Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery
Abstract
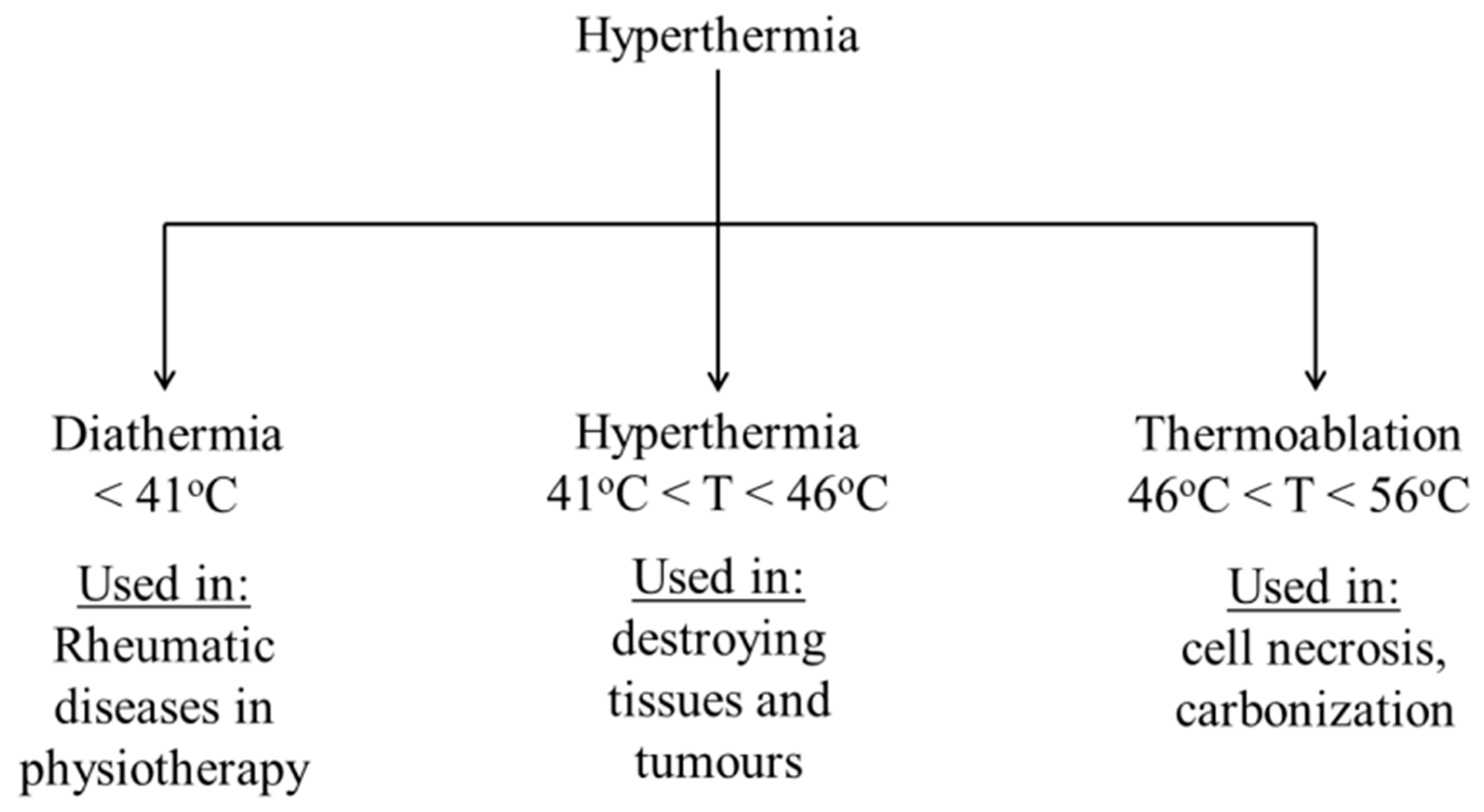
:1. Introduction
2. Magnetic Nanoparticles (MNPs) in Hyperthermia
3. Hydrogels
4. Fabrication of Magnetic Hydrogels
4.1. Fabrication of Magnetic Hydrogels by Blending
4.2. Fabrication of Magnetic Hydrogels by the In Situ Method
4.3. Fabrication of Magnetic Hydrogel by the Grafting-Onto Method
5. Hyperthermia-Based Cancer Treatment
6. Applications in Drug Delivery
7. Outlook and Future Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sounderya, N.; Zhang, Y. Use of core/shell structured nanoparticles for biomedical applications. Recent Pat. Biomed. Eng. Discontin. 2008, 1, 34–42. [Google Scholar] [CrossRef]
- Dutta, J.; Kundu, B.; Yook, S.-J. Three-dimensional thermal assessment in cancerous tumors based on local thermal non-equilibrium approach for hyperthermia treatment. Int. J. Therm. Sci. 2021, 159, 106591. [Google Scholar] [CrossRef]
- van Loo, G.; Saelens, X.; Van Gurp, M.; MacFarlane, M.; Martin, S.; Vandenabeele, P. The role of mitochondrial factors in apoptosis: A Russian roulette with more than one bullet. Cell Death Differ. 2002, 9, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, K.; Arkan, E.; Derakhshankhah, H.; Haghshenas, B.; Jahanban-Esfahlan, R.; Jaymand, M. A novel bioreducible and pH-responsive magnetic nanohydrogel based on β-cyclodextrin for chemo/hyperthermia therapy of cancer. Carbohydr. Polym. 2021, 252, 117229. [Google Scholar] [CrossRef]
- Goldstein, L.; Dewhirst, M.; Repacholi, M.; Kheifets, L. Summary, conclusions and recommendations: Adverse temperature levels in the human body. Int. J. Hyperth. 2003, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rai, K. Analysis of DPL Bioheat Transfer Model During Thermal Treatment. Int. J. Appl. Comput. Math. 2021, 7, 44. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol./Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Roohi, R.; Heydari, M.; Avazzadeh, Z. Optimal control of hyperthermia thermal damage based on tumor configuration. Results Phys. 2021, 23, 103992. [Google Scholar] [CrossRef]
- Edwards, M. Hyperthermia as a teratogen: A review of experimental studies and their clinical significance. Teratog. Carcinog. Mutagenesis 1986, 6, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Dyrbye, B.A.; Overdijk, L.E.; van Kesteren, P.J.; de Haan, P.; Riezebos, R.K.; Bakkum, E.A.; Rademaker, B.M. Gas embolism during hysteroscopic surgery using bipolar or monopolar diathermia: A randomized controlled trial. Am. J. Obstet. Gynecol. 2012, 207, 271.e1–271.e6. [Google Scholar] [CrossRef]
- Borys, N.; Dewhirst, M.W. Drug Development of Lyso-thermosensitive Liposomal Doxorubicin: Combining Hyperthermia and Thermosensitive Drug Delivery. Adv. Drug Deliv. Rev. 2021, 178, 113985. [Google Scholar] [CrossRef] [PubMed]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia–description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Honda, H.; Kobayashi, T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol. Immunother. 2006, 55, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Mbugua, S.N.; Njenga, L.W.; Odhiambo, R.A.; Wandiga, S.O.; Onani, M.O. Beyond DNA-targeting in Cancer Chemotherapy. Emerging Frontiers-A Review. Curr. Top. Med. Chem. 2021, 21, 28–47. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, X.; Unger, M.; Patties, I.; Melzer, A.; Landgraf, L. Focused ultrasound-induced cavitation sensitizes cancer cells to radiation therapy and hyperthermia. Cells 2020, 9, 2595. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Saavedra, E.; del Rosario, H.; González, I.; Hernández, I.; Estévez, F.; Quintana, J. Ethanol Enhances Hyperthermia-Induced Cell Death in Human Leukemia Cells. Int. J. Mol. Sci. 2021, 22, 4948. [Google Scholar] [CrossRef] [PubMed]
- Skandalakis, G.P.; Rivera, D.R.; Rizea, C.D.; Bouras, A.; Jesu Raj, J.G.; Bozec, D.; Hadjipanayis, C.G. Hyperthermia treatment advances for brain tumors. Int. J. Hyperth. 2020, 37, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Hanusch, K.-U.; Janssen, C.H.; Billheimer, D.; Jenkins, I.; Spurgeon, E.; Lowry, C.A.; Raison, C.L. Whole-body hyperthermia for the treatment of major depression: Associations with thermoregulatory cooling. Am. J. Psychiatry 2013, 170, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Ebbini, E.S.; Cain, C.A. A spherical-section ultrasound phased array applicator for deep localized hyperthermia. IEEE Trans. Biomed. Eng. 1991, 38, 634–643. [Google Scholar] [CrossRef]
- He, C.; Yu, L.; Ding, L.; Yao, H.; Chen, Y.; Hao, Y. Lysine demethylase KDM3A regulates nanophotonic hyperthermia resistance generated by 2D silicene in breast cancer. Biomaterials 2020, 255, 120181. [Google Scholar] [CrossRef]
- Santos-Marques, M.J.; Carvalho, F.; Sousa, C.; Remião, F.; Vitorino, R.; Amado, F.; Ferreira, R.; Duarte, J.A.; de Lourdes Bastos, M. Cytotoxicity and cell signalling induced by continuous mild hyperthermia in freshly isolated mouse hepatocytes. Toxicology 2006, 224, 210–218. [Google Scholar] [CrossRef]
- Raaphorst, G.; Freeman, M.; Dewey, W. Radiosensitivity and recovery from radiation damage in cultured CHO cells exposed to hyperthermia at 42.5 or 45.5 C. Radiat. Res. 1979, 79, 390–402. [Google Scholar] [CrossRef]
- Scutigliani, E.M.; Liang, Y.; Crezee, H.; Kanaar, R.; Krawczyk, P.M. Modulating the Heat Stress Response to Improve Hyperthermia-Based Anticancer Treatments. Cancers 2021, 13, 1243. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 2: Hyperthermia techniques. Crit. Rev. Biomed. Eng. 2006, 34. [Google Scholar] [CrossRef]
- Falk, M.; Issels, R. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, J.; Peer-Valstar, J.N.; Rietveld, P.J.; de Graaf-Strukowska, L.; van Rhoon, G.C. Practical limitations of interstitial thermometry during deep hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1205–1212. [Google Scholar] [CrossRef]
- Kurdtabar, M.; Koutenaee, R.N.; Bardajee, G.R. Synthesis and characterization of a novel pH-responsive nanocomposite hydrogel based on chitosan for targeted drug release. J. Polym. Res. 2018, 25, 119. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-based controlled drug delivery for cancer treatment: A review. Mol. Pharm. 2019, 17, 373–391. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Review on magnetic natural polymer constructed hydrogels as vehicles for drug delivery. Biomacromolecules 2020, 21, 2574–2594. [Google Scholar] [CrossRef]
- Vishnubhakthula, S.; Elupula, R.; Durán-Lara, E.F. Recent advances in hydrogel-based drug delivery for melanoma cancer therapy: A mini review. J. Drug Deliv. 2017, 2017, 7275985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Xu, F.; Han, Z.; Wei, D.; Jia, D.; Zhou, Y. Tough magnetic chitosan hydrogel nanocomposites for remotely stimulated drug release. Biomacromolecules 2018, 19, 3351–3360. [Google Scholar] [CrossRef]
- Xue, W.; Liu, X.-L.; Ma, H.; Xie, W.; Huang, S.; Wen, H.; Jing, G.; Zhao, L.; Liang, X.-J.; Fan, H.M. AMF responsive DOX-loaded magnetic microspheres: Transmembrane drug release mechanism and multimodality postsurgical treatment of breast cancer. J. Mater. Chem. B 2018, 6, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H.; Soleymani, F. Magnetic/pH-responsive beads based on caboxymethyl chitosan and κ-carrageenan and controlled drug release. Carbohydr. Polym. 2015, 128, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Uva, M.; Pasqui, D.; Mencuccini, L.; Fedi, S.; Barbucci, R. Influence of alternating and static magnetic fields on drug release from hybrid hydrogels containing magnetic nanoparticles. J. Biomater. Nanobiotechnol. 2014, 2014, 44960. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, K.; Tokuda, A.; Nakamura, J.; Sugawara-Narutaki, A.; Ohtsuki, C. Tearable and Fillable Composite Sponges Capable of Heat Generation and Drug Release in Response to Alternating Magnetic Field. Materials 2020, 13, 3637. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ivkov, R.; Blumenthal, R. Magnetically triggered drug release from Liposome Embedded gel. J. Nanomed. Biother. Discov. 2014, 4, 1. [Google Scholar]
- Selawry, O.S.; Goldstein, M.N.; McCormick, T. Hyperthermia in tissue-cultured cells of malignant origin. Cancer Res. 1957, 17, 785–791. [Google Scholar]
- García-Hevia, L.; Casafont, Í.; Oliveira, J.; Terán, N.; Fanarraga, M.L.; Gallo, J.; Bañobre-López, M. Magnetic lipid nanovehicles synergize the controlled thermal release of chemotherapeutics with magnetic ablation while enabling non-invasive monitoring by MRI for melanoma theranostics. Bioact. Mater. 2022, 8, 153–164. [Google Scholar] [CrossRef]
- Datta, N.R.; Kok, H.P.; Crezee, H.; Gaipl, U.S.; Bodis, S. Integrating loco-regional hyperthermia into the current oncology practice: SWOT and TOWS analyses. Front. Oncol. 2020, 10, 819. [Google Scholar] [CrossRef]
- Walter, E.; Watt, P.W.; Gibson, O.R.; Wilmott, A.G.; Mitchell, D.; Moreton, R.; Maxwell, N.S. Exercise hyperthermia induces greater changes in gastrointestinal permeability than equivalent passive hyperthermia. Physiol. Rep. 2021, 9, e14945. [Google Scholar] [CrossRef]
- Bachmann, C.; Sautkin, I.; Nadiradze, G.; Archid, R.; Weinreich, F.; Königsrainer, A.; Reymond, M. Technology development of hyperthermic pressurized intraperitoneal aerosol chemotherapy (hPIPAC). Surg. Endosc. 2021, 35, 6358–6365. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A. The Capacitive Coupling Modalities for Oncological Hyperthermia. Open J. Biophys. 2021, 11, 252–313. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/poly (norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Interfaces 2021, 13, 31038–31050. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Mondal, S.; Ghorai, U.K.; Maity, P.P.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Banerjee, S.; Das, N.C. Dual doped biocompatible multicolor luminescent carbon dots for bio labeling, UV-active marker and fluorescent polymer composite. Luminescence 2018, 33, 1136–1145. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J.; Schirra, H.; Schmidt, H.; Deger, S.; Loening, S. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2001, 225, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Gavazzi, S.; van Lier, A.L.; Zachiu, C.; Jansen, E.; Lagendijk, J.J.W.; Stalpers, L.J.A.; Crezee, H.; Kok, H.P. Advanced patient-specific hyperthermia treatment planning. Int. J. Hyperth. 2020, 37, 992–1007. [Google Scholar] [CrossRef]
- Suleman, M.; Riaz, S. In silico study of enhanced permeation and retention effect and hyperthermia of porous tumor. Med. Eng. Phys. 2020, 86, 128–137. [Google Scholar] [CrossRef]
- Molcan, M.; Kaczmarek, K.; Kubovcikova, M.; Gojzewski, H.; Kovac, J.; Timko, M.; Józefczak, A. Magnetic hyperthermia study of magnetosome chain systems in tissue-mimicking phantom. J. Mol. Liq. 2020, 320, 114470. [Google Scholar] [CrossRef]
- Wolburg, H.; Noell, S.; Fallier-Becker, P.; Mack, A.F.; Wolburg-Buchholz, K. The disturbed blood–brain barrier in human glioblastoma. Mol. Asp. Med. 2012, 33, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Tailor made magnetic nanolights: Fabrication to cancer theranostics applications. Nanoscale Adv. 2021. [Google Scholar] [CrossRef]
- Varmazyar, M.; Habibi, M.; Amini, M.; Pordanjani, A.H.; Afrand, M.; Vahedi, S.M. Numerical simulation of magnetic nanoparticle-based drug delivery in presence of atherosclerotic plaques and under the effects of magnetic field. Powder Technol. 2020, 366, 164–174. [Google Scholar] [CrossRef]
- Schürch, C.M.; Bhate, S.S.; Barlow, G.L.; Phillips, D.J.; Noti, L.; Zlobec, I.; Chu, P.; Black, S.; Demeter, J.; McIlwain, D.R. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 2020, 182, 1341–1359.e1319. [Google Scholar] [CrossRef]
- Kuhn, J.; Papanastasiou, G.; Tai, C.-W.; Moran, C.M.; Jansen, M.A.; Tavares, A.A.; Lennen, R.J.; Corral, C.A.; Wang, C.; Thomson, A.J. Tri-modal imaging of gold-dotted magnetic nanoparticles for magnetic resonance imaging, computed tomography and intravascular ultrasound: An in vitro study. Nanomedicine 2020, 15, 2433–2445. [Google Scholar] [CrossRef]
- Amara, D.; Felner, I.; Nowik, I.; Margel, S. Synthesis and characterization of Fe and Fe3O4 nanoparticles by thermal decomposition of triiron dodecacarbonyl. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 106–110. [Google Scholar] [CrossRef]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Remotely controlled magneto-regulation of therapeutics from magnetoelastic gel matrices. Biotechnol. Adv. 2020, 44, 107611. [Google Scholar] [CrossRef]
- Molinari, S.; Magro, M.; Baratella, D.; Salviulo, G.; Ugolotti, J.; Filip, J.; Petr, M.; Tucek, J.; Zoppellaro, G.; Zboril, R. Smart synthetic maghemite nanoparticles with unique surface properties encode binding specificity toward AsIII. Sci. Total. Environ. 2020, 741, 140175. [Google Scholar] [CrossRef] [PubMed]
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials 2010, 31, 741–747. [Google Scholar] [CrossRef]
- Ganguly, S.; Grinberg, I.; Margel, S. Layer by layer controlled synthesis at room temperature of tri-modal (MRI, fluorescence and CT) core/shell superparamagnetic IO/human serum albumin nanoparticles for diagnostic applications. Polym. Adv. Technol. 2021, 32, 3909–3921. [Google Scholar] [CrossRef]
- Ziv, O.; Avtalion, R.R.; Margel, S. Immunogenicity of bioactive magnetic nanoparticles: Natural and acquired antibodies. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 85, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziv-Polat, O.; Skaat, H.; Shahar, A.; Margel, S. Novel magnetic fibrin hydrogel scaffolds containing thrombin and growth factors conjugated iron oxide nanoparticles for tissue engineering. Int. J. Nanomed. 2012, 7, 1259. [Google Scholar] [CrossRef] [Green Version]
- Skaat, H.; Margel, S. Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-β fibrils detection and removal by a magnetic field. Biochem. Biophys. Res. Commun. 2009, 386, 645–649. [Google Scholar] [CrossRef]
- Zhang, H.; Moore, L.R.; Zborowski, M.; Williams, P.S.; Margel, S.; Chalmers, J.J. Establishment and implications of a characterization method for magnetic nanoparticle using cell tracking velocimetry and magnetic susceptibility modified solutions. Analyst 2005, 130, 514–527. [Google Scholar] [CrossRef]
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized Thin Coating of Polypyrrole/Graphene Nanofiber/Iron Oxide onto Nonpolar Plastic for Flexible Electromagnetic Radiation Shielding, Strain Sensing, and Non-Contact Heating Applications. Adv. Mater. Interfaces 2021, 2101255. [Google Scholar] [CrossRef]
- Skaat, H.; Sorci, M.; Belfort, G.; Margel, S. Effect of maghemite nanoparticles on insulin amyloid fibril formation: Selective labeling, kinetics, and fibril removal by a magnetic field. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 342–351. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Synthesis of a novel pH responsive phyllosilicate loaded polymeric hydrogel based on poly (acrylic acid-co-N-vinylpyrrolidone) and polyethylene glycol for drug delivery: Modelling and kinetics study for the sustained release of an antibiotic drug. RSC Adv. 2015, 5, 18312–18327. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Das, N.C. Characterization tools and techniques of hydrogels. In Hydrogels Based on Natural Polymers; Elsevier: New York, NY, USA, 2020; pp. 481–517. [Google Scholar] [CrossRef]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, T.K.; Mondal, S.; Das, N. Synthesis of polydopamine-coated halloysite nanotube-based hydrogel for controlled release of a calcium channel blocker. RSC Adv. 2016, 6, 105350–105362. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-synthesized polysaccharide-derived carbon dots as therapeutic cargoes and toughening agents for elastomeric gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Rheological properties of polymer–carbon composites. In Carbon-Containing Polymer Composites; Springer: Singapore, 2019; pp. 271–294. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Das, T.K.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.K.; Das, N.C. Acoustic cavitation assisted destratified clay tactoid reinforced in situ elastomer-mimetic semi-IPN hydrogel for catalytic and bactericidal application. Ultrason. Sonochem. 2020, 60, 104797. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, K.; Dhamodharan, R. Advances in chitosan-based hydrogels: Evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React. Funct. Polym. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Lopez Hernandez, H.; Souza, J.W.; Appel, E.A. A Quantitative Description for Designing the Extrudability of Shear-Thinning Physical Hydrogels. Macromol. Biosci. 2021, 21, 2000295. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Zhou, Y.; Pan, Y.; Lu, T.; Song, X.; Hu, J. Fatigue behaviors of physical hydrogels based on hydrogen bonds. Extrem. Mech. Lett. 2021, 46, 101320. [Google Scholar] [CrossRef]
- Ozay, H.; Ilgin, P.; Sezgintürk, M.K.; Ozay, O. Ruthenium nanoparticles supported in the network of HES-p (AMPS) IPN hydrogel as efficient catalyst for hydrogen production from the hydrolysis of ethylenediamine bisborane. Int. J. Hydrogen Energy 2020, 45, 9892–9902. [Google Scholar] [CrossRef]
- Scalet, J.M.; Suekama, T.C.; Jeong, J.; Gehrke, S.H. Enhanced Mechanical Properties by Ionomeric Complexation in Interpenetrating Network Hydrogels of Hydrolyzed Poly (N-vinyl Formamide) and Polyacrylamide. Gels 2021, 7, 80. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Soleimani, P. Synthesis and characterization of novel semi-IPN nanocomposite hydrogels based on guar gum, partially hydrolyzed poly (acrylamide), and pristine montmorillonite. Polym. Bull. 2021, 78, 5923–5952. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I.; Hosseini, F. Effect of zinc content on structural, functional, morphological, and thermal properties of kappa-carrageenan/NaCMC nanocomposites. Polym. Test. 2021, 93, 106922. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Nazari, Z.; Mobini, P.; Khatir, N.M. Investigation of acyclovir-loaded, acrylamide-based hydrogels for potential use as vaginal ring. Mater. Today Commun. 2018, 16, 274–280. [Google Scholar] [CrossRef]
- Yang, J.; Liang, G.; Xiang, T.; Situ, W. Effect of crosslinking processing on the chemical structure and biocompatibility of a chitosan-based hydrogel. Food Chem. 2021, 354, 129476. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. Temperature/pH dual sensitive Hericium erinaceus residue carboxymethyl chitin/poly (N-isopropyl acrylamide) sequential IPN hydrogels. Cellulose 2020, 27, 825–838. [Google Scholar] [CrossRef]
- O’Brien, S.; Brannigan, R.P.; Ibanez, R.; Wu, B.; O’Dwyer, J.; O’Brien, F.J.; Cryan, S.-A.; Heise, A. Biocompatible polypeptide-based interpenetrating network (IPN) hydrogels with enhanced mechanical properties. J. Mater. Chem. B 2020, 8, 7785–7791. [Google Scholar] [CrossRef]
- Das, B.; Chattopadhyay, D.; Rana, D. The gamut of perspectives, challenges, and recent trends for in situ hydrogels: A smart ophthalmic drug delivery vehicle. Biomater. Sci. 2020, 8, 4665–4691. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.W.; Endud, C.; Mayanar, R. Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Tavares, L.; Flores, E.E.E.; Rodrigues, R.C.; Hertz, P.F.; Noreña, C.P.Z. Effect of deacetylation degree of chitosan on rheological properties and physical chemical characteristics of genipin-crosslinked chitosan beads. Food Hydrocoll. 2020, 106, 105876. [Google Scholar] [CrossRef]
- Parvathy, P.; Ayobami, A.V.; Raichur, A.M.; Sahoo, S.K. Methacrylated alkali lignin grafted P (Nipam-Co-Aac) copolymeric hydrogels: Tuning the mechanical and stimuli-responsive properties. Int. J. Biol. Macromol. 2021, 192, 180–196. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Gao, Q.; Liu, X.; Tong, Z. Magnetic hydrogels with supracolloidal structures prepared by suspension polymerization stabilized by Fe2O3 nanoparticles. Acta Biomater. 2010, 6, 275–281. [Google Scholar] [CrossRef]
- Brunsen, A.; Utech, S.; Maskos, M.; Knoll, W.; Jonas, U. Magnetic composite thin films of FexOy nanoparticles and photocrosslinked dextran hydrogels. J. Magn. Magn. Mater. 2012, 324, 1488–1497. [Google Scholar] [CrossRef]
- Brulé, S.; Levy, M.; Wilhelm, C.; Letourneur, D.; Gazeau, F.; Ménager, C.; Le Visage, C. Doxorubicin release triggered by alginate embedded magnetic nanoheaters: A combined therapy. Adv. Mater. 2011, 23, 787–790. [Google Scholar] [CrossRef]
- Lü, T.; Ma, R.; Ke, K.; Zhang, D.; Qi, D.; Zhao, H. Synthesis of gallic acid functionalized magnetic hydrogel beads for enhanced synergistic reduction and adsorption of aqueous chromium. Chem. Eng. J. 2021, 408, 127327. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jahanban-Esfahlan, R.; Vandghanooni, S.; Akbari-Nakhjavani, S.; Massoumi, B.; Haghshenas, B.; Rezaei, A.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. A bio-inspired gelatin-based pH-and thermal-sensitive magnetic hydrogel for in vitro chemo/hyperthermia treatment of breast cancer cells. J. Appl. Polym. Sci. 2021, 138, 50578. [Google Scholar] [CrossRef]
- Hu, X.; Nian, G.; Liang, X.; Wu, L.; Yin, T.; Lu, H.; Qu, S.; Yang, W. Adhesive tough magnetic hydrogels with high Fe3O4 content. ACS Appl. Mater. Interfaces 2019, 11, 10292–10300. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Inda, M.E.; Lin, S.; Wu, J.; Kim, Y.; Chen, X.; Ma, D.; Lu, T.K.; Zhao, X. Magnetic Living Hydrogels for Intestinal Localization, Retention, and Diagnosis. Adv. Funct. Mater. 2021, 31, 2010918. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; Soleimani, K.; Derakhshankhah, H.; Haghshenas, B.; Rezaei, A.; Massoumi, B.; Farnudiyan-Habibi, A.; Samadian, H.; Jaymand, M. Multi-stimuli-responsive magnetic hydrogel based on Tragacanth gum as a de novo nanosystem for targeted chemo/hyperthermia treatment of cancer. J. Mater. Res. 2021, 36, 858–869. [Google Scholar] [CrossRef]
- Farzaneh, S.; Hosseinzadeh, S.; Samanipour, R.; Hatamie, S.; Ranjbari, J.; Khojasteh, A. Fabrication and characterization of cobalt ferrite magnetic hydrogel combined with static magnetic field as a potential bio-composite for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102525. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhang, Y.; Zhou, L.; Liu, Z.; Jiang, Z.; Liu, Y.; Tang, L. Mechanical behaviors and probabilistic multiphase network model of polyvinyl alcohol hydrogel after being immersed in sodium hydroxide solution. RSC Adv. 2021, 11, 11468–11480. [Google Scholar] [CrossRef]
- Nardecchia, S.; Jiménez, A.; Morillas, J.R.; de Vicente, J. Synthesis and rheological properties of 3D structured self-healing magnetic hydrogels. Polymer 2021, 218, 123489. [Google Scholar] [CrossRef]
- Dresco, P.A.; Zaitsev, V.S.; Gambino, R.J.; Chu, B. Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 1999, 15, 1945–1951. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Almomani, F. Application of Fe3O4 magnetite nanoparticles grafted in silica (SiO2) for oil recovery from oil in water emulsions. Chemosphere 2021, 265, 129054. [Google Scholar] [CrossRef]
- Nagireddy, N.R.; Yallapu, M.M.; Kokkarachedu, V.; Sakey, R.; Kanikireddy, V.; Alias, J.P.; Konduru, M.R. Preparation and characterization of magnetic nanoparticles embedded in hydrogels for protein purification and metal extraction. J. Polym. Res. 2011, 18, 2285–2294. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I. Physical and chemical characterisation of acrylamide-based hydrogels, Aam, Aam/NaCMC and Aam/NaCMC/MgO. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1439–1449. [Google Scholar] [CrossRef]
- Hernández, R.; Mijangos, C. In situ Synthesis of Magnetic Iron Oxide Nanoparticles in Thermally Responsive Alginate-Poly (N-isopropylacrylamide) Semi-Interpenetrating Polymer Networks. Macromol. Rapid Commun. 2009, 30, 176–181. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mahvi, A.H.; Khatibi, A.D.; Balarak, D. Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron (III) oxide magnetic nanoparticles. J. Porous Mater. 2021, 28, 835–852. [Google Scholar] [CrossRef]
- Li, K.; Yan, J.; Zhou, Y.; Li, B.; Li, X. β-cyclodextrin and magnetic graphene oxide modified porous composite hydrogel as a superabsorbent for adsorption cationic dyes: Adsorption performance, adsorption mechanism and hydrogel column process investigates. J. Mol. Liq. 2021, 335, 116291. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A 2021. [Google Scholar] [CrossRef]
- Mondal, S.; Ganguly, S.; Das, P.; Bhawal, P.; Das, T.K.; Nayak, L.; Khastgir, D.; Das, N.C. High-performance carbon nanofiber coated cellulose filter paper for electromagnetic interference shielding. Cellulose 2017, 24, 5117–5131. [Google Scholar] [CrossRef]
- Zhao, W.; Odelius, K.; Edlund, U.; Zhao, C.; Albertsson, A.-C. In situ synthesis of magnetic field-responsive hemicellulose hydrogels for drug delivery. Biomacromolecules 2015, 16, 2522–2528. [Google Scholar] [CrossRef]
- Hu, F.; Neoh, K.G.; Cen, L.; Kang, E.-T. Cellular response to magnetic nanoparticles “PEGylated” via surface-initiated atom transfer radical polymerization. Biomacromolecules 2006, 7, 809–816. [Google Scholar] [CrossRef]
- Barbucci, R.; Pasqui, D.; Giani, G.; De Cagna, M.; Fini, M.; Giardino, R.; Atrei, A. A novel strategy for engineering hydrogels with ferromagnetic nanoparticles as crosslinkers of the polymer chains. Potential applications as a targeted drug delivery system. Soft Matter 2011, 7, 5558–5565. [Google Scholar] [CrossRef]
- Messing, R.; Frickel, N.; Belkoura, L.; Strey, R.; Rahn, H.; Odenbach, S.; Schmidt, A.M. Cobalt ferrite nanoparticles as multifunctional cross-linkers in PAAm ferrohydrogels. Macromolecules 2011, 44, 2990–2999. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Ghosh, S.; Remanan, S.; Ghosh, S.K.; Das, N.C. In-situ synthesis of magnetic nanoparticle immobilized heterogeneous catalyst through mussel mimetic approach for the efficient removal of water pollutants. Colloid Interface Sci. Commun. 2019, 33, 100218. [Google Scholar] [CrossRef]
- Filipcsei, G.; Csetneki, I.; Szilagyi, A.; Zrinyi, M. Magnetic field-responsive smart polymer composites. Adv. Polym. Sci. 2007, 137–189. [Google Scholar]
- Wang, Y.; Li, B.; Zhou, Y.; Jia, D. Chitosan-induced synthesis of magnetite nanoparticles via iron ions assembly. Polym. Adv. Technol. 2008, 19, 1256–1261. [Google Scholar] [CrossRef]
- Sapir, Y.; Cohen, S.; Friedman, G.; Polyak, B. The promotion of in vitro vessel-like organization of endothelial cells in magnetically responsive alginate scaffolds. Biomaterials 2012, 33, 4100–4109. [Google Scholar] [CrossRef] [Green Version]
- Shamim, N.; Hong, L.; Hidajat, K.; Uddin, M. Thermosensitive polymer (N-isopropylacrylamide) coated nanomagnetic particles: Preparation and characterization. Colloids Surf. B Biointerfaces 2007, 55, 51–58. [Google Scholar] [CrossRef]
- Rego, G.N.; Nucci, M.P.; Mamani, J.B.; Oliveira, F.A.; Marti, L.C.; Filgueiras, I.S.; Ferreira, J.M.; Real, C.C.; de Paula Faria, D.; Espinha, P.L.; et al. Therapeutic efficiency of multiple applications of magnetic hyperthermia technique in glioblastoma using aminosilane coated iron oxide nanoparticles: In vitro and in vivo study. Int. J. Mol. Sci. 2020, 21, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Tomar, R.; Chakraverty, S.; Sharma, D. Effect of manganese doping on the hyperthermic profile of ferrite nanoparticles using response surface methodology. RSC Adv. 2021, 11, 16942–16954. [Google Scholar] [CrossRef]
- Dhiman, N.; Shagaghi, N.; Bhave, M.; Sumer, H.; Kingshott, P.; Rath, S.N. Indirect co-culture of lung carcinoma cells with hyperthermia-treated mesenchymal stem cells influences tumor spheroid growth in a collagen-based 3-dimensional microfluidic model. Cytotherapy 2021, 23, 25–36. [Google Scholar] [CrossRef]
- Senturk, F.; Cakmak, S.; Kocum, I.C.; Gumusderelioglu, M.; Ozturk, G.G. GRGDS-conjugated and curcumin-loaded magnetic polymeric nanoparticles for the hyperthermia treatment of glioblastoma cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126648. [Google Scholar] [CrossRef]
- Chandunika, R.; Vijayaraghavan, R.; Sahu, N.K. Magnetic hyperthermia application of MnFe2O4 nanostructures processed through solvents with the varying boiling point. Mater. Res. Express 2020, 7, 064002. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, D. (Carboxymethyl-stevioside)-coated magnetic dots for enhanced magnetic hyperthermia and improved glioblastoma treatment. Colloids Surf. B Biointerfaces 2021, 205, 111870. [Google Scholar] [CrossRef]
- Frenster, J.D.; Desai, S.; Placantonakis, D.G. In vitro evidence for glioblastoma cell death in temperatures found in the penumbra of laser-ablated tumors. Int. J. Hyperth. 2020, 37, 20–26. [Google Scholar] [CrossRef]
- Lao, L.; Ramanujan, R. Magnetic and hydrogel composite materials for hyperthermia applications. J. Mater. Sci. Mater. Med. 2004, 15, 1061–1064. [Google Scholar] [CrossRef]
- Ang, K.; Venkatraman, S.; Ramanujan, R. Magnetic PNIPA hydrogels for hyperthermia applications in cancer therapy. Mater. Sci. Eng. C 2007, 27, 347–351. [Google Scholar] [CrossRef]
- Meenach, S.A.; Hilt, J.Z.; Anderson, K.W. Poly (ethylene glycol)-based magnetic hydrogel nanocomposites for hyperthermia cancer therapy. Acta Biomater. 2010, 6, 1039–1046. [Google Scholar] [CrossRef]
- Meenach, S.A.; Otu, C.G.; Anderson, K.W.; Hilt, J.Z. Controlled synergistic delivery of paclitaxel and heat from poly (β-amino ester)/iron oxide-based hydrogel nanocomposites. Int. J. Pharm. 2012, 427, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, L.; Song, L.; Ma, M.; Gu, N.; Zhang, Y. Enhanced tumor synergistic therapy by injectable magnetic hydrogel mediated generation of hyperthermia and highly toxic reactive oxygen species. ACS Nano 2019, 13, 14013–14023. [Google Scholar] [CrossRef]
- Chen, B.; Xing, J.; Li, M.; Liu, Y.; Ji, M. DOX@ Ferumoxytol-Medical Chitosan as magnetic hydrogel therapeutic system for effective magnetic hyperthermia and chemotherapy in vitro. Colloids Surf. B Biointerfaces 2020, 190, 110896. [Google Scholar] [CrossRef] [PubMed]
- Salloum, M.; Ma, R.; Weeks, D.; Zhu, L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperth. 2008, 24, 337–345. [Google Scholar] [CrossRef]
- Qian, K.-Y.; Song, Y.; Yan, X.; Dong, L.; Xue, J.; Xu, Y.; Wang, B.; Cao, B.; Hou, Q.; Peng, W. Injectable ferrimagnetic silk fibroin hydrogel for magnetic hyperthermia ablation of deep tumor. Biomaterials 2020, 259, 120299. [Google Scholar] [CrossRef]
- Le Renard, P.-E.; Jordan, O.; Faes, A.; Petri-Fink, A.; Hofmann, H.; Ruefenacht, D.; Bosman, F.; Buchegger, F.; Doelker, E. The in vivo performance of magnetic particle-loaded injectable, in situ gelling, carriers for the delivery of local hyperthermia. Biomaterials 2010, 31, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Le Renard, P.-E.; Lortz, R.; Senatore, C.; Rapin, J.-P.; Buchegger, F.; Petri-Fink, A.; Hofmann, H.; Doelker, E.; Jordan, O. Magnetic and in vitro heating properties of implants formed in situ from injectable formulations and containing superparamagnetic iron oxide nanoparticles (SPIONs) embedded in silica microparticles for magnetically induced local hyperthermia. J. Magn. Magn. Mater. 2011, 323, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, S.; Maity, T.; Mondal, S.; Das, P.; Das, N.C. Starch functionalized biodegradable semi-IPN as a pH-tunable controlled release platform for memantine. Int. J. Biol. Macromol. 2017, 95, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug Deliv. 2009, 6, 165–176. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Synthesis of Mussel Inspired Polydopamine Coated Halloysite Nanotubes Based Semi-IPN: An Approach to Fine Tuning in Drug Release and Mechanical Toughening. In Proceedings of the Macromolecular Symposia; Wiley: Hoboken, NJ, USA, 2018; p. 1800076. [Google Scholar] [CrossRef]
- Ganguly, S.; Maity, P.P.; Mondal, S.; Das, P.; Bhawal, P.; Dhara, S.; Das, N.C. Polysaccharide and poly (methacrylic acid) based biodegradable elastomeric biocompatible semi-IPN hydrogel for controlled drug delivery. Mater. Sci. Eng. C 2018, 92, 34–51. [Google Scholar] [CrossRef]
- Ganguly, S. Preparation/processing of polymer-graphene composites by different techniques. In Polymer Nanocomposites Containing Graphene; Elsevier: New York, NY, USA, 2022; pp. 45–74. [Google Scholar] [CrossRef]
- Falk, B.; Garramone, S.; Shivkumar, S. Diffusion coefficient of paracetamol in a chitosan hydrogel. Mater. Lett. 2004, 58, 3261–3265. [Google Scholar] [CrossRef]
- Lee, P.I. Kinetics of drug release from hydrogel matrices. J. Control. Release 1985, 2, 277–288. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Agarwal, T.; Maity, P.; Ghosh, S.; Choudhary, S.; Gangopadhyay, S.; Maiti, T.K.; Dhara, S.; Banerjee, S. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater. Chem. Phys. 2019, 237, 121860. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Das, S.; Ghorai, U.; Bose, M.; Ghosh, S.; Mondal, M.; Das, A.K.; Banerjee, S.; Das, N.C. Microwave assisted green synthesis of Zwitterionic photolumenescent N-doped carbon dots: An efficient ‘on-off’chemosensor for tracer Cr (+6) considering the inner filter effect and nano drug-delivery vector. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123604. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Prabaharan, M.; Mano, J.F. Stimuli-responsive hydrogels based on polysaccharides incorporated with thermo-responsive polymers as novel biomaterials. Macromol. Biosci. 2006, 6, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, N.C. Water uptake kinetics and control release of agrochemical fertilizers from nanoclay-assisted semi-interpenetrating sodium acrylate-based hydrogel. Polym.-Plast. Technol. Eng. 2017, 56, 744–761. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Hu, S.-H.; Liu, K.-H.; Liu, D.-M.; Chen, S.-Y. Preparation and characterization of smart magnetic hydrogels and its use for drug release. J. Magn. Magn. Mater. 2006, 304, e397–e399. [Google Scholar] [CrossRef]
- Hernández, R.; Sacristán, J.; Asín, L.; Torres, T.; Ibarra, M.; Goya, G.; Mijangos, C. Magnetic hydrogels derived from polysaccharides with improved specific power absorption: Potential devices for remotely triggered drug delivery. J. Phys. Chem. B 2010, 114, 12002–12007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.; Raju, K.M. Magnetic and electric responsive hydrogel–magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2011, 122, 1364–1375. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, M.; Chen, J.; Gao, C.; Gong, Q. A novel magnetic triple-responsive composite semi-IPN hydrogels for targeted and controlled drug delivery. Eur. Polym. J. 2012, 48, 1734–1744. [Google Scholar] [CrossRef]
- Sayadnia, S.; Arkan, E.; Jahanban-Esfahlan, R.; Sayadnia, S.; Jaymand, M. Tragacanth gum-based pH-responsive magnetic hydrogels for “smart” chemo/hyperthermia therapy of solid tumors. Polym. Adv. Technol. 2021, 32, 262–271. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, N.; Xiao, Y.; Huang, R.; Li, Y.; Wu, L. Hybrid Magnetic Hydrogels Used as Artificial Marine Animals for Noncontact Cleaning. ACS Appl. Polym. Mater. 2021, 3, 1182–1189. [Google Scholar] [CrossRef]
- Dai, G.; Sun, L.; Xu, J.; Zhao, G.; Tan, Z.; Wang, C.; Sun, X.; Xu, K.; Zhong, W. Catechol–metal coordination-mediated nanocomposite hydrogels for on-demand drug delivery and efficacious combination therapy. Acta Biomater. 2021, 21, 958. [Google Scholar] [CrossRef] [PubMed]










| Hydrogel Matrix | MNPs | Concentration | Method | Ref. |
|---|---|---|---|---|
| Chitosan | Fe3O4 | 11.1–13.6 wt% | In situ | [117] |
| Alginate/PNIPAM | γ-Fe2O3 | - | In situ | [106] |
| PAAm-GA | Fe3O4 | 8.3–14.04 wt% | In situ | [104] |
| Fibrin | Fe3O4 | 2.5 mg/mL | Blending | [60] |
| Dextran | CoFe2O4 | 2.5–15 wt% | Blending | [92] |
| Alginate | FePt | 8 wt% | Blending | [118] |
| PNIPAM | CoPt | 1 wt% | Blending | [91] |
| PAAm | CoFe2O4 | 2 wt% | Grafting onto | [114] |
| NIPAM | Fe3O4 | <50% | Grafting onto | [119] |
| NIPAM | γ-Fe2O3 | ~50% | Grafting onto | [119] |
| CMC | CoFe2O4 | 1.5 wt% | Grafting onto | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, S.; Margel, S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers 2021, 13, 4259. https://doi.org/10.3390/polym13234259
Ganguly S, Margel S. Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers. 2021; 13(23):4259. https://doi.org/10.3390/polym13234259
Chicago/Turabian StyleGanguly, Sayan, and Shlomo Margel. 2021. "Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery" Polymers 13, no. 23: 4259. https://doi.org/10.3390/polym13234259
APA StyleGanguly, S., & Margel, S. (2021). Design of Magnetic Hydrogels for Hyperthermia and Drug Delivery. Polymers, 13(23), 4259. https://doi.org/10.3390/polym13234259







