline stability by incorporating ionic liquid modified carbon nanotubes. J. Membr. Sci. 2018, 573, 1–10. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, B.; Wu, H.; Cao, L.; He, X.; Jiang, Z. Imidazolium-functionalized carbon nanotubes crosslinked with imidazole poly(ether ether ketone) for fabricating anion exchange membranes with high hydroxide conductivity and dimension stability. Electrochim. Acta 2019, 318, 572–580. [Google Scholar] [CrossRef]
- Gong, X.; Dai, Y.; Yan, X.; Wu, X.; Wang, Q.; Zhen, D.; Li, T.; Chen, W.; He, G. Electrospun imidazolium functionalized multiwalled carbon nanotube/polysulfone inorganic-organic nanofibers for reinforced anion exchange membranes. Int. J. Hydrogen Energy 2018, 43, 21547–21559. [Google Scholar] [CrossRef]
- Di Vona, M.L.; Casciola, M.; Donnadio, A.; Nocchetti, M.; Pasquini, L.; Narducci, R.; Knauth, P. Anionic conducting composite membranes based on aromatic polymer and layered double hydroxides. Int. J. Hydrogen Energy 2017, 42, 3197–3205. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.; Li, Y. Synthesis and characterization of crosslinked poly (vinyl alcohol)/layered double hydroxide composite polymer membranes for alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2012, 37, 18425–18432. [Google Scholar] [CrossRef]
- He, X.Y.; Cao, L.; He, G.W.; Zhao, A.Q.; Mao, X.L.; Huang, T.; Li, Y.; Wu, H.; Sun, J.; Jiang, Z.Y. A highly conductive and robust anion conductor obtained via synergistic manipulation in intra- and inter-laminate of layered double hydroxide nanosheets. J. Mater. Chem. A 2018, 6, 10277–10285. [Google Scholar] [CrossRef]
- Hu, Y.; Tsen, W.-C.; Chuang, F.-S.; Jang, S.-C.; Zhang, B.; Zheng, G.; Wen, S.; Liu, H.; Qin, C.; Gong, C. Glycine betaine intercalated layered double hydroxide modified quaternized chitosan/polyvinyl alcohol composite membranes for alkaline direct methanol fuel cells. Carbohydr. Polym. 2018, 213, 320–328. [Google Scholar] [CrossRef]
- Zhao, S.; Tsen, W.-C.; Hu, F.; Zhong, F.; Liu, H.; Wen, S.; Zheng, G.; Qin, C.; Gong, C. Layered double hydroxide-coated silica nanospheres with 3D architecture-modified composite anion exchange membranes for fuel cell applications. J. Mater. Sci. 2019, 55, 2967–2983. [Google Scholar] [CrossRef]
- Gong, C.; Zhao, S.; Tsen, W.-C.; Hu, F.; Zhong, F.; Zhang, B.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Hierarchical layered double hydroxide coated carbon nanotube modified quaternized chitosan/polyvinyl alcohol for alkaline direct methanol fuel cells. J. Power Sources 2019, 441, 227176. [Google Scholar] [CrossRef]
- Liu, W.; Liang, N.; Peng, P.; Qu, R.; Chen, D.; Zhang, H. Anion-exchange membranes derived from quaternized polysulfone and exfoliated layered double hydroxide for fuel cells. J. Solid State Chem. 2017, 246, 324–328. [Google Scholar] [CrossRef]
- Pizzoferrato, R.; Ciotta, E.; Ferrari, I.V.; Narducci, R.; Pasquini, L.; Varone, A.; Richetta, M.; Antonaroli, S.; Braglia, M.; Knauth, P.; et al. Layered Double Hydroxides Containing an Ionic Liquid: Ionic Conductivity and Use in Composite Anion Exchange Membranes. ChemElectroChem 2018, 5, 2781–2788. [Google Scholar] [CrossRef]
- Chen, N.; Long, C.; Li, Y.; Wang, D.; Lu, C.; Zhu, H.; Yu, J. Three-Decker Strategy Based on Multifunctional Layered Double Hydroxide to Realize High-Performance Hydroxide Exchange Membranes for Fuel Cell Applications. ACS Appl. Mater. Interfaces 2018, 10, 18246–18256. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.J.; Long, C.; Li, Y.X.; Wang, D.; Zhu, H. High-performance layered double hydroxide/poly(2,6-dimethyl-1,4-phenyleneoxide) membrane with porous sandwich structure for anion exchange membrane fuel cell applications. J. Membr. Sci. 2018, 552, 51–60. [Google Scholar] [CrossRef]
- Zhu, H.; Li, R.; Chen, N.; Wang, F.; Wang, Z.; Han, K. Electrorheological effect induced quaternized poly(2,6-dimethyl phenylene oxide)-layered double hydroxide composite membranes for anion exchange membrane fuel cells. RSC Adv. 2016, 6, 85486–85494. [Google Scholar] [CrossRef]
- Wang, D.; Chen, N.; Long, C.; Lu, C.; Li, Y.; Zhu, H.; Wang, F. Electric-field-aligned functionalized-layered double hydroxide/polyphenyl ether composite membrane for ion transport. Int. J. Hydrogen Energy 2019, 44, 13852–13863. [Google Scholar] [CrossRef]
- Pasquini, L.; Becerra-Arciniegas, R.; Narducci, R.; Sgreccia, E.; Gressel, V.; Di Vona, M.; Knauth, P. Properties and Alkaline Stability of Composite Anion Conducting Ionomers Based on Poly(phenylene oxide) Grafted with DABCO and Mg/Al Lamellar Double Hydroxide. ChemElectroChem 2020, 7, 2917–2924. [Google Scholar] [CrossRef]
- Sailaja, G.S.; Zhang, P.; Anilkumar, G.M.; Yamaguchi, T. Aniosotropically Organized LDH on PVDF: A Geometrically Templated Electrospun Substrate for Advanced Anion Conducting Membranes. ACS Appl. Mater. Interfaces 2015, 7, 6397–6401. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, C.; Yao, N.; Zhang, P.; Hong, T.; Xu, C.; Cheng, J. Quaternary Ti3C2Tx enhanced ionic conduction in quaternized polysulfone membrane for alkaline anion exchange membrane fuel cells. J. Membr. Sci. 2018, 563, 882–887. [Google Scholar] [CrossRef]
- Wang, L.; Shi, B. Hydroxide Conduction Enhancement of Chitosan Membranes by Functionalized MXene. Materials 2018, 11, 2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.-S.; Cheng, M.-Y.; Xie, X.-L.; Rick, J.; Huang, Y.-J.; Chang, F.-C.; Hwang, B.-J. Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J. Power Sources 2013, 239, 424–432. [Google Scholar] [CrossRef]
- Yang, J.-M.; Wang, S.-A. Preparation of graphene-based poly(vinyl alcohol)/chitosan nanocomposites membrane for alkaline solid electrolytes membrane. J. Membr. Sci. 2015, 477, 49–57. [Google Scholar] [CrossRef]
- Sharma, P.P.; Gahlot, S.; Bhil, B.M.; Gupta, H.; Kulshrestha, V. An environmentally friendly process for the synthesis of an fGO modified anion exchange membrane for electro-membrane applications. RSC Adv. 2015, 5, 38712–38721. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Influence of Graphene Oxide on the Ethanol Permeability and Ionic Conductivity of QPVA-Based Membrane in Passive Alkaline Direct Ethanol Fuel Cells. Nanoscale Res. Lett. 2019, 14, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.-T.; Kumar, S.R.; Hsu, C.-T.; Shih, C.-M.; Tsai, S.-W.; Yang, C.-C.; Liu, Y.-L.; Lue, S.J. Magnetic field-assisted alignment of graphene oxide nanosheets in a polymer matrix to enhance ionic conduction. J. Membr. Sci. 2018, 563, 259–269. [Google Scholar] [CrossRef]
- Lin, J.-S.; Kumar, S.R.; Ma, W.-T.; Shih, C.-M.; Teng, L.-W.; Yang, C.-C.; Lue, S.J. Gradiently distributed iron oxide@graphene oxide nanofillers in quaternized polyvinyl alcohol composite to enhance alkaline fuel cell power density. J. Membr. Sci. 2017, 543, 28–39. [Google Scholar] [CrossRef]
- Liu, L.; Tong, C.; He, Y.; Zhao, Y.; Lü, C. Enhanced properties of quaternized graphenes reinforced polysulfone based composite anion exchange membranes for alkaline fuel cell. J. Membr. Sci. 2015, 487, 99–108. [Google Scholar] [CrossRef]
- Luo, Z.; Gong, Y.; Liao, X.; Pan, Y.; Zhang, H. Nanocomposite membranes modified by graphene-based materials for anion exchange membrane fuel cells. RSC Adv. 2016, 6, 13618–13625. [Google Scholar] [CrossRef]
- Liu, J.; Qu, R.; Peng, P.; Liu, W.; Chen, D.; Zhang, H.; Liu, X. Covalently functionalized graphene oxide and quaternized polysulfone nanocomposite membranes for fuel cells. RSC Adv. 2016, 6, 71305–71310. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, J.; Zhang, K.; Ma, L.; Qaisrani, N.A.; Zhang, F.; He, G. Hybrid anion exchange membrane of hydroxyl-modified polysulfone incorporating guanidinium-functionalized graphene oxide. Ionics 2017, 23, 3085–3096. [Google Scholar] [CrossRef]
- Hu, B.; Miao, L.; Zhao, Y.; Lü, C. Azide-assisted crosslinked quaternized polysulfone with reduced graphene oxide for highly stable anion exchange membranes. J. Membr. Sci. 2017, 530, 84–94. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.; Ndungu, P.G.; Ramontja, J. Quaternized poly (2.6 dimethyl—1.4 phenylene oxide)/Polysulfone anion exchange membrane reinforced with graphene oxide for methanol alkaline fuel cell application. J. Polym. Res. 2018, 25, 143. [Google Scholar] [CrossRef]
- Mao, X.; Li, Z.; He, G.; Li, Z.; Zhao, J.; Zhang, Y.; Jiang, Z. Enhancing hydroxide conductivity of anion exchange membrane via incorporating densely imidazolium functionalized graphene oxide. Solid State Ionics 2019, 333, 83–92. [Google Scholar] [CrossRef]
- Hu, B.; Liu, L.; Zhao, Y.; Lü, C. A facile construction of quaternized polymer brush-grafted graphene modified polysulfone based composite anion exchange membranes with enhanced performance. RSC Adv. 2016, 6, 51057–51067. [Google Scholar] [CrossRef]
- Miao, L.; Bai, Y.; Yuan, Y.; Lü, C. Mussel-inspired strategy towards functionalized reduced graphene oxide-crosslinked polysulfone-based anion exchange membranes with enhanced properties. Int. J. Hydrogen Energy 2018, 43, 17461–17474. [Google Scholar] [CrossRef]
- Bai, Y.; Yuan, Y.; Miao, L.; Lü, C. Functionalized rGO as covalent crosslinkers for constructing chemically stable polysulfone-based anion exchange membranes with enhanced ion conductivity. J. Membr. Sci. 2018, 570–571, 481–493. [Google Scholar] [CrossRef]
- Bai, Y.; Yuan, Y.; Yang, Y.; Lü, C. A facile fabrication of functionalized rGO crosslinked chemically stable polysulfone-based anion exchange membranes with enhanced performance. Int. J. Hydrogen Energy 2019, 44, 6618–6630. [Google Scholar] [CrossRef]
- Shukla, G.; Shahi, V.K. Amine functionalized graphene oxide containing C16 chain grafted with poly(ether sulfone) by DABCO coupling: Anion exchange membrane for vanadium redox flow battery. J. Membr. Sci. 2019, 575, 109–117. [Google Scholar] [CrossRef]
- Yadav, V.; Sharma, P.P.; Kulshrestha, V. Facile synthesis of Br-PPO/f GO based polymer electrolyte membranes for electrochemical applications. Int. J. Hydrogen Energy 2017, 42, 26511–26521. [Google Scholar] [CrossRef]
- Das, G.; Park, B.J.; Kim, J.; Kang, D.; Yoon, H.H. Quaternized cellulose and graphene oxide crosslinked polyphenylene oxide based anion exchange membrane. Sci. Rep. 2019, 9, 9572. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lin, C.X.; Liu, F.H.; Li, L.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Poly (2,6-dimethyl-1,4-phenylene oxide)/ionic liquid functionalized graphene oxide anion exchange membranes for fuel cells. J. Membr. Sci. 2018, 552, 367–376. [Google Scholar] [CrossRef]
- Ion-Ebrasu, D.; Pollet, B.G.; Caprarescu, S.; Chitu, A.; Trusca, R.; Niculescu, V.; Gabor, R.; Carcadea, E.; Varlam, M.; Vasile, B.S. Graphene inclusion effect on anion-exchange membranes properties for alkaline water electrolyzers. Int. J. Hydrogen Energy 2020, 45, 17057–17066. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Ruan, H.; Xue, L.; Van der Bruggen, B.; Gao, C.; Shen, J. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J. Membr. Sci. 2017, 536, 167–175. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; An, L.; Zhao, G.; Yan, X.H. A high-performance sandwiched-porous polybenzimidazole membrane with enhanced alkaline retention for anion exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 2768–2774. [Google Scholar] [CrossRef]
- Yu, B.-C.; Wang, Y.-C.; Lu, H.-C.; Lin, H.-L.; Shih, C.-M.; Kumar, S.R.; Lue, S.J. Hydroxide-ion selective electrolytes based on a polybenzimidazole/graphene oxide composite membrane. Energy 2017, 134, 802–812. [Google Scholar] [CrossRef]
- Chang, W.T.; Chao, Y.H.; Li, C.W.; Lin, K.L.; Wang, J.J.; Kumar, S.R.; Lue, S.J. Graphene oxide synthesis using microwave-assisted vs. modified Hummer’s methods: Efficient fillers for improved ionic conductivity and suppressed methanol permeability in alkaline methanol fuel cell electrolytes. J. Power Sources 2019, 414, 86–95. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Graphene-mediated organic-inorganic composites with improved hydroxide conductivity and outstanding alkaline stability for anion exchange membranes. Compos. Part B Eng. 2018, 164, 324–332. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Jin, C.; Zhang, Z. Cyclodextrin edge functionalized graphene oxide modified poly(phthalazinone ether ketone) composite membrane with enhanced properties for anion exchange membrane. Solid State Ion. 2018, 320, 360–368. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Wu, H.; Cao, L.; He, X.; Li, Y.; Xu, M.; Jiang, Z. Incorporating imidazolium-functionalized graphene oxide into imidazolium-functionalized poly(ether ether ketone) for enhanced hydroxide conductivity. J. Membr. Sci. 2018, 565, 233–240. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Improved electrochemical performance of composite anion exchange membranes for fuel cells through cross linking of the polymer chain with functionalized graphene oxide. J. Membr. Sci. 2020, 611, 118385. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Hu, Y.; Gong, T.; Li, H.; Zhang, Y. Ionic-Liquid-Functionalized Graphene Nanoribbons for Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2017, 164, F433–F440. [Google Scholar] [CrossRef]
- Dai, P.; Mo, Z.-H.; Xu, R.-W.; Zhang, S.; Lin, X.; Lin, W.-F.; Wu, Y.-X. Development of a cross-linked quaternized poly(styrene-b-isobutylene-b-styrene)/graphene oxide composite anion exchange membrane for direct alkaline methanol fuel cell application. RSC Adv. 2016, 6, 52122–52130. [Google Scholar] [CrossRef] [Green Version]
- Mo, Z.-H.; Yang, R.; Hong, S.; Wu, Y.-X. In-situ preparation of cross-linked hybrid anion exchange membrane of quaternized poly (styrene-b-isobutylene-b-styrene) covalently bonded with graphene. Int. J. Hydrogen Energy 2018, 43, 1790–1804. [Google Scholar] [CrossRef]
- Ouadah, A.; Luo, T.; Wang, J.; Gao, S.; Wang, X.; Zhang, X.; Fang, Z.; Wu, Z.; Wang, J.; Zhu, C. Imidazolium-grafted graphene oxide via free radical polymerization: An efficient and simple method for an interpenetrating polymer network as electrolyte membrane. Compos. Sci. Technol. 2018, 164, 204–213. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.; Selyanchyn, R.; Daio, T.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S. Alkaline anion exchange membranes based on KOH-treated multilayer graphene oxide. J. Membr. Sci. 2016, 508, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Ingabire, P.B.; Pan, X.; Haragirimana, A.; Li, N.; Hu, Z.; Chen, S. Enhanced conduction capability of nanocomposite membrane of quaternized poly (arylene ether sulfone)s covalently bonded with graphitic carbon nitride nanosheets for fuel cells. React. Funct. Polym. 2019, 144, 104260. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, X.; Li, N.; Hu, Z.; Chen, S. Improved performance of quaternized poly(arylene ether ketone)s/graphitic carbon nitride nanosheets composite anion exchange membrane for fuel cell applications. Appl. Surf. Sci. 2019, 503, 144071. [Google Scholar] [CrossRef]
- Rathod, N.H.; Yadav, V.; Rajput, A.; Sharma, J.; Shukla, D.; Kulshrestha, V. New class of composite anion exchange membranes based on Quaternized poly (phenylene oxide) and functionalized boron nitride. Colloid Interface Sci. Commun. 2020, 36, 100265. [Google Scholar] [CrossRef]
- Li, C.; Sun, G.; Ren, S.; Liu, J.; Wang, Q.; Wu, Z.; Sun, H.; Jin, W. Casting Nafion–sulfonated organosilica nano-composite membranes used in direct methanol fuel cells. J. Membr. Sci. 2006, 272, 50–57. [Google Scholar] [CrossRef]
- Adjemian, K.T.; Lee, S.J.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Silicon oxide Nafion composite membranes for proton-exchange membrane fuel cell operation at 80–140 °C. J. Electrochem. Soc. 2002, 149, A256–A261. [Google Scholar] [CrossRef]
- Sgreccia, E.; Narducci, R.; Knauth, P.; Di Vona, M. Silica Containing Composite Anion Exchange Membranes by Sol–Gel Synthesis: A Short Review. Polymers 2021, 13, 1874. [Google Scholar] [CrossRef]
- Zuo, X.; Yu, S.; Xu, X.; Xu, J.; Bao, R.; Yan, X. New PVDF organic–inorganic membranes: The effect of SiO2 nanoparticles content on the transport performance of anion-exchange membranes. J. Membr. Sci. 2009, 340, 206–213. [Google Scholar] [CrossRef]
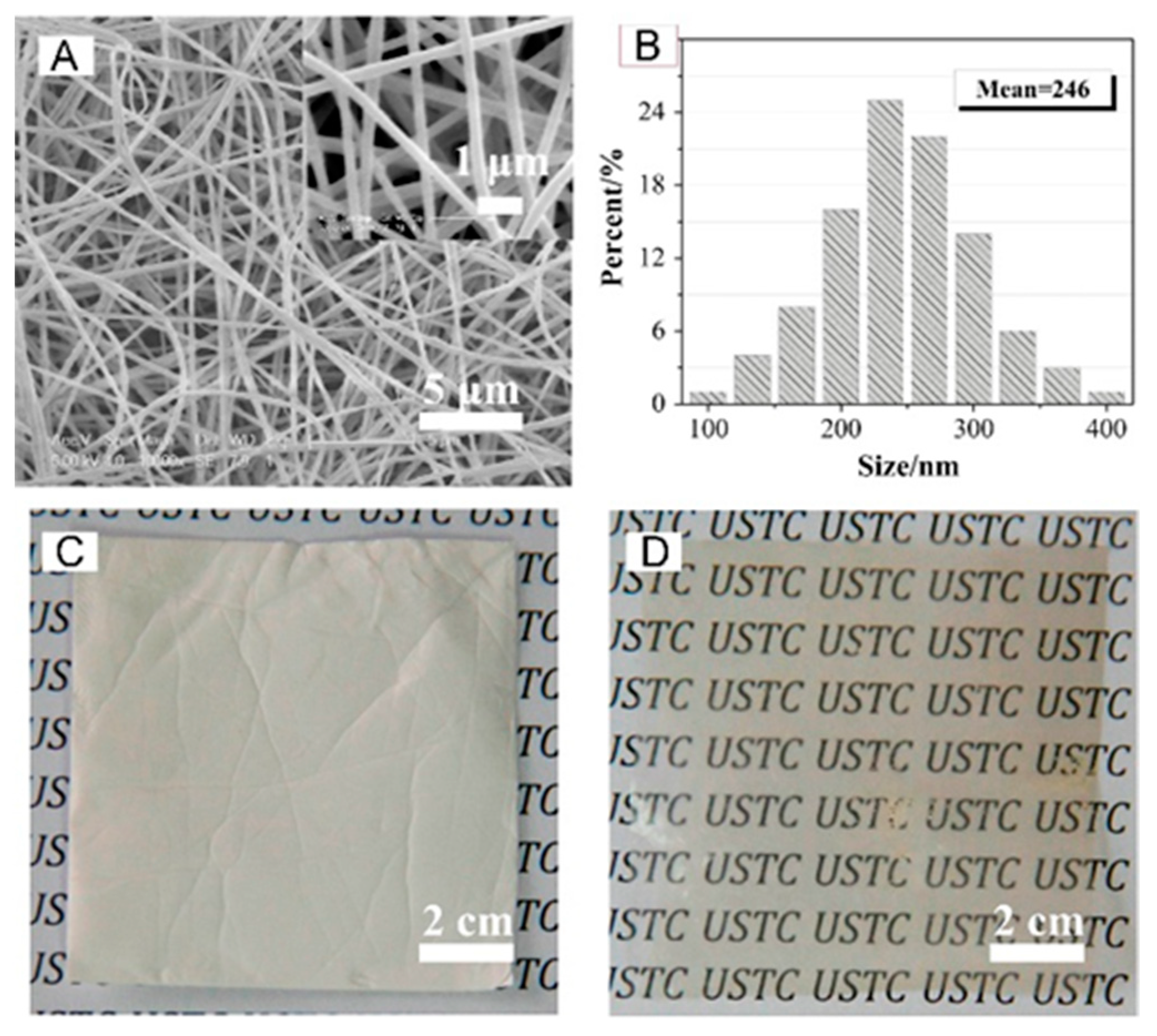
- Liu, G.; Tsen, W.-C.; Jang, S.-C.; Hu, F.; Zhong, F.; Zhang, B.; Wang, J.; Liu, H.; Wang, G.; Wen, S.; et al. Composite membranes from quaternized chitosan reinforced with surface-functionalized PVDF electrospun nanofibers for alkaline direct methanol fuel cells. J. Membr. Sci. 2020, 611, 118242. [Google Scholar] [CrossRef]
- Wang, E.D.; Zhao, T.S.; Yang, W.W. Poly (vinyl alcohol)/3-(trimethylammonium) propyl-functionalized silica hybrid membranes for alkaline direct ethanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 2183–2189. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chiu, S.-S.; Kuo, S.-C.; Liou, T.-H. Fabrication of anion-exchange composite membranes for alkaline direct methanol fuel cells. J. Power Sources 2012, 199, 37–45. [Google Scholar] [CrossRef]
- Kumar, S.R.; Juan, C.-H.; Liao, G.-M.; Lin, J.-S.; Yang, C.-C.; Ma, W.-T.; You, J.-H.; Lue, S.J. Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells. Energies 2015, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Armentrout, A.A.; Li, J.; Tekinalp, H.L.; Nanda, J.; Ozcan, S. A cellulose nanocrystal-based composite electrolyte with superior dimensional stability for alkaline fuel cell membranes. J. Mater. Chem. A 2015, 3, 13350–13356. [Google Scholar] [CrossRef]
- García-Cruz, L.; Casado-Coterillo, C.; Iniesta, J.; Montiel, V.; Irabien, A. Chitosan:poly (vinyl) alcohol composite alkaline membrane incorporating organic ionomers and layered silicate materials into a PEM electrochemical reactor. J. Membr. Sci. 2016, 498, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.F.; He, Y.B.; Wu, L.; Jiang, C.X.; Wu, B.; Mondal, A.N.; Cheng, C.L.; Xu, T.W. Anion exchange membranes from hot-pressed electrospun QPPO-SiO2 hybrid nanofibers for acid recovery. J. Membr. Sci. 2015, 480, 115–121. [Google Scholar] [CrossRef]
- Moghadasi, M.; Mortaheb, H.R. Incorporating functionalized silica nanoparticles in polyethersulfone-based anion exchange nanocomposite membranes. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Chen, N.; Long, C.; Li, Y.; Wang, D.; Zhu, H. A hamburger-structure imidazolium-modified silica/polyphenyl ether composite membrane with enhancing comprehensive performance for anion exchange membrane applications. Electrochimica Acta 2018, 268, 295–303. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Sangeetha, D. A quaternized mesoporous silica/polysulfone composite membrane for an efficient alkaline fuel cell application. RSC Adv. 2015, 5, 42828–42835. [Google Scholar] [CrossRef]
- Liu, L.; Tong, C.; He, Y.; Zhao, Y.; Hu, B.; Lü, C. Novel quaternized mesoporous silica nanoparticle modified polysulfone-based composite anion exchange membranes for alkaline fuel cells. RSC Adv. 2015, 5, 43381–43390. [Google Scholar] [CrossRef]
- Elumalai, V.; Dharmalingam, S. Synthesis characterization and performance evaluation of ionic liquid immobilized SBA-15/quaternised polysulfone composite membrane for alkaline fuel cell. Microporous Mesoporous Mater. 2016, 236, 260–268. [Google Scholar] [CrossRef]
- Liao, X.; Ren, L.; Chen, D.; Liu, X.; Zhang, H. Nanocomposite membranes based on quaternized polysulfone and functionalized montmorillonite for anion-exchange membranes. J. Power Sources 2015, 286, 258–263. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhou, S.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J.; Zhang, Q.; Yang, D. Enhancement of hydroxide conductivity by incorporating nanofiber-like palygorskite into quaternized polysulfone as anion exchange membranes. Appl. Clay Sci. 2020, 195, 105702. [Google Scholar] [CrossRef]
- Yun, S.; Parrondo, J.; Ramani, V. Composite anion exchange membranes based on quaternized cardo-poly(etherketone) and quaternized inorganic fillers for vanadium redox flow battery applications. Int. J. Hydrogen Energy 2016, 41, 10766–10775. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Chiu, S.J.; Chien, W.C.; Chiu, S.S. Quaternized poly(vinyl alcohol)/alumina composite polymer membranes for alkaline direct methanol fuel cells. J. Power Sources 2010, 195, 2212–2219. [Google Scholar] [CrossRef]
- Pérez-Prior, M.T.; García-García, T.; Varez, A.; Levenfeld, B. Preparation and characterization of ammonium-functionalized polysulfone/Al2O3 composite membranes. J. Mater. Sci. 2015, 50, 5893–5903. [Google Scholar] [CrossRef]
- Vinodh, R.; Purushothaman, M.; Sangeetha, D. Novel quaternized polysulfone/ZrO2 composite membranes for solid alkaline fuel cell applications. Int. J. Hydrogen Energy 2011, 36, 7291–7302. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Arangadi, A.F.; Velu, S.; Fawzi, B.; Show, P.L. ZrO2 incorporated polysulfone anion exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2020, 45, 29668–29680. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Meng, Y. Novel Quaternized Poly(arylene ether sulfone)/Nano-ZrO2 Composite Anion Exchange Membranes for Alkaline Fuel Cells. ACS Appl. Mater. Interfaces 2013, 5, 1414–1422. [Google Scholar] [CrossRef]
- Li, X.H.; Tao, J.X.; Nie, G.H.; Wang, L.C.; Li, L.H.; Liao, S.J. Cross-linked multiblock copoly(arylene ether sulfone) ionomer/nano-ZrO2 composite anion exchange membranes for alkaline fuel cells. RSC Adv. 2014, 4, 41398–41410. [Google Scholar] [CrossRef]
- Xu, S.; Jiang, R.; Jiang, S.; Gao, Y. Communication—Anion-Conductive Perfluoroheteroaromatic Composite Membranes: High Chemical Stability under Strong Alkaline Conditions. J. Electrochem. Soc. 2016, 163, F688–F690. [Google Scholar] [CrossRef]
- Vinodh, R.; Sangeetha, D. Comparative study of composite membranes from nano-metal-oxide-incorporated polymer electrolytes for direct methanol alkaline membrane fuel cells. J. Appl. Polym. Sci. 2012, 128, 1930–1938. [Google Scholar] [CrossRef]
- Nonjola, P.T.; Mathe, M.K.; Modibedi, R.M. Chemical modification of polysulfone: Composite anionic exchange membrane with TiO2 nano-particles. Int. J. Hydrogen Energy 2013, 38, 5115–5121. [Google Scholar] [CrossRef]
- Xie, F.; Gao, X.; Hao, J.; Yu, H.; Shao, Z.; Yi, B. Preparation and properties of amorphous TiO2 modified anion exchange membrane by impregnation-hydrolysis method. React. Funct. Polym. 2019, 144, 104348. [Google Scholar] [CrossRef]
- Msomi, P.; Nonjola, P.; Ndungu, P.; Ramontja, J. Poly (2, 6-dimethyl-1, 4-phenylene)/polysulfone anion exchange membrane blended with TiO2 with improved water uptake for alkaline fuel cell application. Int. J. Hydrogen Energy 2020, 45, 29465–29476. [Google Scholar] [CrossRef]
- Derbali, Z.; Fahs, A.; Chailan, J.-F.; Ferrari, I.; Di Vona, M.; Knauth, P. Composite anion exchange membranes with functionalized hydrophilic or hydrophobic titanium dioxide. Int. J. Hydrogen Energy 2017, 42, 19178–19189. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, Y.; Chen, N.; Wang, F.; Zhu, H. A new method for improving the ion conductivity of anion exchange membranes by using TiO2 nanoparticles coated with ionic liquid. RSC Adv. 2016, 6, 96768–96777. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, N.; Li, R.; Zhang, Y.; Li, K.; Wang, F.; Zhu, H. A new method for improving the conductivity of alkaline membrane by incorporating TiO2-ionic liquid composite particles. Electrochim. Acta 2017, 255, 335–346. [Google Scholar] [CrossRef]
- Tao, H.-C.; Sun, X.-N.; Xiong, Y. A novel hybrid anion exchange membrane for high performance microbial fuel cells. RSC Adv. 2014, 5, 4659–4663. [Google Scholar] [CrossRef]
- Moly, P.P.; Jeena, C.B.; Elsa, P.J.; Ambily, K.J.; Joy, V.T. High performance polyvinyl alcohol/calcium titanate nanocomposite anion-exchange membranes as separators in redox flow batteries. Polym. Bull. 2018, 75, 4409–4428. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K. An intermediate-temperature alkaline fuel cell using an Sn0.92Sb0.08P2O7-based hydroxide-ion-conducting electrolyte and electrodes. J. Mater. Chem. A 2012, 1, 1134–1140. [Google Scholar] [CrossRef]
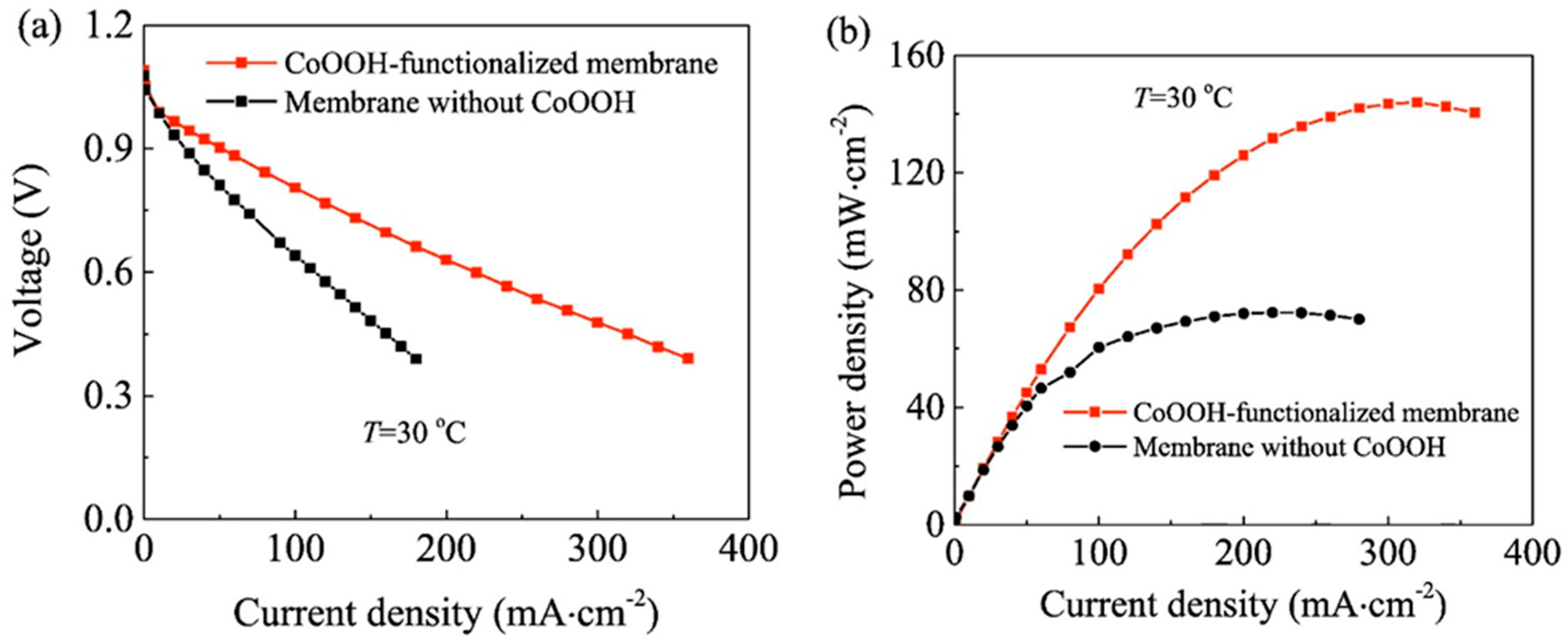
- Qin, H.; Hu, Y.; Zhu, C.; Chu, W.; Sheng, H.; Dong, Z.; He, Y.; Wang, J.; Li, A.; Chi, H.; et al. Functionalization of polyvinyl alcohol composite membrane by CoOOH for direct borohydride fuel cells. Electrochem. Commun. 2017, 77, 1–4. [Google Scholar] [CrossRef]
- Jiang, X.C.; Sun, Y.J.; Zhang, H.X.; Hou, L.X. Preparation and characterization of quaternized poly(vinyl alcohol)/chitosan/MoS2 composite anion exchange membranes with high selectivity. Carbohydr. Polym. 2018, 180, 96–103. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.; Ndungu, P.; Ramonjta, J. Quaternized poly(2.6 dimethyl-1.4 phenylene oxide)/polysulfone blend composite membrane doped with ZnO-nanoparticles for alkaline fuel cells. J. Appl. Polym. Sci. 2017, 135, 45959. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Liu, F.; Chen, H.; Wang, X.; Mao, T.; Wang, D.; Liu, G.; Wang, Z. Flame-retardant AEMs based on organic-inorganic composite polybenzimidazole membranes with enhanced hydroxide conductivity. J. Membr. Sci. 2019, 591, 117306. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2018, 6, 1801146. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.-Y.V.; Chan, K.-Y.; Chen, Z.-N. Metal–Organic Framework Threaded with Aminated Polymer Formed in Situ for Fast and Reversible Ion Exchange. J. Am. Chem. Soc. 2014, 136, 7209–7212. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, G.; Zhao, C.; Li, X.; Li, M.; Na, H. MOFs synthesized by the ionothermal method addressing the leaching problem of IL–polymer composite membranes. Chem. Commun. 2014, 50, 14121–14124. [Google Scholar] [CrossRef]
- Liu, C.; Feng, S.; Zhuang, Z.; Qi, D.; Li, G.; Zhao, C.; Li, X.; Na, H. Towards basic ionic liquid-based hybrid membranes as hydroxide-conducting electrolytes under low humidity conditions. Chem. Commun. 2015, 51, 12629–12632. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.R.; Chang, C.-H.; Wu, K.C.-W.; Tung, K.-L.; Lue, S.J. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Ge, L.; Yu, D.; Hou, L.; Li, Q.; Yang, Z.; Xu, T. Cationic metal–organic framework porous membranes with high hydroxide conductivity and alkaline resistance for fuel cells. J. Mater. Chem. A 2016, 4, 14545–14549. [Google Scholar] [CrossRef]
- He, X.; Gang, M.; Li, Z.; He, G.; Yin, Y.; Cao, L.; Zhang, B.; Wu, H.; Jiang, Z. Highly conductive and robust composite anion exchange membranes by incorporating quaternized MIL-101(Cr). Sci. Bull. 2017, 62, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Hu, B.; Tong, C.; Bai, Y.; Lü, C. Novel quaternized carbon dots modified polysulfone-based anion exchange membranes with improved performance. Int. J. Hydrogen Energy 2019, 44, 22181–22193. [Google Scholar] [CrossRef]
- Yadav, V.; Raj, S.K.; Rathod, N.H.; Kulshrestha, V. Polysulfone/graphene quantum dots composite anion exchange membrane for acid recovery by diffusion dialysis. J. Membr. Sci. 2020, 611, 118331. [Google Scholar] [CrossRef]
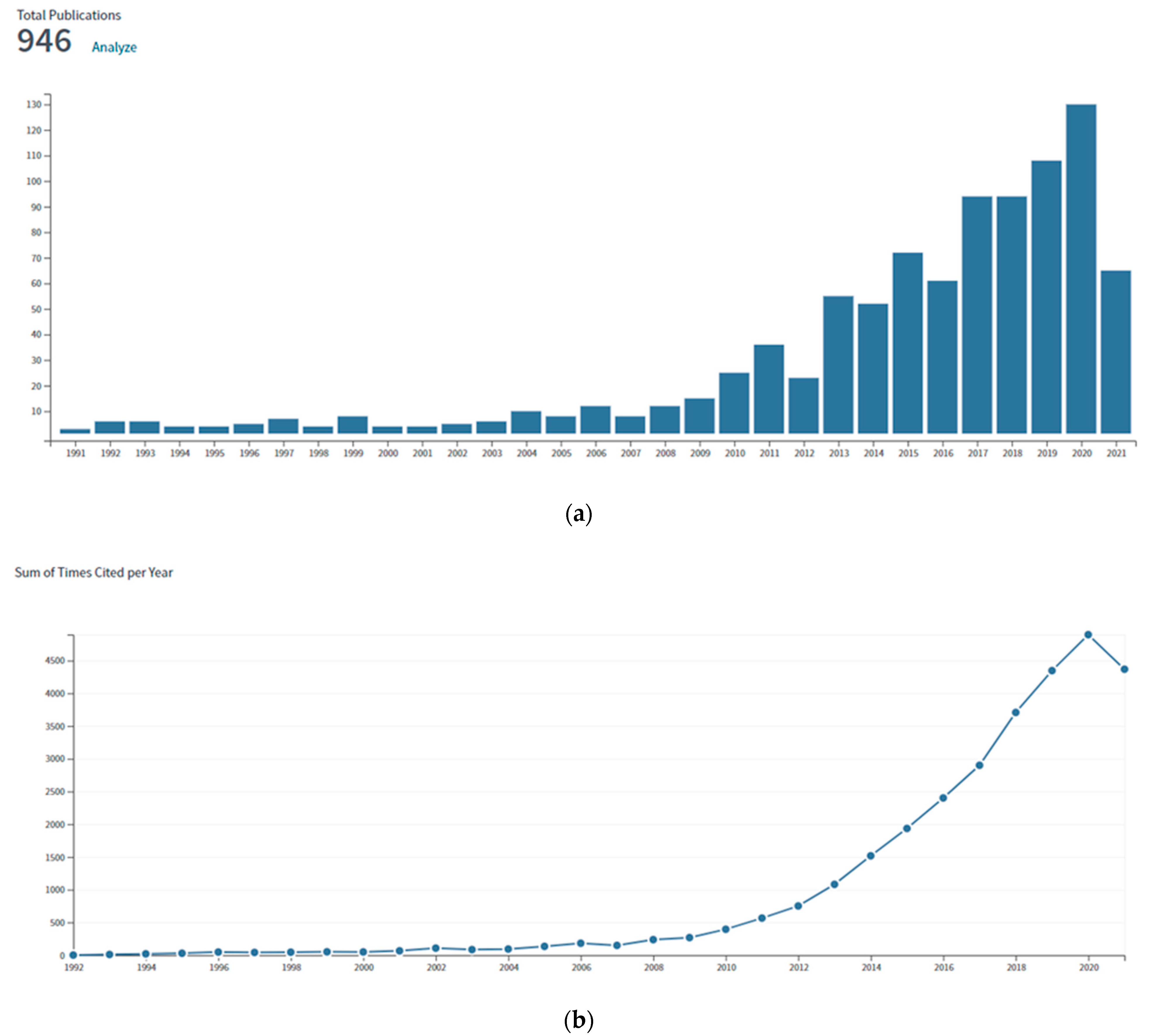


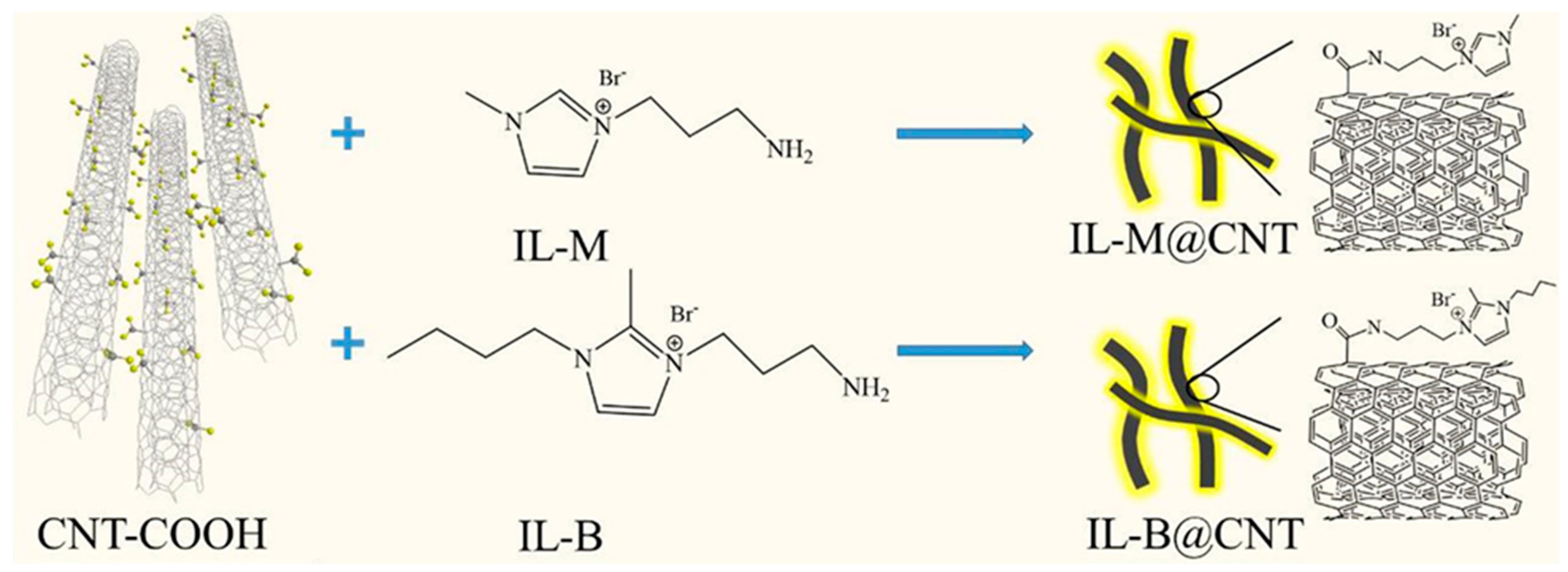
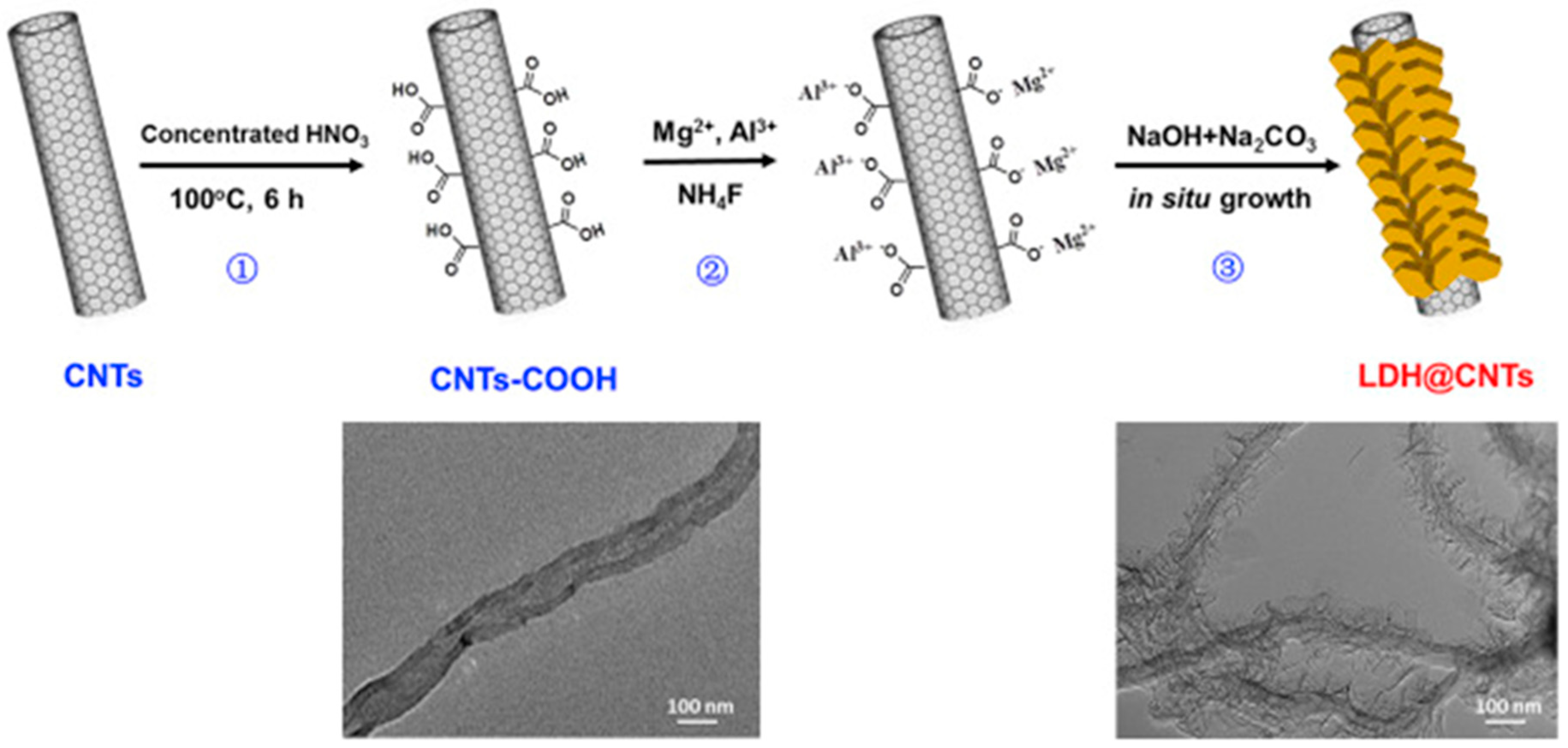



| Polymer | 1D Filler | Remark | Ref |
|---|---|---|---|
| TEA-PSU | triethylammonium TNT | maximum power density 285 mW cm−2 at 60 °C | [20] |
| TEA-PSU | 1-methyl-3-(3-trimethoxysilylpropyl) Im-chloride TNT | 302 mW cm−2 at 60 °C (5 wt%) | [21] |
| TMA-PAEK | trimethylammonium (3-chloropropyl)-trimethoxysilane TNT | conductivity 52.5 mS cm−1 at 80 °C | [22] |
| CS | QHNT | TS 52 MPa, Young’s modulus 1100 MPa (5 wt%) | [23] |
| PVA | MWCNT | methanol permeability 3.57 × 10−7 cm2 s−1 | [24] |
| PVA | grafted MWCNT | KOH 8 M solution uptake 108 % | [25] |
| PVA | FeCNT | maximum power density 88 mW cm−2 at 60 °C (DMAFC) | [26] |
| PVA | grafted MWCNT | maximum power density 65 mW cm−2 at 60 °C (DEAFC) | [27] |
| PVA/poly(diallyldimethylammonium chloride) | MWCNTs-OH | TS 40 MPa (3 wt%) | [28] |
| CS | MWCNTs-OH | conductivity 6 mS cm−1 at RT | [29] |
| glycidyltrimethylammonium chloride-CS (GTA, AR) | CNT | TS 32 MPa | [30] |
| 2,3-epoxypropyl trimethyl ammonium chloride-CS | QSiO2-CNT | maximum power density 81 mW cm−2 at 60 °C (DMAFC) | [31] |
| Q-trimethylamine polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene | TMA-MWCNT | maximum power density 187 mW cm−2 at 60 °C (DMAFC) | [32] |
| Im-PPO | PIL(BF4) MWCNT | conductivity 56 mS cm−1 at 75 °C | [33] |
| Im-PEEK | Im-CNT | conductivity 135 mS cm−1 at 70 °C, 100% RH | [34] |
| Im-PEEK | poly(vinyl imidazole)-CNT | maximum power density129 mW cm−2 at 60 °C (AEMFC) | [35] |
| Im-PSU | Im-MWCNTs | TS 24 MPa (0.2 wt%) | [36] |
| Polymer | 2D Filler | Remarks | Ref |
|---|---|---|---|
| (TMA, DABCO)-PSU | MgAl-LDH (Cl−) | Young’s modulus 620 MPa (100% RH) | [37] |
| XL glutaraldehyde-PVA | MgAl-LDH (CO32−) | EtOH permeability 1.8 × 10−7 cm2 s−1 | [38] |
| 2,3-epoxypropyltrimethylammonium chloride-PVA | MgAl-LDH(CO32−, NO3−) | NO3− conductivity 156 mS cm−1 at 80 °C, TS 48 MPa | [39] |
| glycidyltrimethylammonium chloride-CS/PVA | MgAl-LDH (NO3−) | TS 24 MPa | [40] |
| (2,3-epoxypropyl trimethyl ammonium chloride)-CS/PVA | CNT coated with MgAl-LDH (CO32−) | maximum power density 107 mW cm−2 (DMAFC) | [42] |
| glycidyl trimethyl ammonium chloride-CS/PVA | MgAl-LDH (CO32−) wrapped on quaternized SiO2 nanospheres | CO32− conductivity 11 mS cm−1 at 80 °C | [41] |
| TMA-PSU | MgAl-LDH (NO3−) | TS 21 MPa | [43] |
| DABCO-PSU | ZnAl-LDH (BmimSO4) | conductivity 16 mS cm−1 at 25 °C | [44] |
| 1-methylimidazole PPO | MgAl-LDH (CO32−) | tensile strength 29.5 MPa | [47] |
| 1-(N′,N′-dimethylamino)-6,11-(N, N,N-trimethylammonium) undecane-PPO | MgAl-LDH with N,N,N-trimethylpropyltriethoxysilane ammonium chloride | conductivity 122 mS cm−1 at 80 °C, maximum power density 267 mW cm−2 at 60 °C | [46] |
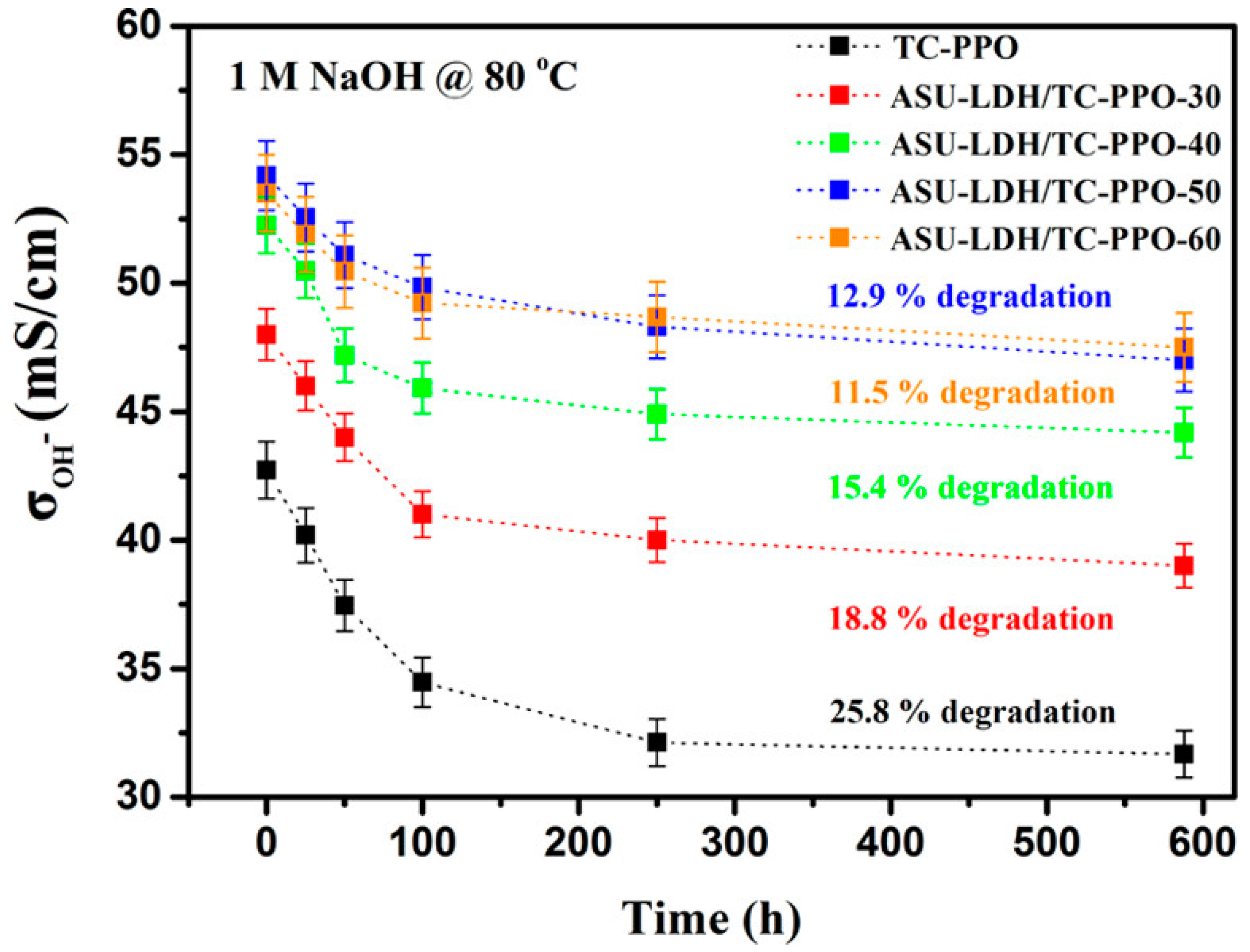
| 1-(N′,N′-dimethylamino)-6-(N,N′-dimethylammonium)-11-(N,N′,N″-trimethyl ammonium)undecane-PPO (TC-PPO) | MgAl-LDH, 3-hydroxy-6-azaspiro [5.5] undecane, N,N,N-trimethyl-3-(triethoxysilyl)propan-1-aminium bromide (ASU-LDH) | IEC values 3.11–3.90 meq g−1 | [45] |
| TC-PPO | MgAl-LDH (ASU-LDH) electric-field-aligned | conductivity 110 mS cm−1 at 80 °C | [48] |
| DABCO-PPO | MgAl-LDH | Young’s modulus 320 ± 60 MPa (100% RH) | [49] |
| electrospun PVDF | Mg4Al2(OH)12CO3·3H2O | conductivity 87 mS cm–1 at 70 °C, 100% RH | [50] |
| triethylene diamine-PSU | LiF-Ti3C2Tx; NH4HF2-Ti3C2Tx | maximum power density 101 mW cm−2 at 60 °C | [52] |
| CS | Im brush-functionalized MXene | TS 41.0 MPa (7.5 wt%) | [53] |
| Polymer | 2D Filler | Remarks | Ref |
|---|---|---|---|
| PVA | exfoliated graphene | maximum power density 46 mW cm−2 at 60 °C | [54] |
| PVA/CS | graphene, sulfonated graphene | conductivity 47 mS cm−1 at 25 °C | [55] |
| QPEI/PVA | silica functionalized GO | conductivity 72 mS cm−1 at 30 °C | [56] |
| XL glycidyl-trimethylammonium chloride-PVA | GO | EtOH permeability 3.65 × 10−7 cm2 s−1 at 60 °C | [57] |
| glycidytrimethyl ammonium chloride PVA | aligned GO-Fe3O4 | maximum power density 172 mW cm−2 at 60 °C (0.1 wt%) | [58] |
| glycidytrimethyl ammonium-PVA (QPVA) | GO-Fe3O4 | conductivity 47–55 mS cm−1 at 30–60 °C (0.1 wt%) | [59] |
| TMA-PSU | QGs | TS 205 MPa (0.25 wt%) | [60] |
| TMA-QPSU | dopamine modified GO (DGO) | TS 13 MPa, elongation at break 33% (1 wt%) | [61] |
| TMA-QPSU | XL-GO | swelling ratio 3.9%, water uptake 19% at 60 °C (2 wt%) | [62] |
| diethanolamine-modified PSU (HPSU) | guanidinium-GO | swelling of 9% at 60 °C | [63] |
| 3-azidopropyl-N,N-dimethylamine (Ap-DMA) and TMA (AMPSU) QPSU | (azide XL) rGO | Young’s modulus 2890 MPa, RH 100% (0.1 wt%) | [64] |
| TMA-PPO/PSU | GO | IEC 3.21 meq g−1 (2 wt%) | [65] |
| Im-functionalized bisphenol PSU | Im-GO | maximum power density 79 mW cm−2 at 60 °C (0.2 wt%) | [66] |
| TMA-PSU | QPbGs (polymer brush funzionalized graphenes) | conductivity 56 mS cm−1 at 80 °C (1 wt%) | [67] |
| XL 3-(dimethylamino)-1-propylamine PSU | polydopamine-reduced GO (PDArGO) | Young’s modulus 1843 MPa (1.5 wt%) | [68] |
| TMA-PSU | XL reduced and functionalized GO (rGO) | conductivity 140 mS cm−1 at 80 °C (2 wt%) | [69] |
| TMA-PSU | rGO modified with pyrene-containing tertiary amines | conductivity 140 mS cm−1 80 °C (2 wt%) | [70] |
| DABCO-PSU | C16 GO | 19 × 105 S min cm−3 selectivity for VRFB | [71] |
| BrPPO | (PEI)-GO | IEC 3.59 meq g−1 | [72] |
| cellulose/DABCO-PPO | GO | conductivity 215 mS cm−1 at 80 °C | [73] |
| Im-PPO | 1-(3-aminopropyl)-3-methylimidazolium bromine (IL-GO) | IEC decreased from 1.90 to 1.34 meq g−1 in 2 M NaOH, 80 °C, 480 h | [74] |
| Fumion® FAA-3 | graphene with surface area of 500 m2/g | conductivity 113 mS cm−1 at 80 °C | [75] |
| JAM-II-07 (Yanrun, China) | sulfonated rGO | area specific resistance 3.72 Ω cm2 | [76] |
| sp-PBI | rGO | maximum power density 544 mW cm−2 at 90 °C | [77] |
| PBI | spin-coated GO | TS 50 MPa | [78] |
| PBI | MGO and NGO | conductivity 24 mS cm−1 (NGO) at 80 °C (1 wt%) | [79] |
| TMA-PAEK | rGO | Conductivity 115 mS cm−1 at 90 °C (1 wt%) | [80] |
| PPEK | QA-CDβ@GO | Young’s modulus 1243 MPa (10 wt%) | [81] |
| Im-PEEK | Im-GO | maximum power density 50 mW cm−2 at 50 °C (4 wt%) | [82] |
| DABCO-PAE | QGO | maximum power density 136 mW cm−2 at 70 °C (0.7 wt%) | [83] |
| perfluorinated AEM (I-PFSO2NH2-Cl) | IGNRs (GNRs grafted APTMS and MIMC) | conductivity 121 mS cm−1 at 80 °C (1 wt%) | [84] |
| XL-QSIBS | GO quaternized with: octadecylamine (GOA), octadecylamine + N,N-dimethyl-1,3-propanediamine (GOAN) | conductivity 19.5 mS cm−1 (GOAN 0.50 wt%) at 60 °C | [85] |
| QSIBS | poly (vinylbenzyl chloride) grafted graphene (GN-g-PVBC) | conductivity 18 mS cm−1 at 60 °C, storage modulus 418 MPa (0.55 wt%) | [86] |
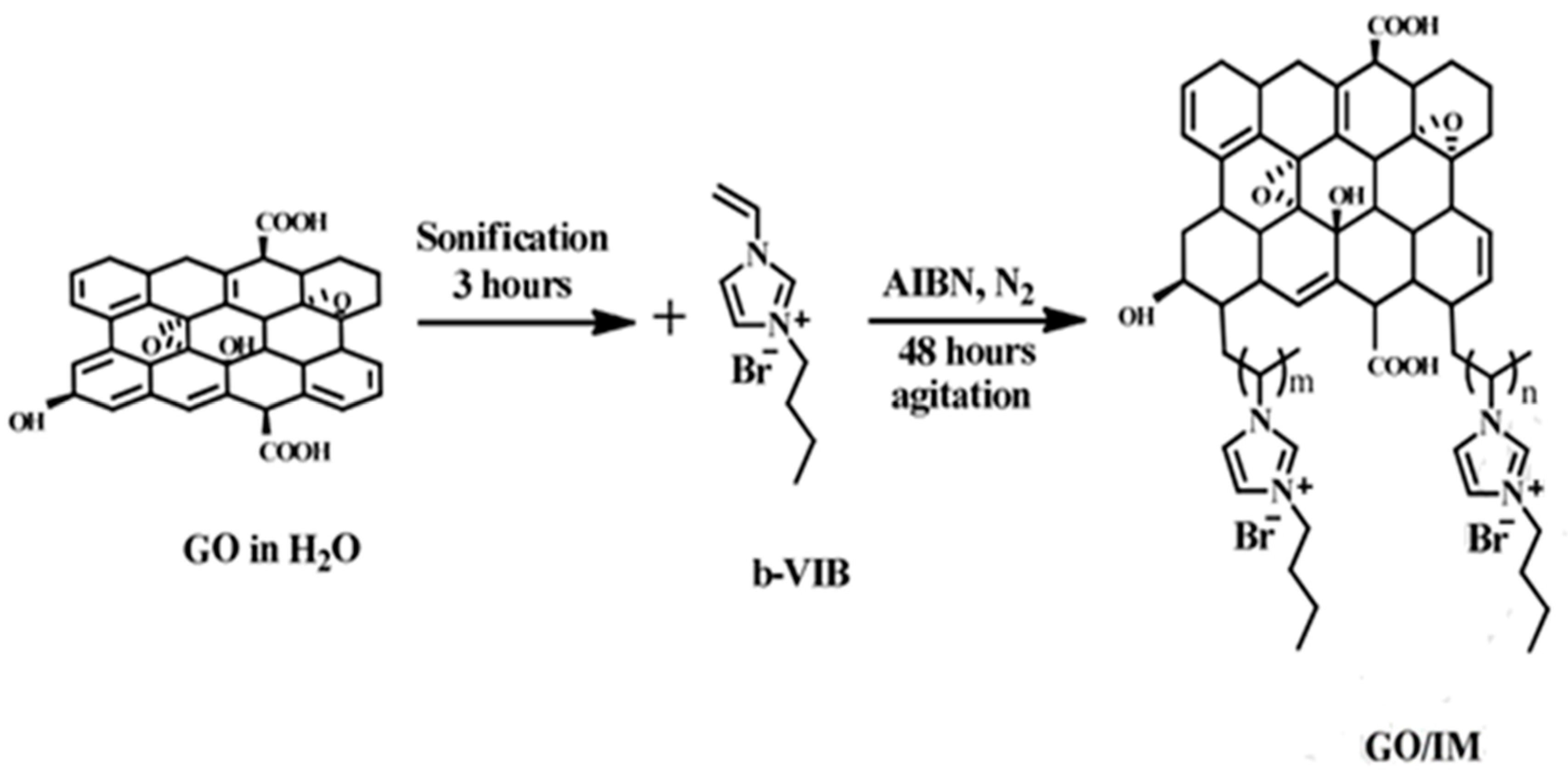
| copolymer PMS/b-VIB/DPEBI | butylvinylimidazolium GO | conductivity 102 mS cm−1 at 100 °C | [87] |
| GO multilayer membranes | GO and GOKOH | water uptake GOKOH 1099 wt% | [88] |
| TMA-PAES | g-C3N4 | maximum power density 68 mW cm−2 at 60 °C (0.6 wt%) | [89] |
| TMA-PAEK | g-C3N4 nanosheets | conductivity 35 mS cm−1 at 80 °C (0.5 wt%) | [90] |
| N-methyl morpholine-PPO | f-BN | Yield stress 37 MPa (5 wt%) | [91] |
| Polymer | 3D Filler | Remarks | Ref |
|---|---|---|---|
| XL TMA-PVDF | SiO2 |
conductivity 3 mS cm−1 at RT (2 wt%) | [95] |
| QCS | SiO2 coated PVDF grafted with trimethyl-3-(trimethoxysilyl) propyl ammonium chloride |
conductivity 41 mS cm−1 at 80 °C (10.6 wt%) | [96] |
| XL PVA/3-(trimethylammonium) propyl-functionalized silica | SiO2 | maximum power density 50 mW cm−2 at 60 °C (DEAFC) | [97] |
| 3-(trimethyl ammonium)-PVA | 3-(trimethyl ammonium) propyl-functionalized SiO2 | storage modulus 172 MPa at 100 °C (20 wt%) | [98] |
| GTMAC-PVA | FS | conductivity 35 mS cm−1 at 60 °C (5 wt%) | [99] |
| CNC-PVA | SiO2 | conductivity 65 mS cm−1 at 60 °C (40 wt%) | [100] |
| PVA and CS | AM-4, 4VP, AS4, UZAR-S3 | conductivity 1 mS cm−1 at RT (4VP/CS:PVA) | [101] |
| TMA-PPO | SiO2 | UH 0.041 m h−1, S 49 | [102] |
| TMA-PES | functionalized SiO2 ASi-I, ASi-II, ASi-III | conductivity 46 mS cm−1 at 25 °C (3 wt%, ASi-II) | [103] |
| PPO | Im-SiO2 | conductivity 105 m S cm−1 at 80 °C | [104] |
| TMA-PSU | TEA-SiO2 (QSBA) | OCV 0.86 V, power density 298 mW cm−2 (3 wt%) | [105] |
| TMA-PSU | TMA-SiO2 (QMSNs) | Young’s modulus 2250 MPa (20 wt%) | [106] |
| TEA-PSU | Im-mesoporous SiO2 | maximum power density 278 mW cm−2 at 60 °C | [107] |
| TMA-PSU | modified montmorillonite | conductivity 47 mS cm−1 at 95 °C (5 wt%) | [108] |
| TMA-PSU | palygorskite | conductivity 93 mS cm−1 at 80 °C (0.5 wt%) | [109] |
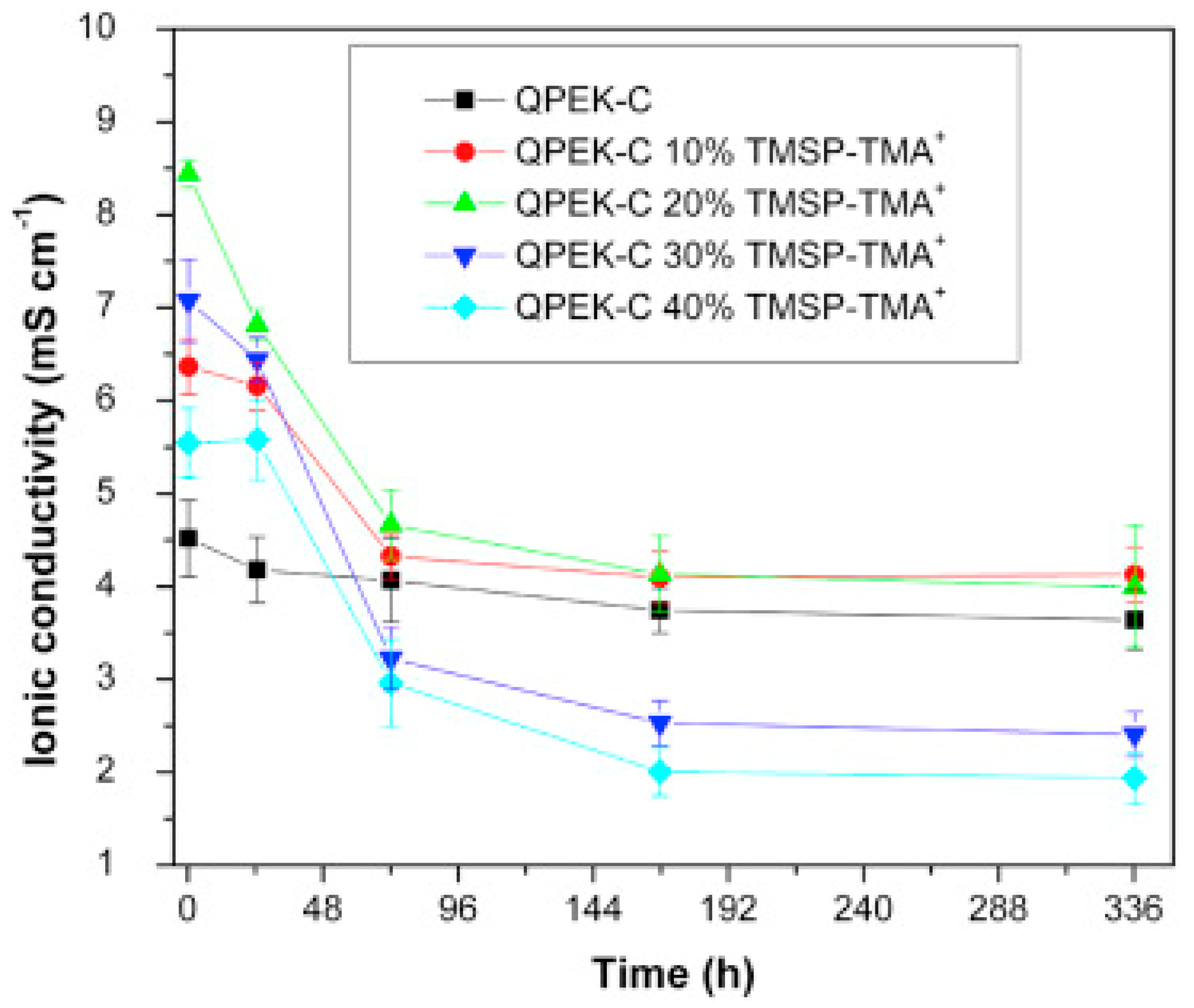
| TMA-cardo-poly(etherketone) (QPEK-C) | N-(trimethoxysilylpropyl)-N,N,N-trimethylammonium | TS 26 MPa, elongation at break 32% | [110] |
| Polymer | 3D Filler | Remarks | Ref |
|---|---|---|---|
| GTMAC-PVA | Al2O3 | conductivity 48 mS cm−1 at 70 °C | [111] |
| TMA-PSU | Al2O3 | TS 31 MPa (4 wt%) | [112] |
| TMA-PSU | ZrO2 | conductivity 15 mS cm−1 at RT | [113] |
| Im-PSU | ZrO2 | conductivity 80 mS cm−1 at 50 °C | [114] |
| TMA-PAES | nano ZrO2 |
conductivity 48 mS cm–1 at 80 °C (10 wt%) | [115] |
| XL TMA-coPAES | nano ZrO2 | Young’s modulus 492 MPa (10 wt%) | [116] |
| Perfluoro(phenyl 2,2:6,2-terpyridine); 2,2:6,2-terpyridine | ZrO(ClO4)2 | IEC 0.76 meq g−1 | [117] |
| TMA polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (PSEBS)/TMA-PSU | SiO2, ZrO2, TiO2 | maximum power density 75 mW cm−2 at 60 °C (7.5% TiO2) | [118] |
| TMA-PSU | TiO2 | conductivity 13 mS cm−1 at 21 °C (10 wt% TiO2) | [119] |
| vinylbenzyl chloride-divinylbenzene copolymers | amorphous TiO2 | conductivity 43 mS cm−1 at 30 °C | [120] |
| TMA-PPO/PSU | TiO2 | maximum power density 118 mW cm−2 at 60 °C (2 wt%) | [121] |
| DABCO-PSU | TMP-TiO2 PMHS-TiO2 | conductivity 39 mS cm−1 (PMHS-TiO2), 34 mS cm−1 (TMP-TiO2) at 25 °C in KOH 2M | [122] |
| TEA-PPO | 1-methy-3-methylimidazolium-TiO2 | Young’s modulus 921 MPa (TEA-PPO-1TiO2-15IL) | [123] |
| TEA-PPO | 1-methy-3-methylimidazolium-TiO2 | conductivity 52 mS cm−1 at 80 °C | [124] |
| TMA-PVA | TiO2 |
maximum power density 125 mW cm−2 at 35 °C | [125] |
| PVA | CaTiO3 | conductivity 66 mS cm−1 at RT | [126] |
| PTFE | Sn0.92Sb0.08P2O7 | maximum power density 147 mW cm−2 at 200 °C | [127] |
| CoOOH-PVA | CoSO4 | maximum power density 144 mW cm−2 at 30 °C | [128] |
| TMA-PVA/CS | MoS2 | TS 33 MPa | [129] |
| TMA-PPO/PSU | ZnO | maximum power density 69 mW cm−2 at RT | [130] |
| PBI | 1-butyl-3-methyl imidazolium phosphotungstate | conductivity 76 mS cm−1 at 80 °C (20 wt%, PBI/(PWA-IL1:5)) | [131] |
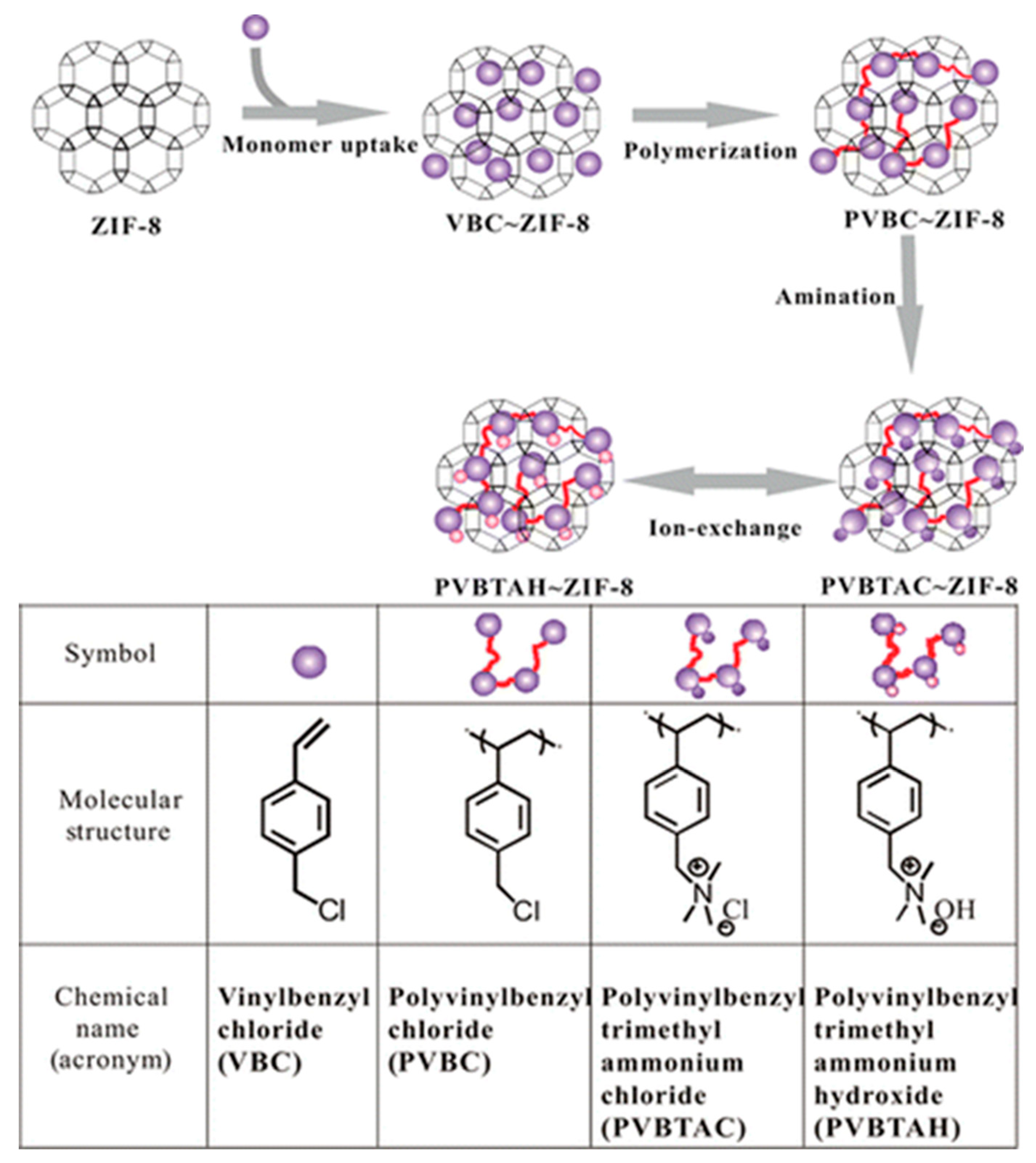
| poly vinyl benzyl trimethylammonium hydroxide | ZIF-8 | BET 1045 to 600 m2 g−1 | [133] |
| PVA | ZIF-8 | conductivity 0.3 mS cm−1 at 60 °C | [134] |
| IL/PVA | IL@ZIF-8 | conductivity 1.0 mS cm−1 at 60 °C (20 mol%) | [135] |
| PVA | ZIF-8 | maximum power density 173 mW cm−2 at 60 °C (PVA/40.5% ZIF-8) (DMAFC) | [136] |
| BrPPO/PVA | MIL-101-Fe-NH2-F | conductivity 145 mS cm−1 at 80 °C | [137] |
| Im-PEEK | imidazolium MIL-101(Cr) | TS 35 MPa, (10 wt%) | [138] |
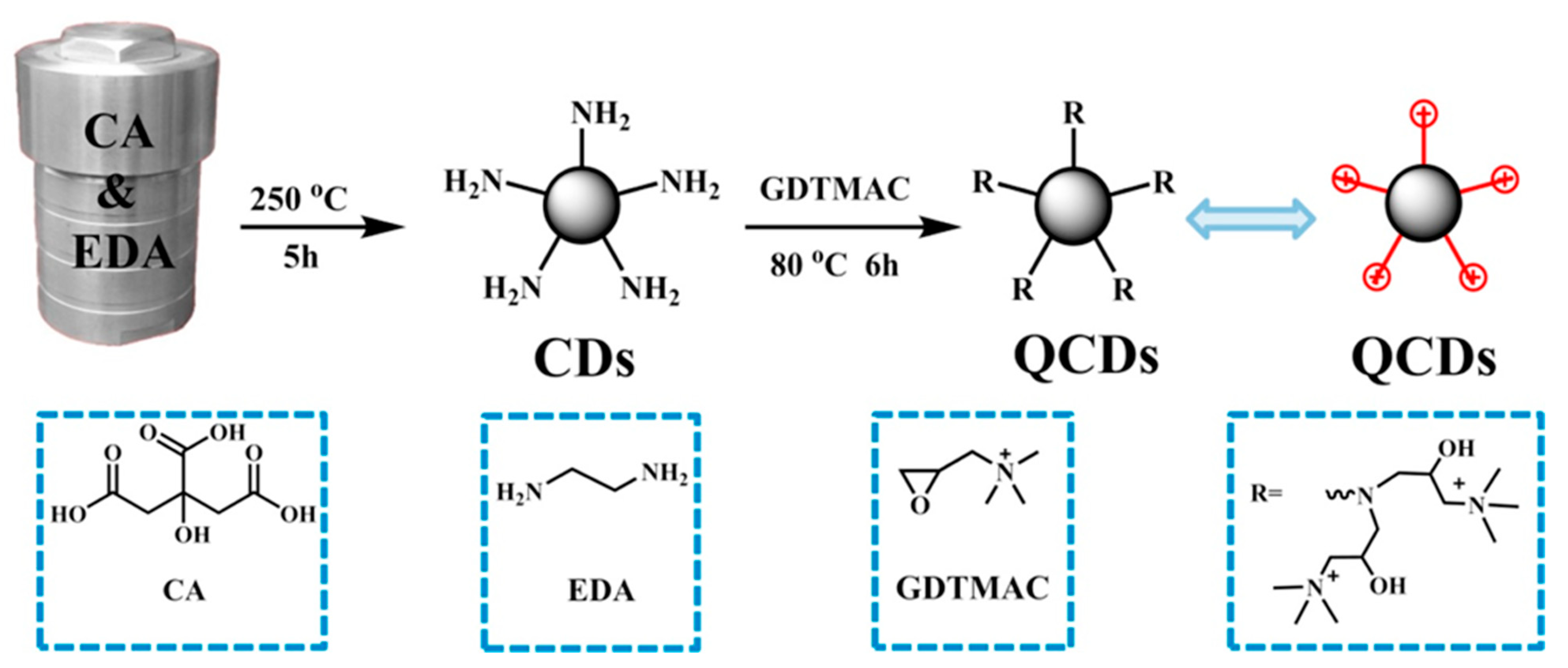
| Im-PSU | QCDs | Young’s modulus 1600 MPa | [139] |
| TMA-PSU | TMA-GQDs | conductivity 15 mS cm−1 at RT (0.1 wt%) | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narducci, R.; Sgreccia, E.; Knauth, P.; Di Vona, M.L. Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers 2021, 13, 3887. https://doi.org/10.3390/polym13223887
Narducci R, Sgreccia E, Knauth P, Di Vona ML. Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers. 2021; 13(22):3887. https://doi.org/10.3390/polym13223887
Chicago/Turabian StyleNarducci, Riccardo, Emanuela Sgreccia, Philippe Knauth, and Maria Luisa Di Vona. 2021. "Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review" Polymers 13, no. 22: 3887. https://doi.org/10.3390/polym13223887
APA StyleNarducci, R., Sgreccia, E., Knauth, P., & Di Vona, M. L. (2021). Anion Exchange Membranes with 1D, 2D and 3D Fillers: A Review. Polymers, 13(22), 3887. https://doi.org/10.3390/polym13223887










