Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
3. Results
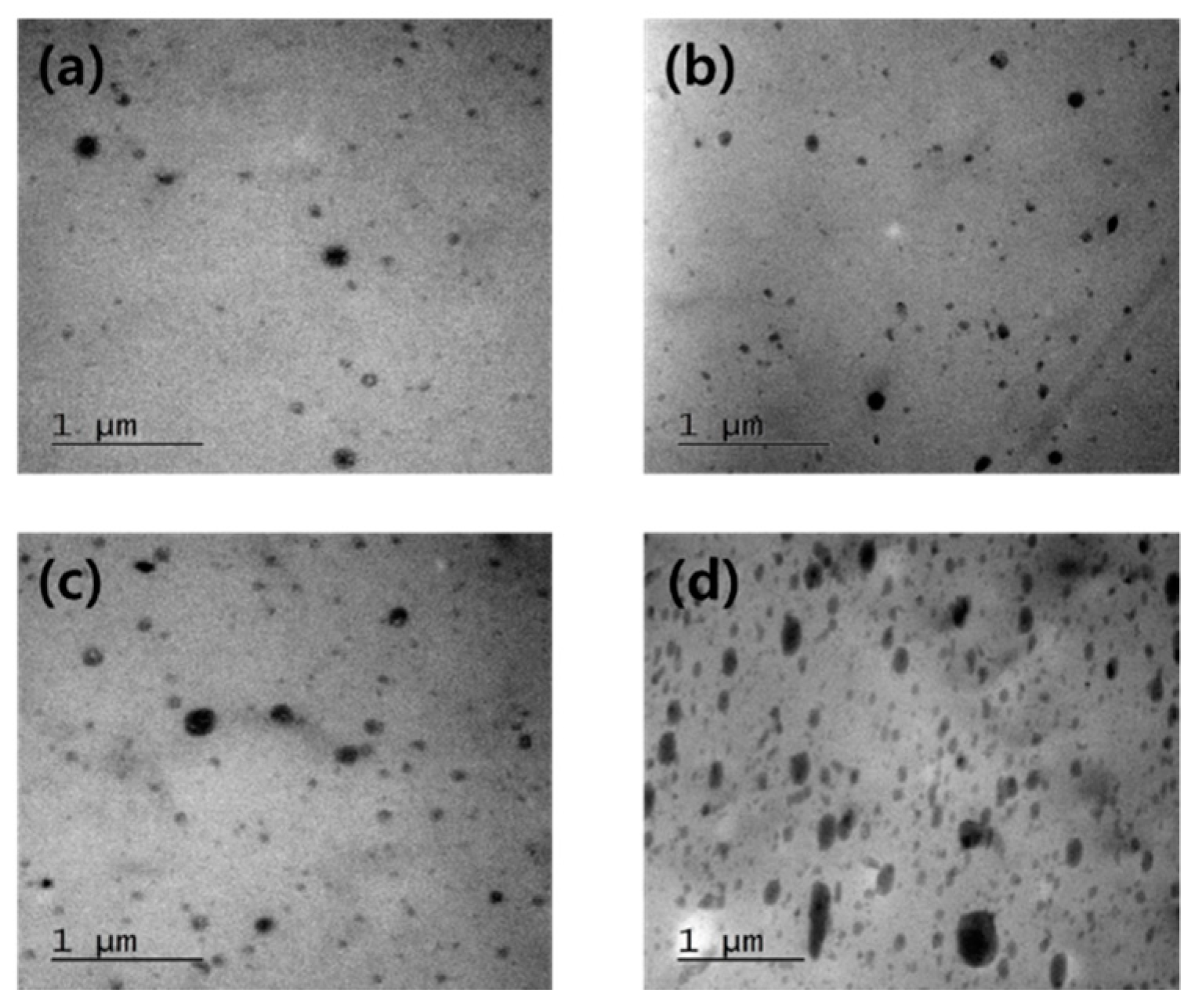
3.1. Phase Morphology
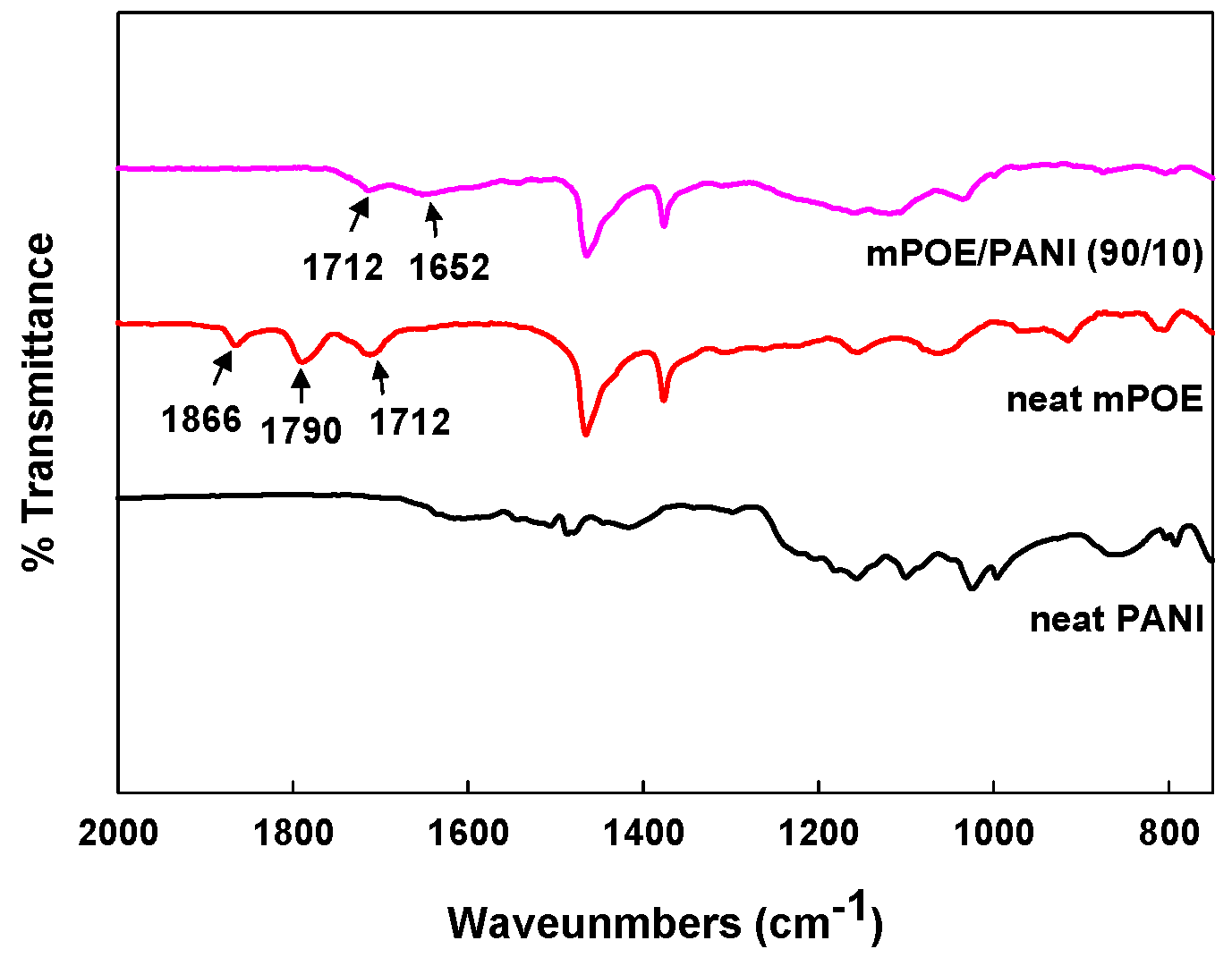
3.2. FT-IR
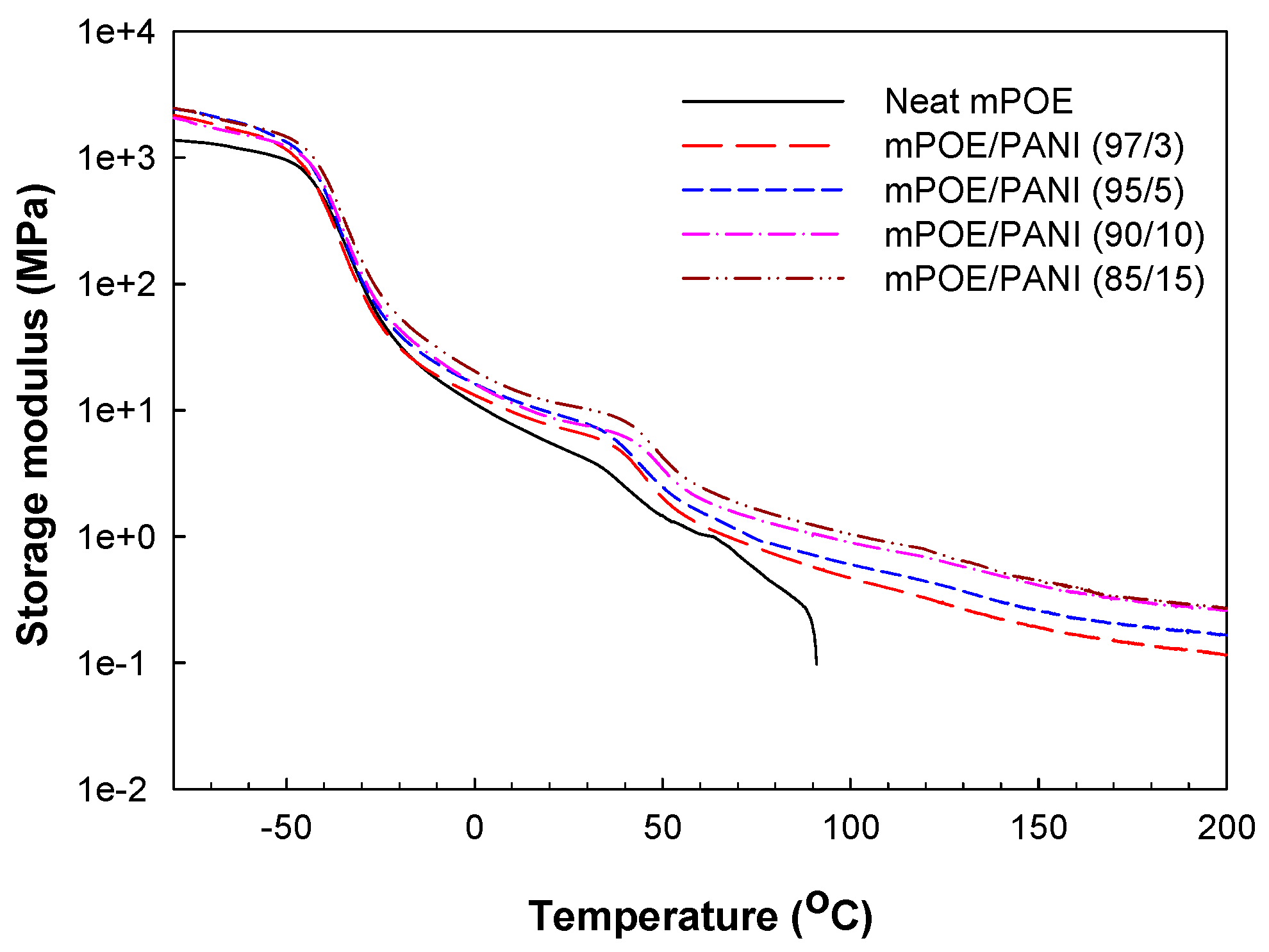
3.3. Dynamic Mechanical Properties
3.4. Thermal Properties
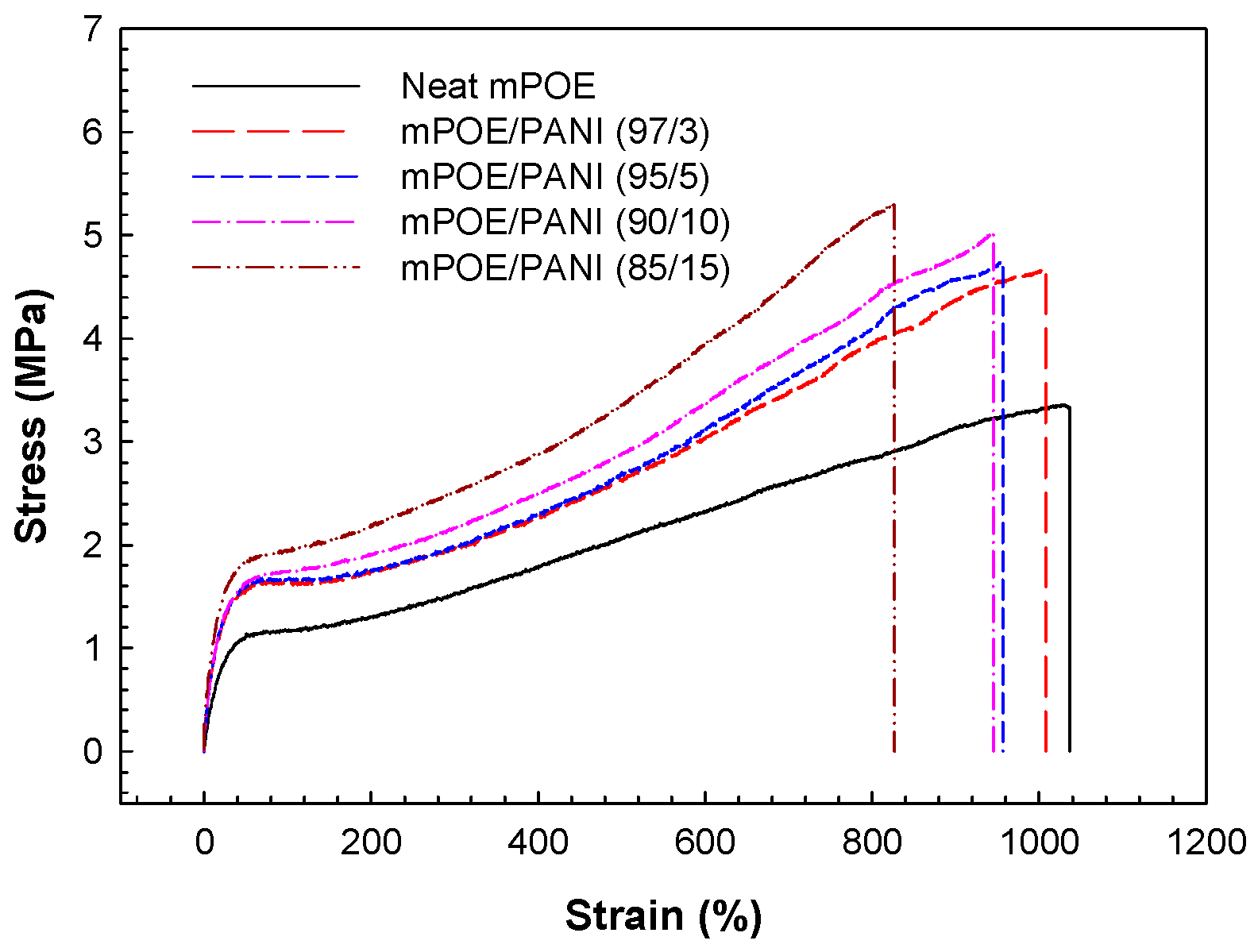
3.5. Tensile Properties
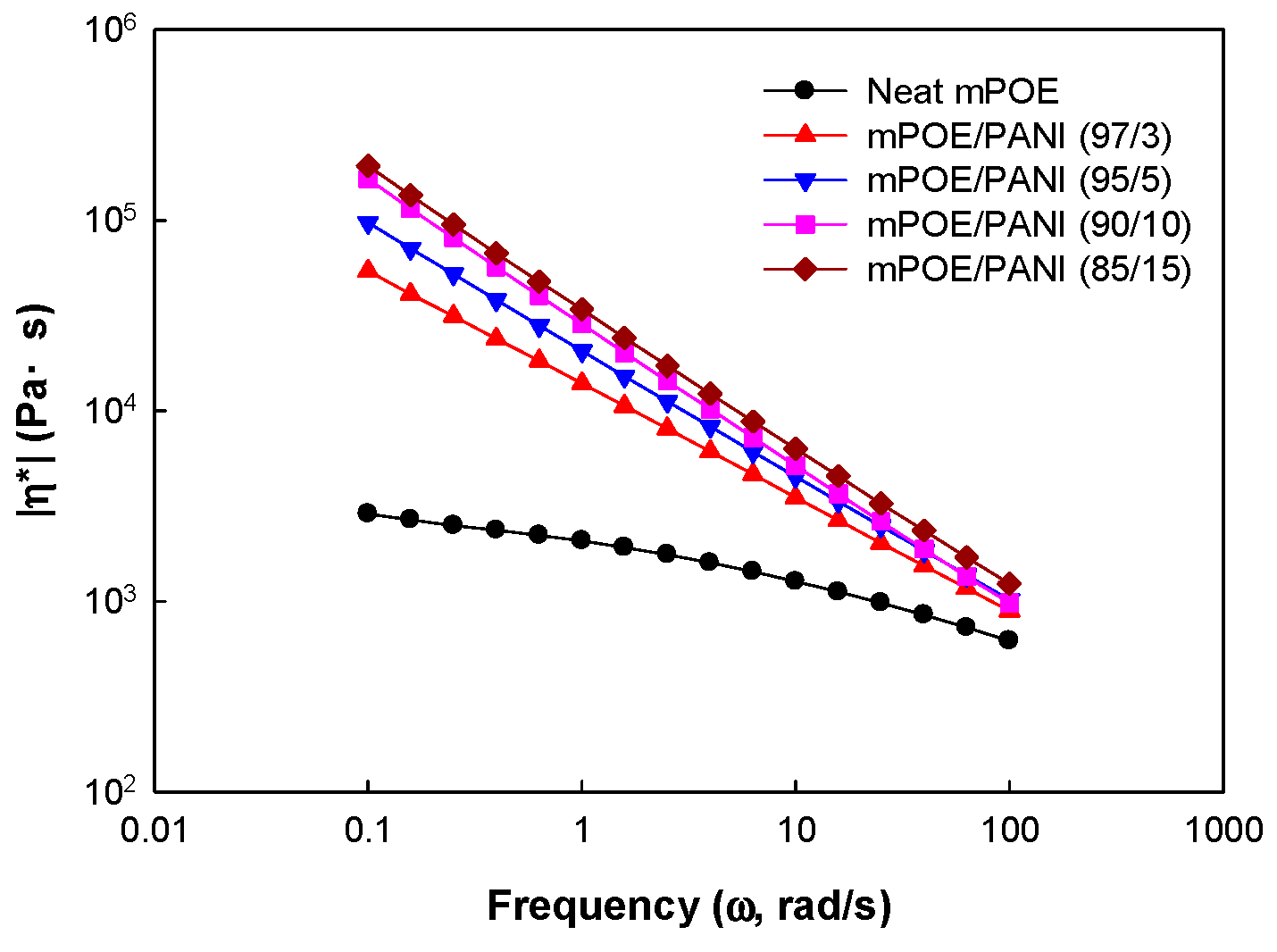
3.6. Melt Rheological Properties
3.7. Photothermal Effects
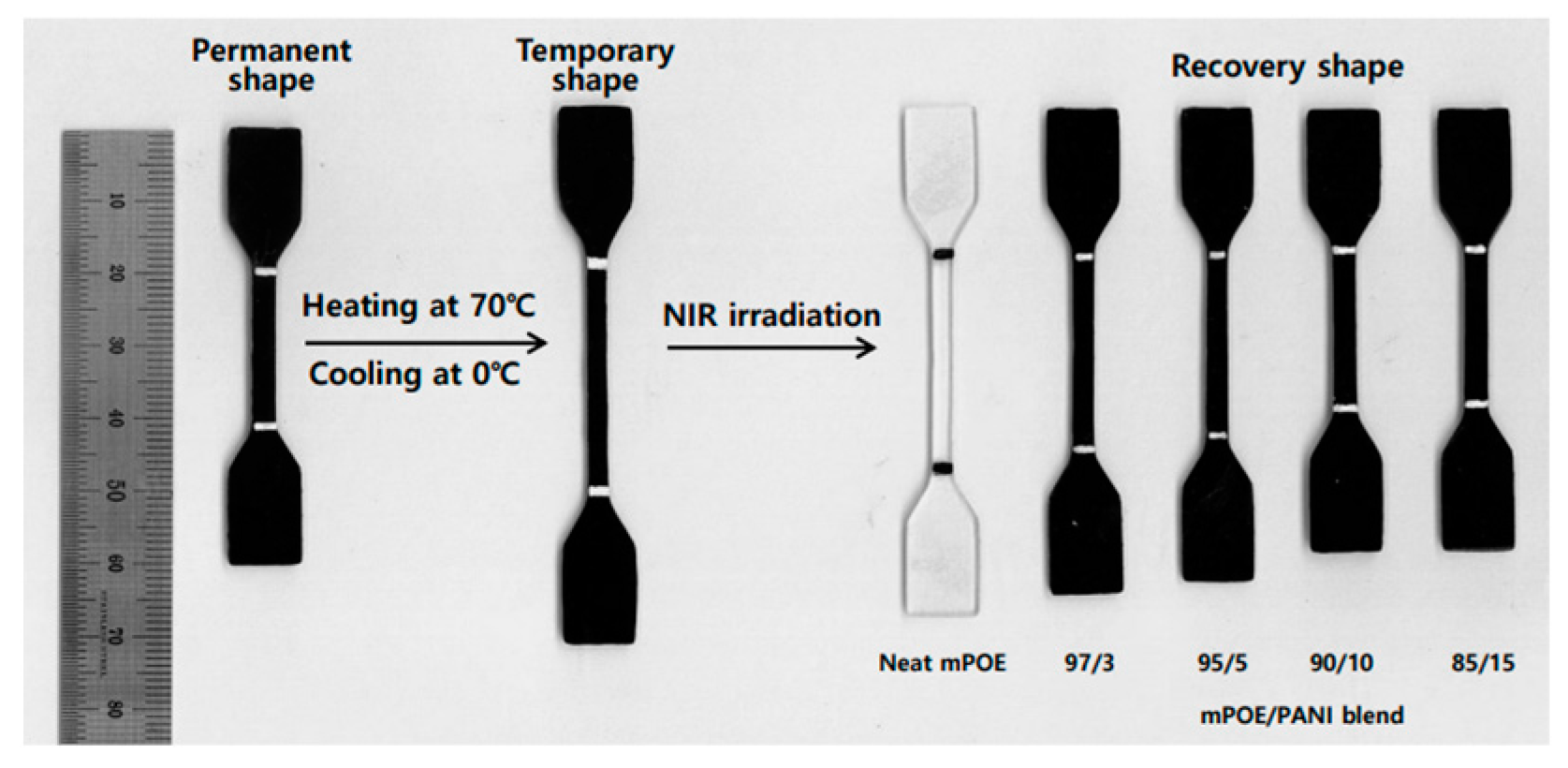
3.8. NIR Light-Induced Shape Memory Effect
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape-memory polymers: Structure, mechanism, functionality, modelling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Behl, M.; Razzaq, M.Y.; Lendlein, A. Multifunctional shape-memory polymers. Adv. Mater. 2010, 22, 3388–3410. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2014, 49–50, 79–120. [Google Scholar] [CrossRef] [Green Version]
- Serrano, M.C.; Ameer, G.A. Recent insights into the biomedical applications of shape-memory polymers. Macromol. Biosci. 2012, 12, 1156–1171. [Google Scholar] [CrossRef]
- Lai, H.; Shang, Y.; Cheng, Z.; Lv, T.; Zhang, E.; Zhang, D.; Wang, J.; Liu, Y. Control of tip nanostructure on superhydrophobic shape memory arrays toward reversibly adjusting water adhesion. Adv. Comp. Hybrid. Mater. 2019, 2, 753–762. [Google Scholar] [CrossRef]
- Wang, W.; Shen, R.; Cui, H.; Cui, Z.; Liu, Y. Two-stage reactive shape memory thiol–epoxy–acrylate system and application in 3D structure design. Adv. Comp. Hybrid. Mater. 2020, 3, 41–48. [Google Scholar] [CrossRef]
- Basak, S. Redesigning the modern applied medical sciences and engineering with shape memory polymers. Adv. Comp. Hybrid. Mater. 2021, 4, 223–234. [Google Scholar] [CrossRef]
- Gao, H.; Li, J.; Liu, Y.; Leng, J. Shape memory polymer solar cells. Adv. Comp. Hybrid. Mater. 2021. [Google Scholar] [CrossRef]
- Herath, M.; Epaarchchi, J.; Islam, M.; Fang, L.; Leng, J. Light activated shape memory polymers and composites: A review. Eur. Polym. J. 2020, 136, 109912. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, H.; Zhao, Y. Optically triggered and spatially controllable shape-memory polymer–gold nanoparticle composite materials. J. Mater. Chem. 2012, 22, 845–849. [Google Scholar] [CrossRef]
- Yenpech, N.; Intasanta, V.; Chirachanchai, S. Laser-triggered shape memory based on thermoplastic and thermoset matrices with silver nanoparticle. Polymer 2019, 182, 121792. [Google Scholar] [CrossRef]
- Ishii, S.; Uto, K.; Niiyama, E.; Ebara, M.; Nagao, T. Hybridizing poly(ε-caprolactone) and plasmonic titanium nitride nanoparticles for broadband photoresponsive shape memory films. ACS Appl. Mater. Interfaces 2016, 8, 5634–5640. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tong, R.; Wang, Z.; Xia, H. Polydopamine paticle-flled shape-memory polyurethane composites with fast near-infrared light responsibility. Chem. Phys. Chem. 2018, 19, 2052–2057. [Google Scholar] [CrossRef]
- Qian, W.; Song, Y.; Shi, D.; Dong, W.; Wang, X.; Zhang, H. Photothermal-triggered shape memory polymer prepared by cross-linking porphyrin-loaded micellar particles. Materials 2019, 12, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, B. Infrared light actuated shape memory effects in crystalline polyurethane/graphene chemical hybrids. Smart Mater. Struct. 2014, 23, 025038. [Google Scholar] [CrossRef]
- Kashif, M.; Chang, Y.-W. Supramolecular hydrogen-bonded polyolefin elastomer/modified graphene nanocomposites with near infrared responsive shape memory and healing properties. Eur. Polym. J. 2015, 66, 273–281. [Google Scholar] [CrossRef]
- Koerner, H.; Price, G.; Pearce, N.; Alexander, M.; Vaia, R.A. Remotely actuated polymer nanocomposites-stress-recovery of carbon-nanotube-filled thermoplastic elastomers. Nat. Mater. 2004, 3, 115–120. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.; Sun, Y.; Pang, X.; Zhang, X.; Zhuang, J.; Zhang, H.; Hu, C.; Lei, B.; Liu, Y. Facile fabrication of a CD/PVA composite polymer to access light-responsive shape-memory effects. J. Mater. Chem. C 2020, 8, 8935–8941. [Google Scholar] [CrossRef]
- Chen, M.; Fang, X.; Tang, S.; Zheng, N. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem. Comm. 2012, 48, 8934–8936. [Google Scholar] [CrossRef]
- Li, L.; Liang, K.; Hua, Z.; Zou, M.; Chen, K.; Wang, W. A green route to water-soluble polyaniline for photothermal therapy catalyzed by iron phosphates peroxidase mimic. Polym. Chem. 2015, 6, 2290–2296. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Z.; Zhu, X.; Wang, X.; Liao, Y.; Ma, Z.; Li, F. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials 2013, 34, 9584–9592. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ma, Y.; Zheng, Z.; Wu, C.; Wang, Y.; Liang, G. Facile syntheses of conjugated polymers for photothermal tumour therapy. Nat. Commun. 2019, 10, 1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.; Zhang, J.; Chen, X. A thermal-, water-, and near-infrared light-induced shape memory composite based on polyvinyl alcohol and polyaniline fibers. ACS Appl. Mater. Interfaces 2018, 10, 14017–14025. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Geng, C.; Liu, C.; Gao, J.; Zhou, Q. Near-infrared light photothermally induced shape memory and self-healing effects of epoxy resin coating with polyaniline nanofibers. Synth. Met. 2020, 266, 116417. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, S.; Chong, K.C.; Wang, S.; Liu, B. Near-infrared light-induced shape memory, self-healable and anti-bacterial elastomers prepared by incorporation of a diketopyrrolopyrrole-based conjugated polymer. Mater. Chem. Front. 2019, 3, 836. [Google Scholar] [CrossRef]
- Shimizu, S.; Saitoh, T.; Uzawa, M.; Yuasa, M.; Yano, K.; Maruyama, T.; Watanabe, K. Synthesis and applications of sulfonated polyaniline. Synth. Met. 1997, 85, 1337–1338. [Google Scholar] [CrossRef]
- Roy, B.C.; Gupta, M.D.; Bhowmik, L.; Ray, J.K. Studies on water soluble conducting polymer aniline initiated polymerization of m-aminobenzene sulfonic acid. Synth. Met. 1999, 100, 233–236. [Google Scholar] [CrossRef]
- Liao, Y.; Strong, V.; Chian, W.; Wang, X.; Li, X.G.; Kaner, R.B. Sulfonated polyaniline nanostructures synthesized by rapid initiated copolymerization with controllable morphology, size and electrical properties. Macromolecules 2012, 45, 1570–1579. [Google Scholar] [CrossRef]
- Kashif, M.; Chang, Y.W. Preparation of supramolecular thermally repairable elastomer by crosslinking of maleated polyethylene-octene elastomer with 3-amino-1,2,4-triazole. Polym. Int. 2014, 63, 1936–1948. [Google Scholar] [CrossRef]
- Wu, C.S. Preparation and characterization of aromatic polyester/polyaniline composite and its improved counterpart. eXPRESS Polym. Lett. 2012, 6, 465–473. [Google Scholar] [CrossRef]
- Heydaria, M.; Moghadama, P.N.; Fareghia, A.R.; Bahramb, M.; Movagharnezhad, N. Synthesis of water-soluble conductive copolymer based on polyaniline. Polym. Adv. Technol. 2015, 26, 250–254. [Google Scholar] [CrossRef]
- Pernot, H.; Baumert, M.; Court, F.; Leibler, L. Design and properties of co-continuous nanostructured polymers by reactive blending. Nat. Mater. 2002, 1, 54–58. [Google Scholar] [CrossRef]
- Choi, M.C.; Jung, J.Y.; Chang, Y.W. Shape memory thermoplastic elastomer from maleated polyolefin elastomer and nylon 12 blends. Polym. Bull. 2014, 71, 625–635. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Novel tunable super-tough materials from biodegradable polymer blends: Nano-structuring through reactive extrusion. RSC Adv. 2019, 9, 2836–2847. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.W.; Eom, J.P.; Kim, J.; Kim, H.T.; Kim, D.K. Preparation and characterization of shape memory polymer networks based on carboxylated telechelic poly(ε-caprolactone)/epoxidized natural rubber blends. J. Ind. Eng. Chem. 2010, 16, 256–260. [Google Scholar] [CrossRef]
- Liu, C.; Chun, S.B.; Mather, P.T.; Zheng, L.; Haley, E.H.; Coughlin, E.B. Chemically cross-linked polycyclooctene: Synthesis, characterization, and shape memory behavior. Macromolecules 2002, 35, 9868–9874. [Google Scholar] [CrossRef]
- Sung, Y.T.; Kim, C.K.; Lee, H.S.; Kim, J.S.; Yoon, H.G.; Kim, W.N. Effects of crystallinity and crosslinking on the thermal and rheological properties of ethylene vinyl acetate copolymer. Polymer 2005, 46, 11844–11848. [Google Scholar] [CrossRef]
- Sasmal, A.; Sahoo, D.; Nanda, R.; Nayak, P.; Nayak, P.L.; Mishra, J.K.; Chang, Y.W.; Yoon, J.Y. Biodegradable nanocomposites from maleated polycaprolactone/soy protein isolate blend with organoclay: Preparation, characterization, and properties. Polym. Comp. 2009, 30, 708–714. [Google Scholar] [CrossRef]

| Sample | Tm (°C) | ΔHm (J/g) | χc (%) 1 |
|---|---|---|---|
| Neat mPOE | 45.2 | 19.9 | 6.8 |
| mPOE/PANI (97/3) | 44.4 | 18.6 | 6.3 |
| mPOE/PANI (95/5) | 43.8 | 17.1 | 5.8 |
| mPOE/PANI (90/10) | 43.6 | 15.3 | 5.2 |
| mPOE/PANI (85/15) | 43.2 | 14.8 | 5.1 |
| Sample | 100% Tensile Modulus (MPa) | 300% Tensile Modulus (MPa) |
Tensile Strength (MPa) |
Elongation-at-Break (%) |
|---|---|---|---|---|
| Neat mPOE | 1.2 ± 0.1 | 1.5 ± 0.1 | 3.3 ± 0.2 | 1030 ± 70 |
| mPOE/PANI (97/3) | 1.6 ± 0.1 | 2.0 ± 0.2 | 4.6 ± 0.3 | 1000 ± 70 |
| mPOE/PANI (95/5) | 1.7 ± 0.1 | 2.0 ± 0.2 | 4.6 ± 0.3 | 950 ± 50 |
| mPOE/PANI (90/10) | 1.7 ± 0.1 | 2.2 ± 0.2 | 5.0 ± 0.3 | 940 ± 50 |
| mPOE/PANI (85/15) | 1.9 ± 0.1 | 2.5 ± 0.2 | 5.3 ± 0.3 | 820 ± 40 |
| Sample | Rf (%) | Rr (%) |
|---|---|---|
| Neat mPOE | 98.2 | 28.5 |
| mPOE/PANI (97/3) | 99.6 | 44.6 |
| mPOE/PANI (95/5) | 99.7 | 88.5 |
| mPOE/PANI (90/10) | 99.7 | 92.9 |
| mPOE/PANI (85/15) | 99.6 | 96.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, M.-S.; Kim, T.-H.; Chang, Y.-W.; Jang, K.S. Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline. Polymers 2021, 13, 3984. https://doi.org/10.3390/polym13223984
Heo M-S, Kim T-H, Chang Y-W, Jang KS. Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline. Polymers. 2021; 13(22):3984. https://doi.org/10.3390/polym13223984
Chicago/Turabian StyleHeo, Min-Su, Tae-Hoon Kim, Young-Wook Chang, and Keon Soo Jang. 2021. "Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline" Polymers 13, no. 22: 3984. https://doi.org/10.3390/polym13223984
APA StyleHeo, M.-S., Kim, T.-H., Chang, Y.-W., & Jang, K. S. (2021). Near-Infrared Light-Responsive Shape Memory Polymer Fabricated from Reactive Melt Blending of Semicrystalline Maleated Polyolefin Elastomer and Polyaniline. Polymers, 13(22), 3984. https://doi.org/10.3390/polym13223984








