Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Investigation Method
2.2. Biosorbent Preparation and Characterization
2.3. Experimental Design
2.4. Biosorption Kinetics
3. Results and Discussion
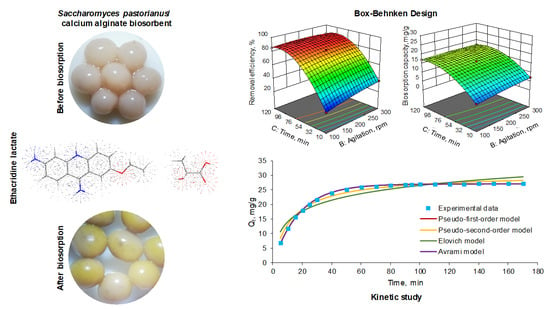
3.1. Biosorbent Preparation and Characterization
3.2. Box–Behnken Design and Model Validation
3.2.1. Box–Behnken Design
3.2.2. Models Validation
3.3. Biosorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: A review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Vinayagam, V.; Murugan, S.; Kumaresan, R.; Narayanan, M.; Sillanpää, M.; Viet N Vo, D.; Kushwaha, O.S.; Jenis, P.; Potdar, P.; Gadiya, S. Sustainable adsorbents for the removal of pharmaceuticals from wastewater: A review. Chemosphere 2022, 300, 134597. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- Smaali, A.; Berkani, M.; Merouane, F.; Le, V.T.; Vasseghian, Y.; Rahim, N.; Kouachi, M. Photocatalytic-persulfate- oxidation for diclofenac removal from aqueous solutions: Modeling, optimization and biotoxicity test assessment. Chemosphere 2021, 266, 129158. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Nguyen, D.T.C.; Le, H.T.N.; Vo, D.-V.N.; Nanda, S.; Nguyen, T.D. Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J. Environ. Sci. 2020, 93, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Madhura, L.; Singh, S.; Kanchi, S.; Sabela, M.; Bisetty, K.; Inamuddin. Nanotechnology-based water quality management for wastewater treatment. Environ. Chem. Lett. 2019, 17, 65–121. [Google Scholar] [CrossRef]
- Femina Carolin, C.; Senthil Kumar, P.; Janet Joshiba, G.; Vinoth Kumar, V. Analysis and removal of pharmaceutical residues from wastewater using membrane bioreactors: A review. Environ. Chem. Lett. 2021, 19, 329–343. [Google Scholar] [CrossRef]
- Sharma, V.K.; Jinadatha, C.; Lichtfouse, E.; Decroly, E.; van Helden, J.; Choi, H.; Chatterjee, P. COVID-19 epidemiologic surveillance using wastewater. Environ. Chem. Lett. 2021, 19, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Hameed, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Wang, M.; Hu, T.; Wang, Z. Enhancement with physicochemical and biological treatments in the removal of pharmaceutically active compounds during sewage sludge anaerobic digestion processes. Chem. Eng. J. 2017, 316, 361–369. [Google Scholar] [CrossRef]
- Marques, S.C.R.; Marcuzzo, J.M.; Baldan, M.R.; Mestre, A.S.; Carvalho, A.P. Pharmaceuticals removal by activated carbons: Role of morphology on cyclic thermal regeneration. Chem. Eng. J. 2017, 321, 233–244. [Google Scholar] [CrossRef]
- Vrinceanu, N.; Hlihor, R.M.; Simion, A.I.; Rusu, L.; Fekete-Kertész, I.; Barka, N.; Favier, L. New Evidence of the Enhanced Elimination of a Persistent Drug Used as a Lipid Absorption Inhibitor by Advanced Oxidation with UV-A and Nanosized Catalysts. Catalysts 2019, 9, 761. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Blaga, A.-C.; Harja, M. Encapsulation of Saccharomyces pastorianus Residual Biomass in Calcium Alginate Matrix with Insights in Ethacridine Lactate Biosorption. Polymers 2022, 14, 170. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Suceveanu, E.M.; Simion, A.-I.; Dediu Botezatu, A.V.; Istrate, B.; Doroftei, I. Eco-friendly biosorbents based on microbial biomass and natural polymers: Synthesis, characterization and application for the removal of drugs and dyes from aqueous solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef] [PubMed]
- Seid, L.; Lakhdari, D.; Berkani, M.; Belgherbi, O.; Chouder, D.; Vasseghian, Y.; Lakhdari, N. High-efficiency electrochemical degradation of phenol in aqueous solutions using Ni-PPy and Cu-PPy composite materials. J. Hazard. Mater. 2022, 423, 126986. [Google Scholar] [CrossRef]
- Zhang, Z.; Ouyang, Z.; Yang, J.; Liu, Y.; Yang, C.; Dang, Z. High mineral adsorption of glyphosate versus diethyl phthalate and tetracycline, during visible light photodegradation with goethite and oxalate. Environ. Chem. Lett. 2019, 17, 1421–1428. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E. Saccharomyces pastorianus immobilized on brewer’s spent grain in continuous system for lead ion biosorption. Int. Biodeterior. Biodegrad. 2014, 96, 191–197. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Dhankhar, R.; Hooda, A. Fungal biosorption—An alternative to meet the challenges of heavy metal pollution in aqueous solutions. Environ. Technol. 2011, 32, 467–491. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.M.; Șuteu, D.; Harja, M. Application of Saccharomyces cerevisiae/calcium alginate composite beads for cephalexin antibiotic biosorption from aqueous solutions. Materials 2021, 14, 4728. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Dediu Botezatu, A.V.; Harja, M. Biosorptive Removal of Ethacridine Lactate from Aqueous Solutions by Saccharomyces pastorianus Residual Biomass/Calcium Alginate Composite Beads: Fixed-Bed Column Study. Materials 2022, 15, 4657. [Google Scholar] [CrossRef]
- Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Istrate, B.; Harja, M. Biosorption Potential of Microbial and Residual Biomass of Saccharomyces pastorianus Immobilized in Calcium Alginate Matrix for Pharmaceuticals Removal from Aqueous Solutions. Polymers 2022, 14, 2855. [Google Scholar] [CrossRef]
- Yasin, S.; Sun, D.; Memon, H.; Zhu, F.; Jian, H.; Bin, Y.; Mingbo, M.; Hussain, M. Optimization of Mechanical and Thermal Properties of iPP and LMPP Blend Fibres by Surface Response Methodology. Polymers 2018, 10, 1135. [Google Scholar] [CrossRef]
- Assadian, F.; Niazi, A.; Ramezani, M. Response Surface Modeling and Optimization of Effective Parameters for Zn(II) Removal From Aqueous Solution Using Gracilaria Corticata. J. Chem. Health Risks 2020, 10, 213–224. [Google Scholar] [CrossRef]
- Alam, Z.; Muyibi, S.A.; Toramae, J. Statistical optimization of adsorption processes for removal of 2,4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. J. Environ. Sci. 2007, 19, 674–677. [Google Scholar] [CrossRef]
- Krishna, D.; Padma Sree, R. Response surface modeling and optimization of chromium (VI) removal from aqueous solution using borasus flabellifer coir powder. Int. J. Appl. Sci. Eng. 2013, 11, 213–226. [Google Scholar]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Ghorbani, A.; Mesgari, S.; Nazarfakhari, M.; Fakhariyan, A. Optimization of Effective Parameters for Cd (II) Removal from Aqueous Solutions by Red Mud Using Design of Experimental (Box-Behnken). J. Basic. Appl. Sci. Res. 2013, 3, 406–412. [Google Scholar]
- Erdem, F.; Ergun, M. Application of Response Surface Methodology for Removal of Remazol Yellow (RR) by Immobilised S. cerevisiae on Pumice Stone. Iran. J. Chem. Chem. Eng. (IJCCE) 2020, 39, 175–187. [Google Scholar] [CrossRef]
- Yasin, S.; Curti, M.; Behary, N.; Perwuelz, A.; Giraud, S.; Rovero, G.; Guan, J.; Chen, G. Process optimization of eco-friendly flame retardant finish for cotton fabric: A Response Surface Methodology approach. Surf. Rev. Lett. 2017, 24, 1750114. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, J.; Huang, H.; Xiong, S.; Gao, J.; Zhang, J.; Feng, S. Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2019, 175, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Choińska-Pulit, A.; Sobolczyk-Bednarek, J.; Łaba, W. Optimization of copper, lead and cadmium biosorption onto newly isolated bacterium using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2018, 149, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Can-Terzi, B.; Goren, A.Y.; Okten, H.E.; Sofuoglu, S.C. Biosorption of methylene blue from water by live Lemna minor. Environ. Technol. Innov. 2021, 22, 101432. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A. The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. React. Kinet. Mech. Catal. 2015, 116, 261–271. [Google Scholar] [CrossRef]
- Zahoor, M. Effect of Agitation Speed on Adsorption of Imidacloprid on Activated Carbon. J. Chem. Soc. Pak 2011, 33, 305–312. [Google Scholar]
- Omri, A.; Wali, A.; Benzina, M. Adsorption of bentazon on activated carbon prepared from Lawsonia inermis wood: Equilibrium, kinetic and thermodynamic studies. Arab. J. Chem. 2016, 9, S1729–S1739. [Google Scholar] [CrossRef]
- Ihsanullah; Asmaly, H.A.; Saleh, T.A.; Laoui, T.; Gupta, V.K.; Atieh, M.A. Enhanced adsorption of phenols from liquids by aluminum oxide/carbon nanotubes: Comprehensive study from synthesis to surface properties. J. Mol. Liq. 2015, 206, 176–182. [Google Scholar] [CrossRef]
- Kumar, P.; Maurya, A.; Garg, S.; Yadav, A.; Mishra, V.; Sharma, R.S. Dead biomass of Morganella morganii acts as an efficient adsorbent to remove Pb(II) from aqueous solution in different aeration–agitation and pH conditions. SN Appl. Sci. 2020, 2, 1258. [Google Scholar] [CrossRef]
- Jing, H.; Huang, X.; Du, X.; Mo, L.; Ma, C.; Wang, H. Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr. Polym. 2022, 278, 118993. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, S.; Dilbaghi, N.; Bhanjana, G.; Chopra, M.; Kaur, H.; Kumar, R.; Manuja, B.; Singh, S.; Yadav, S. Quinapyramine sulfate-loaded sodium alginate nanoparticles show enhanced trypanocidal activity. Nanomedicine 2014, 9, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Jiron, G.; Leal, D.; Matsuhiro, B.; Osorio-Roman, I.O. Vibrational spectroscopy and density functional theory calculations of poly-D-mannuronate and heteropolymeric fractions from sodium alginate. J. Raman Spectrosc. 2011, 42, 870–878. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Merijs Meri, R.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef]
- Moreno Rivas, S.C.; Armenta Corral, R.I.; Frasquillo Félix, M.d.C.; Islas Rubio, A.R.; Vázquez Moreno, L.; Ramos-Clamont Montfort, G. Removal of cadmium from aqueous solutions by Saccharomyces cerevisiae–alginate system. Materials 2019, 12, 4128. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR method for the assessment of immobilization of active substances in the matrix of biomedical materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef]
- Jang, M.-H.; Kim, M.-S.; Han, M.; Kwak, D.-H. Experimental application of a zero-point charge based on pH as a simple indicator of microplastic particle aggregation. Chemosphere 2022, 299, 134388. [Google Scholar] [CrossRef]
- Bagheban Shahri, F.; Niazi, A. Synthesis of modified maghemite nanoparticles and its application for removal of Acridine Orange from aqueous solutions by using Box-Behnken design. J. Magn. Magn. Mater. 2015, 396, 318–326. [Google Scholar] [CrossRef]
- Sadrnia, A.; Orooji, Y.; Behmaneshfar, A.; Darabi, R.; Maghsoudlou Kamali, D.; Karimi-Maleh, H.; Opoku, F.; Govender, P.P. Developing a simple box–behnken experimental design on the removal of doxorubicin anticancer drug using Fe3O4/graphene nanoribbons adsorbent. Environ. Res. 2021, 200, 111522. [Google Scholar] [CrossRef]
- Babas, H.; Khachani, M.; Warad, I.; Ajebli, S.; Guessous, A.; Guenbour, A.; Safi, Z.; Berisha, A.; Bellaouchou, A.; Abdelkader, Z.; et al. Sofosbuvir adsorption onto activated carbon derived from argan shell residue: Optimization, kinetic, thermodynamic and theoretical approaches. J. Mol. Liq. 2022, 356, 119019. [Google Scholar] [CrossRef]
- Ajebli, S.; Kaichouh, G.; Khachani, M.; Babas, H.; El Karbane, M.; Warad, I.; Safi, Z.S.; Berisha, A.; Mehmeti, V.; Guenbour, A.; et al. The adsorption of Tenofovir in aqueous solution on activated carbon produced from maize cobs: Insights from experimental, molecular dynamics simulation, and DFT calculations. Chem. Phys. Lett. 2022, 801, 139676. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Knezevic, Z.; Mojovic, L.; Adnadjevic, B. Palm oil hydrolysis by lipase from Candida cylindracea immobilized on zeolite type Y. Enzym. Microb. Technol. 1998, 22, 275–280. [Google Scholar] [CrossRef]
- Piorkowska, E.; Galeski, A.; Haudin, J.-M. Critical assessment of overall crystallization kinetics theories and predictions. Prog. Polym. Sci. 2006, 31, 549–575. [Google Scholar] [CrossRef]
- George, R.; Sugunan, S. Kinetics of adsorption of lipase onto different mesoporous materials: Evaluation of Avrami model and leaching studies. J. Mol. Catal. B Enzym. 2014, 105, 26–32. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- dos Santos Lins, P.V.; Henrique, D.C.; Ide, A.H.; de Paiva e Silva Zanta, C.L.; Meili, L. Evaluation of caffeine adsorption by MgAl-LDH/biochar composite. Environ. Sci. Pollut. Res. 2019, 26, 31804–31811. [Google Scholar] [CrossRef]
- González-Fernández, L.A.; Medellín-Castillo, N.A.; Ocampo-Pérez, R.; Hernández-Mendoza, H.; Berber-Mendoza, M.S.; Aldama-Aguilera, C. Equilibrium and kinetic modelling of triclosan adsorption on Single-Walled Carbon Nanotubes. J. Environ. Chem. Eng. 2021, 9, 106382. [Google Scholar] [CrossRef]
- Altalhi, T.A.; Ibrahim, M.M.; Mersal, G.A.M.; Mahmoud, M.H.H.; Kumeria, T.; El-Desouky, M.G.; El-Bindary, A.A.; El-Bindary, M.A. Adsorption of doxorubicin hydrochloride onto thermally treated green adsorbent: Equilibrium, kinetic and thermodynamic studies. J. Mol. Struct. 2022, 1263, 133160. [Google Scholar] [CrossRef]
- Nasiri, A.; Rajabi, S.; Amiri, A.; Fattahizade, M.; Hasani, O.; Lalehzari, A.; Hashemi, M. Adsorption of tetracycline using CuCoFe2O4@Chitosan as a new and green magnetic nanohybrid adsorbent from aqueous solutions: Isotherm, kinetic and thermodynamic study. Arab. J. Chem. 2022, 15, 104014. [Google Scholar] [CrossRef]
- Smiljanić, D.; de Gennaro, B.; Daković, A.; Galzerano, B.; Germinario, C.; Izzo, F.; Rottinghaus, G.E.; Langella, A. Removal of non-steroidal anti-inflammatory drugs from water by zeolite-rich composites: The interference of inorganic anions on the ibuprofen and naproxen adsorption. J. Environ. Manag. 2021, 286, 112168. [Google Scholar] [CrossRef] [PubMed]
- Nezhadali, A.; Koushali, S.E.; Divsar, F. Synthesis of polypyrrole—chitosan magnetic nanocomposite for the removal of carbamazepine from wastewater: Adsorption isotherm and kinetic study. J. Environ. Chem. Eng. 2021, 9, 105648. [Google Scholar] [CrossRef]





| Factors Name | Factors Code | Factors Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Ethacridine lactate initial concentration, mg/L | A | 20 | 40 | 60 |
| Agitation speed, rpm | B | 100 | 200 | 300 |
| Biosorption time, min | C | 10 | 65 | 120 |
| Run | Independent Variables | EL Removal Efficiency, % | Biosorption Capacity, mg/g | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||||
| Coded Value | Actual Value | Coded Value | Actual Value | Coded Value | Actual Value | Experimental Value | Predicted Value | Experimental Value | Predicted Value | |
| 1 | −1 | 20 | −1 | 100 | 0 | 65 | 65.91 | 65.81 | 6.49 | 7.58 |
| 2 | 0 | 40 | 0 | 200 | 0 | 65 | 81.61 | 81.54 | 16.04 | 16.07 |
| 3 | 0 | 40 | 0 | 200 | 0 | 65 | 81.78 | 81.54 | 16.11 | 16.07 |
| 4 | −1 | 20 | 0 | 200 | −1 | 10 | 21.28 | 21.47 | 2.10 | 1.27 |
| 5 | +1 | 60 | 0 | 200 | −1 | 10 | 23.76 | 24.37 | 7.06 | 8.30 |
| 6 | +1 | 60 | −1 | 100 | 0 | 65 | 71.41 | 70.89 | 21.11 | 20.13 |
| 7 | 0 | 40 | 0 | 200 | 0 | 65 | 81.11 | 81.54 | 16.11 | 16.07 |
| 8 | 0 | 40 | −1 | 100 | −1 | 10 | 22.55 | 22.45 | 4.48 | 4.23 |
| 9 | 0 | 40 | +1 | 300 | −1 | 10 | 36.48 | 35.76 | 7.19 | 7.04 |
| 10 | −1 | 20 | 0 | 200 | +1 | 120 | 73.96 | 73.34 | 7.29 | 6.06 |
| 11 | 0 | 40 | 0 | 200 | 0 | 65 | 81.12 | 81.54 | 15.94 | 16.07 |
| 12 | +1 | 60 | +1 | 300 | 0 | 65 | 80.69 | 80.79 | 23.95 | 22.86 |
| 13 | 0 | 40 | +1 | 300 | +1 | 120 | 86.31 | 86.41 | 17.02 | 17.27 |
| 14 | 0 | 40 | −1 | 100 | +1 | 120 | 85.13 | 85.85 | 16.91 | 17.06 |
| 15 | +1 | 60 | 0 | 200 | +1 | 120 | 86.73 | 86.54 | 25.74 | 26.57 |
| 16 | 0 | 40 | 0 | 200 | 0 | 65 | 82.10 | 81.54 | 16.14 | 16.07 |
| 17 | −1 | 20 | +1 | 300 | 0 | 65 | 69.26 | 69.78 | 6.89 | 7.87 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Observation |
|---|---|---|---|---|---|---|
| Model | 9272.38 | 9 | 1030.26 | 2279.93 | <0.0001 | Highly significant |
| A | 129.54 | 1 | 129.54 | 286.67 | <0.0001 | Highly significant |
| B | 96.19 | 1 | 96.19 | 212.87 | <0.0001 | Highly significant |
| C | 6502.34 | 1 | 6502.34 | 14389.38 | <0.0001 | Highly significant |
| AB | 8.79 | 1 | 8.79 | 19.44 | 0.0031 | Insignificant |
| AC | 26.48 | 1 | 26.48 | 58.59 | 0.0001 | Significant |
| BC | 40.65 | 1 | 40.65 | 89.96 | <0.0001 | Highly significant |
| A2 | 266.50 | 1 | 266.50 | 589.74 | <0.0001 | Highly significant |
| B2 | 13.19 | 1 | 13.19 | 29.19 | 0.0010 | Significant |
| C2 | 2066.88 | 1 | 2066.88 | 4573.91 | <0.0001 | Highly significant |
| Residual | 3.16 | 7 | 0.4519 | |||
| Lack of Fit | 2.42 | 3 | 0.8081 | 4.37 | 0.0940 | Insignificant |
| Pure Error | 0.7390 | 4 | 0.1847 | |||
| Cor Total | 9275.55 | 16 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value | Observation |
|---|---|---|---|---|---|---|
| Model | 788.47 | 9 | 87.61 | 68.96 | <0.0001 | Highly significant |
| A | 379.27 | 1 | 379.27 | 298.55 | <0.0001 | Highly significant |
| B | 4.59 | 1 | 4.59 | 3.61 | 0.0990 | Insignificant |
| C | 266.05 | 1 | 266.05 | 209.42 | <0.0001 | Highly significant |
| AB | 1.49 | 1 | 1.49 | 1.18 | 0.3142 | Insignificant |
| AC | 45.45 | 1 | 45.45 | 35.78 | 0.0006 | Significant |
| BC | 1.70 | 1 | 1.70 | 1.34 | 0.2855 | Insignificant |
| A2 | 5.64 | 1 | 5.64 | 4.44 | 0.0732 | Insignificant |
| B2 | 0.3879 | 1 | 0.3879 | 0.3053 | 0.5978 | Insignificant |
| C2 | 80.22 | 1 | 80.22 | 63.14 | <0.0001 | Highly significant |
| Residual | 8.89 | 7 | 1.27 | |||
| Lack of Fit | 8.89 | 3 | 2.96 | 474.96 | <0.0001 | Highly significant |
| Pure Error | 0.0249 | 4 | 0.0062 | |||
| Cor Total | 797.36 | 16 |
| Kinetic Model | Pseudo-First-Order | Pseudo-Second-Order | Elovich | Avrami | |
|---|---|---|---|---|---|
| Kinetic Parameters | Qe | 26.9978 | 30.3847 | 26.9978 | |
| k1 | 0.0559 | ||||
| k2 | 0.0002 | ||||
| α | 6.3717 | ||||
| β | 0.1784 | ||||
| kAv | 0.8680 | ||||
| nAv | 0.0645 | ||||
| Statistical Error Function | RMSE | 0.1868 | 0.7605 | 1.7131 | 0.1868 |
| MPSD | 1.5637 | 5.6877 | 14.2008 | 1.6066 | |
| HYBRID | 0.2695 | 3.8475 | 21.4119 | 0.2845 | |
| Χ2 | 0.0525 | 0.6845 | 3.3903 | 0.0525 | |
| R2 | 0.9988 | 0.9817 | 0.9071 | 0.9988 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, L.; Grigoraș, C.-G.; Simion, A.-I.; Suceveanu, E.-M.; Schnakovszky, C.; Favier, L. Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies. Polymers 2022, 14, 3388. https://doi.org/10.3390/polym14163388
Rusu L, Grigoraș C-G, Simion A-I, Suceveanu E-M, Schnakovszky C, Favier L. Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies. Polymers. 2022; 14(16):3388. https://doi.org/10.3390/polym14163388
Chicago/Turabian StyleRusu, Lăcrămioara, Cristina-Gabriela Grigoraș, Andrei-Ionuț Simion, Elena-Mirela Suceveanu, Carol Schnakovszky, and Lidia Favier. 2022. "Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies" Polymers 14, no. 16: 3388. https://doi.org/10.3390/polym14163388
APA StyleRusu, L., Grigoraș, C.-G., Simion, A.-I., Suceveanu, E.-M., Schnakovszky, C., & Favier, L. (2022). Investigation into Biosorption of Pharmaceuticals from Aqueous Solutions by Biocomposite Material Based on Microbial Biomass and Natural Polymer: Process Variables Optimization and Kinetic Studies. Polymers, 14(16), 3388. https://doi.org/10.3390/polym14163388