Immunomodulatory and Antiprotozoal Potential of Fabricated Sesamum radiatum Oil/Polyvinylpyrrolidone/Au Polymeric Bionanocomposite Film
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Botanical Material
2.3. Cell Culture and Parasites
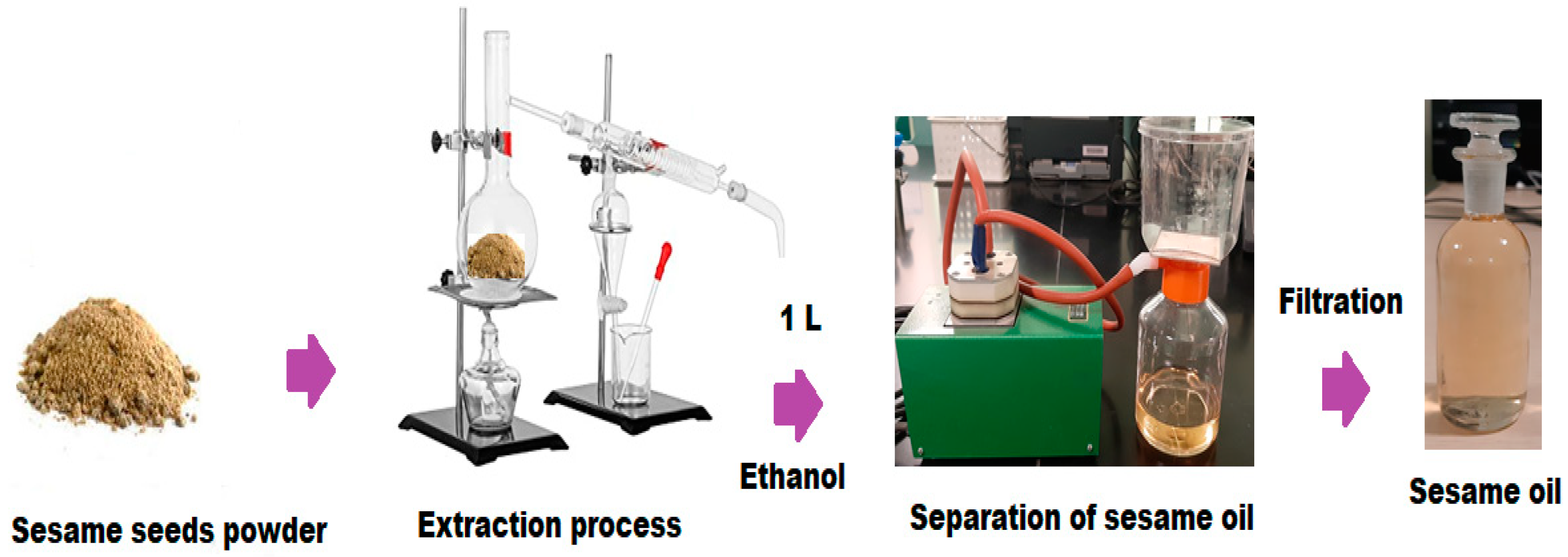
2.4. Extraction and Purification of Sesamum radiatum Oil
2.5. Synthesis of Gold Nanoparticles
2.6. Biogenic Synthesis of Sesame Oil/PVP/Au Bionanocomposite
2.7. GC-MS Analysis of Sesame Oil
2.8. Characterization of AuNPs and Sesame Oil/PVP/Au Bionanocomposite
2.9. Thermal Stability of Sesame Oil/PVP/Au Bionanocomposite
2.10. Anti-Inflammatory Effects of AuNPs and Bionanocomposite
2.11. Cell Viability Assay
2.12. Real-Time Polymerase Chain Reaction (PCR)
2.13. ELISA Measurements
2.14. Immunoblotting
2.15. In Vitro Antiprotozoal Activity of AuNPs and Bionanocomposite
2.16. Morphological Changes in Leishmania spp. and T. cruzi
2.17. Statistical Analysis
3. Results and Discussion
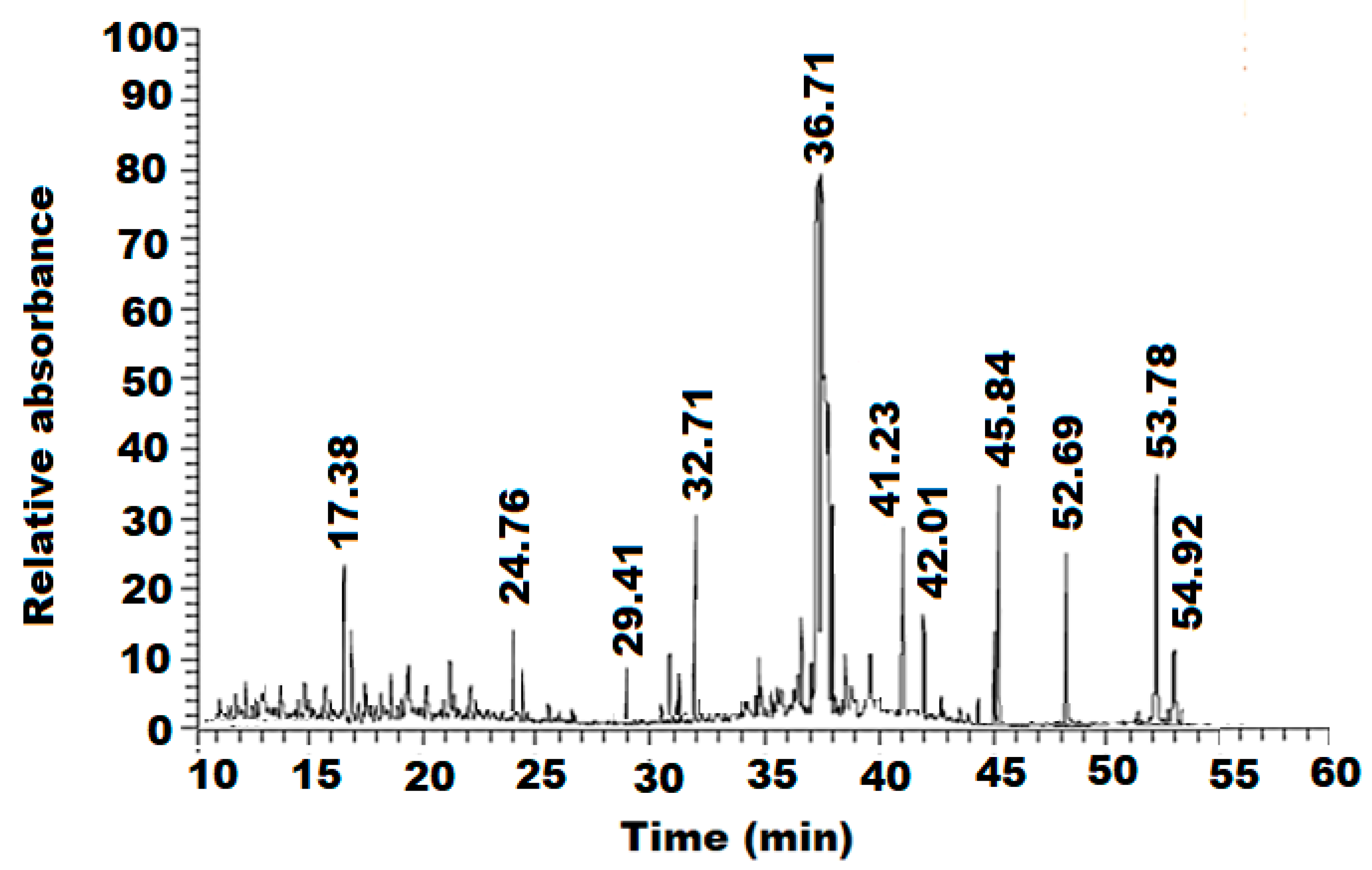
3.1. GC-MS Analysis of Sesame Oil
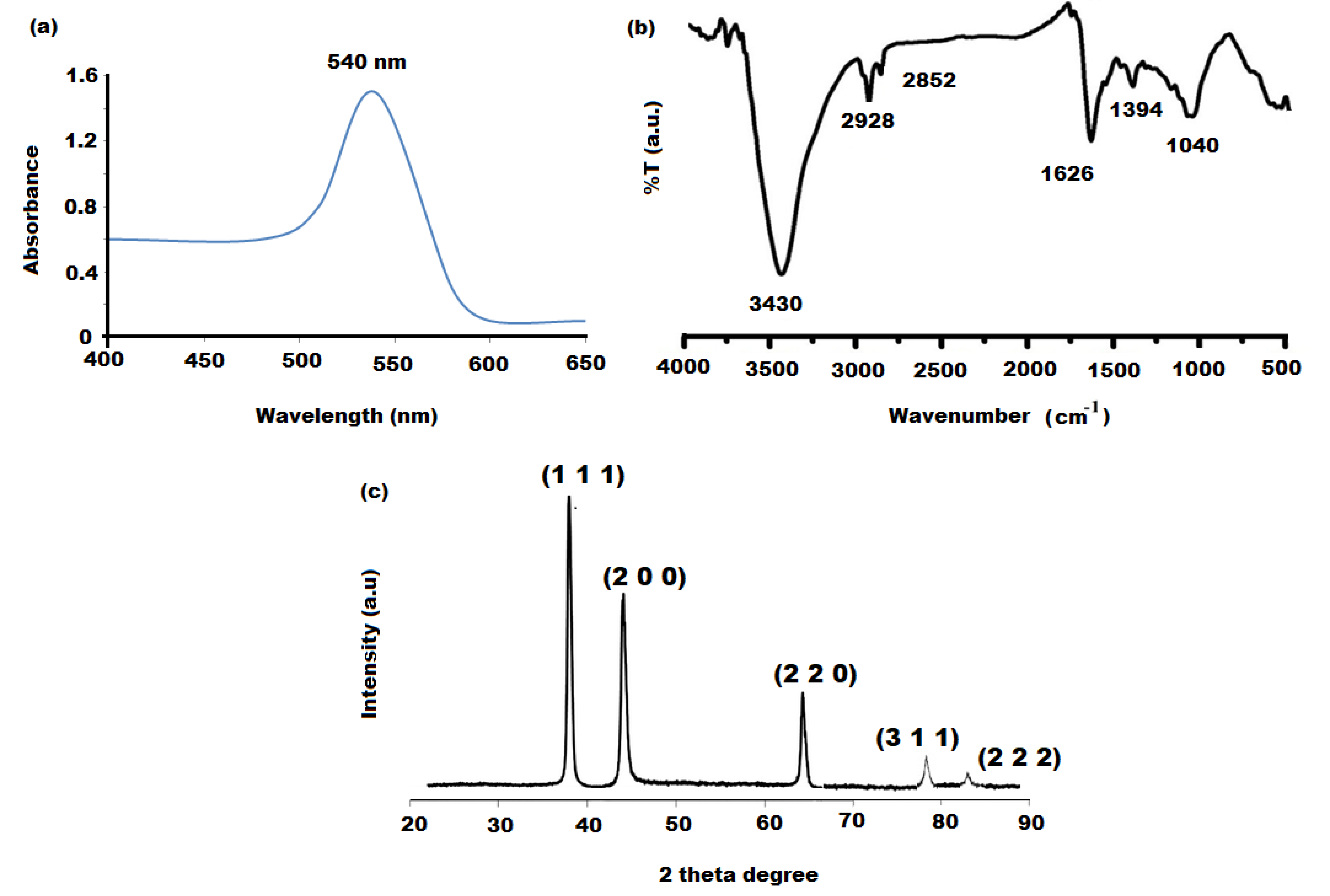
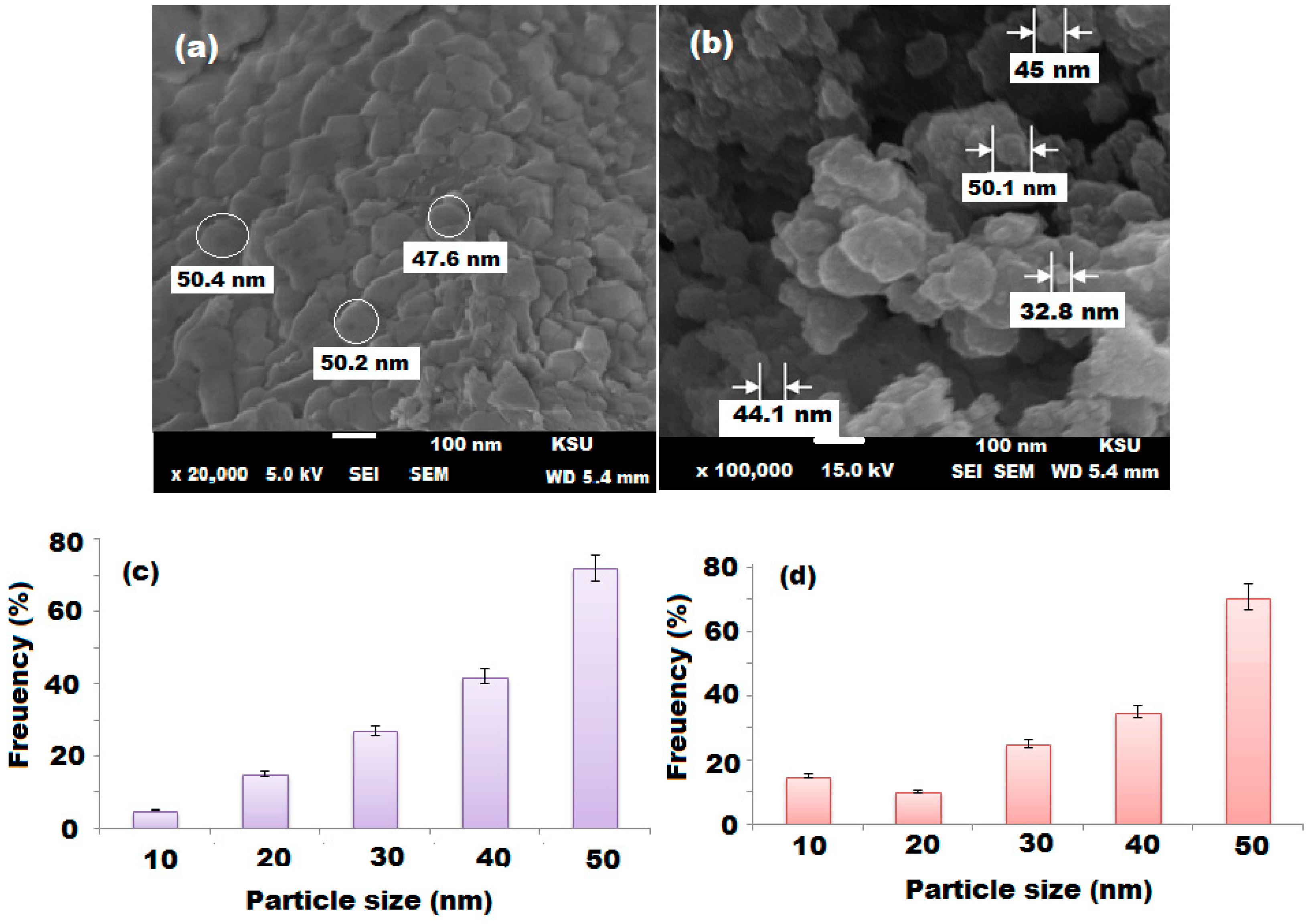
3.2. Characterization of Gold Nanoparticles (AuNPs)
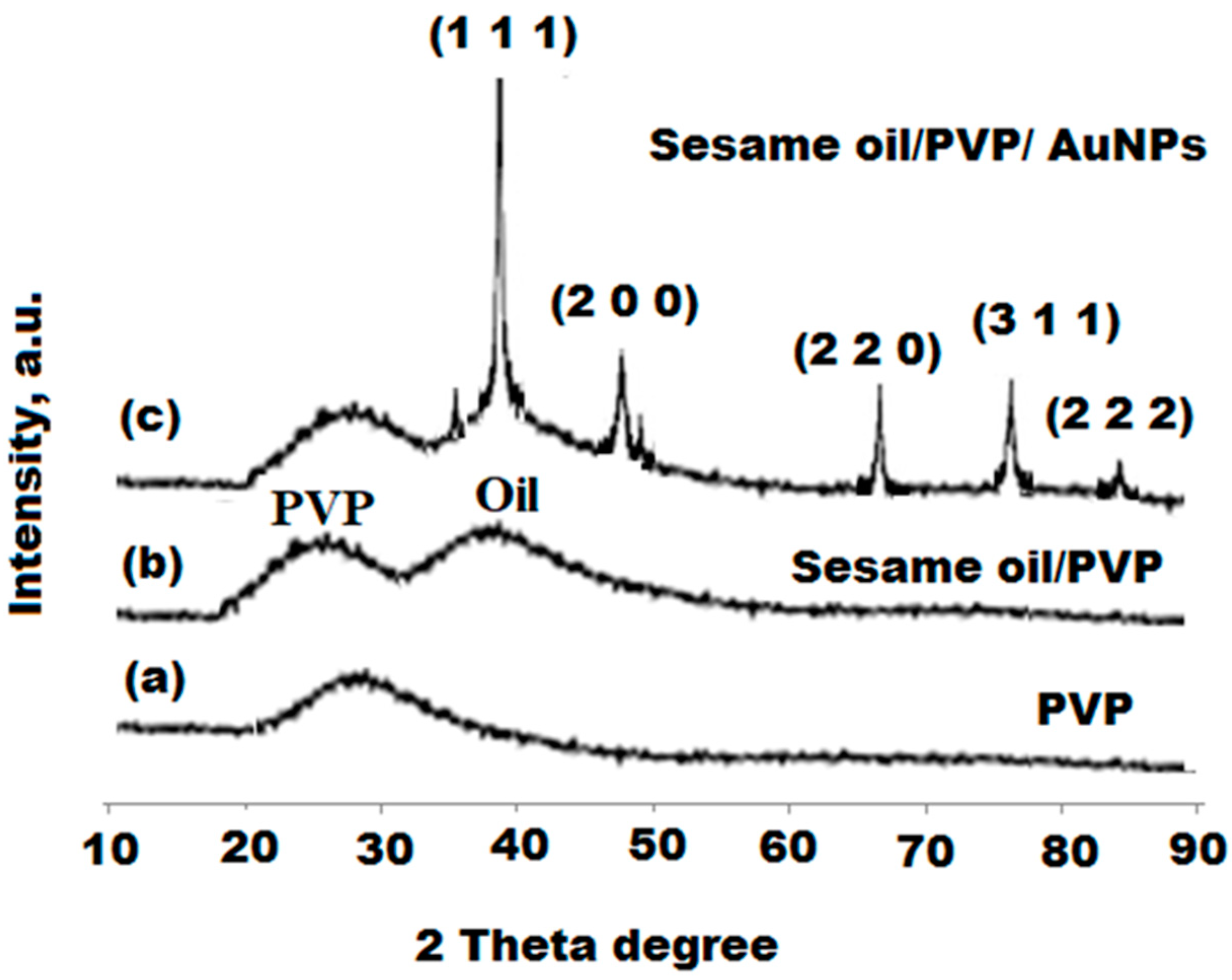
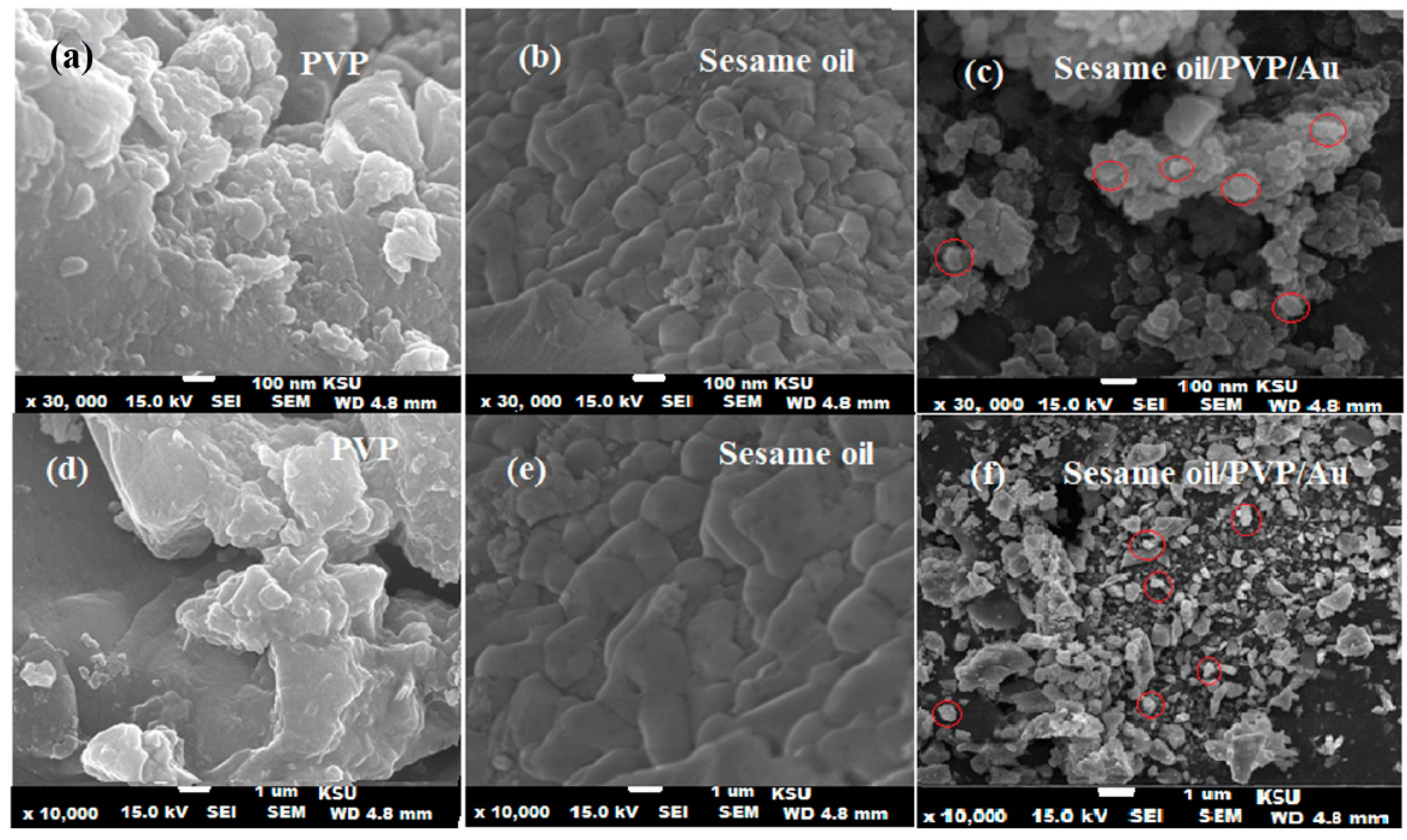
3.3. Characterization of Bionanocomposite
3.4. Thermal Stability
3.5. Immunomodulatory Activity
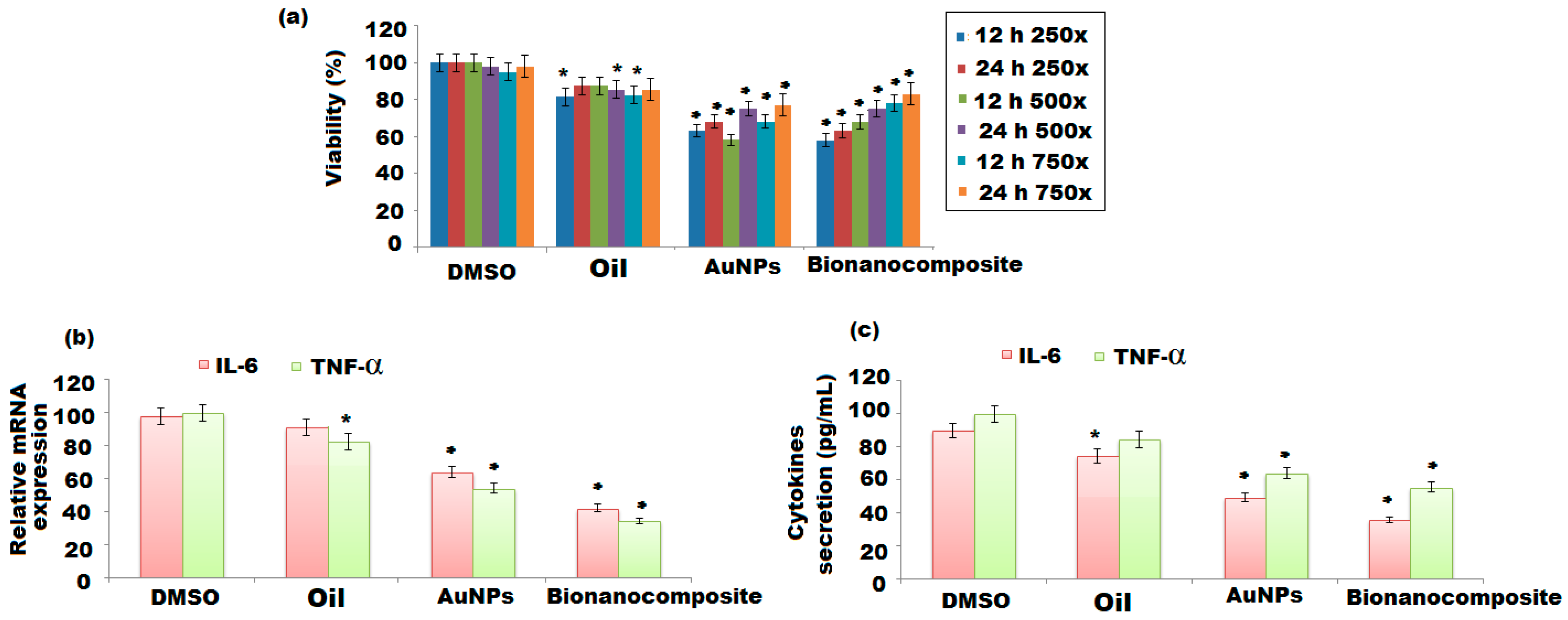
3.5.1. Effects of Nanomaterials on Cell Viability of BV-2 Cells
3.5.2. Effect of Nanomaterials on mRNA Expression, IL-6, and TNFα Secretion
3.5.3. Alteration of mRNA Expression and Secretion of IL-6 and TNFα by Nanomaterials after LPS Pretreatment
3.5.4. Nanomaterials’ Pretreatment Alteration of mRNA Expression and IL-6 and TNFα Secretion of BV-2 Cells Exposed to LPS
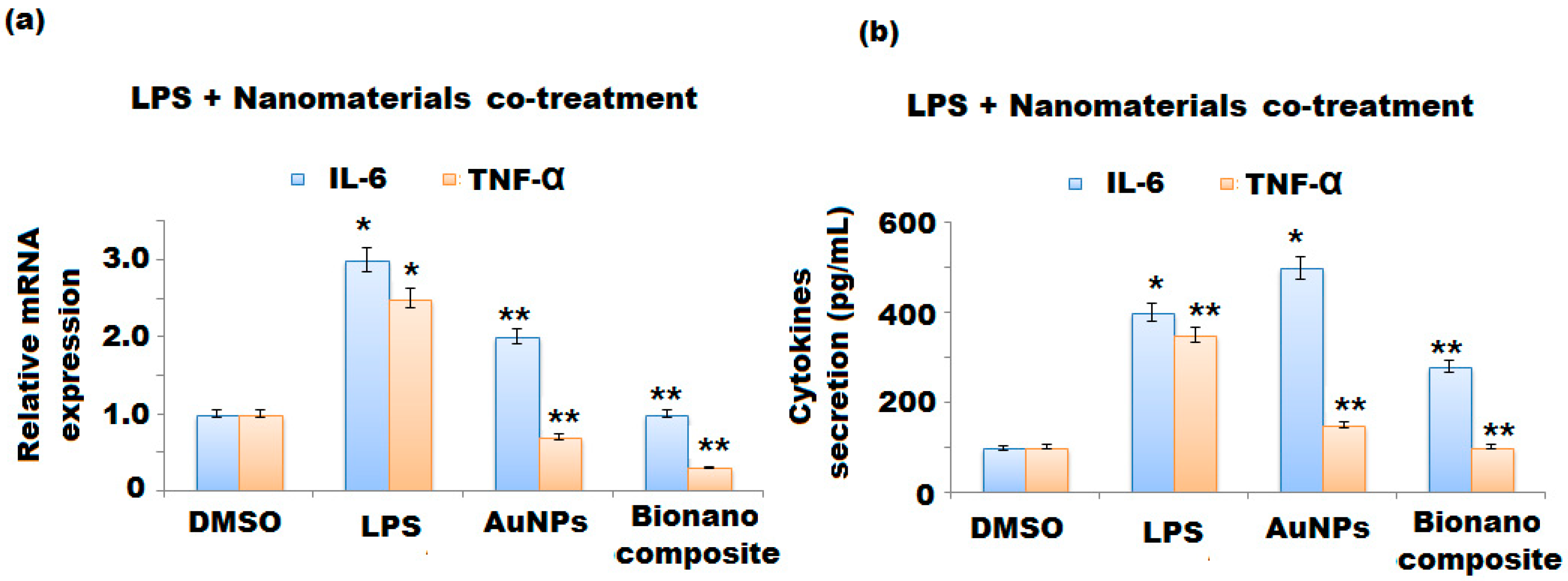
3.5.5. Co-Treatment of LPS and Nanomaterials’ Effect on the mRNA Expression and Secretion of IL-6 and TNFα of BV-2 Cells
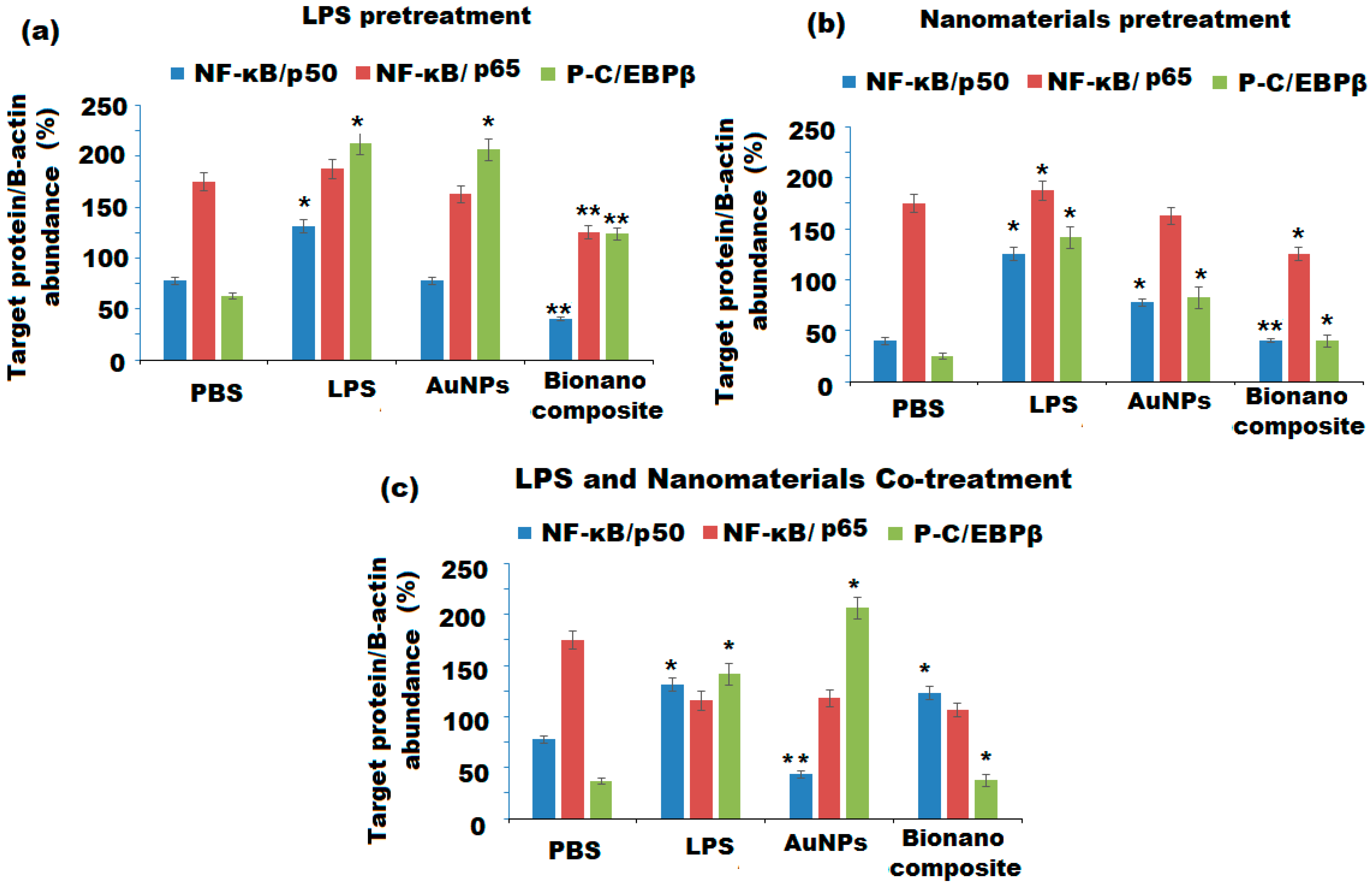
3.5.6. Nanomaterials’ Effect on the NF-κB and Phospho-C/EBPβ Pathways
3.6. In Vitro Antiprotozoal Activity of AuNPs and Bionanocomposite
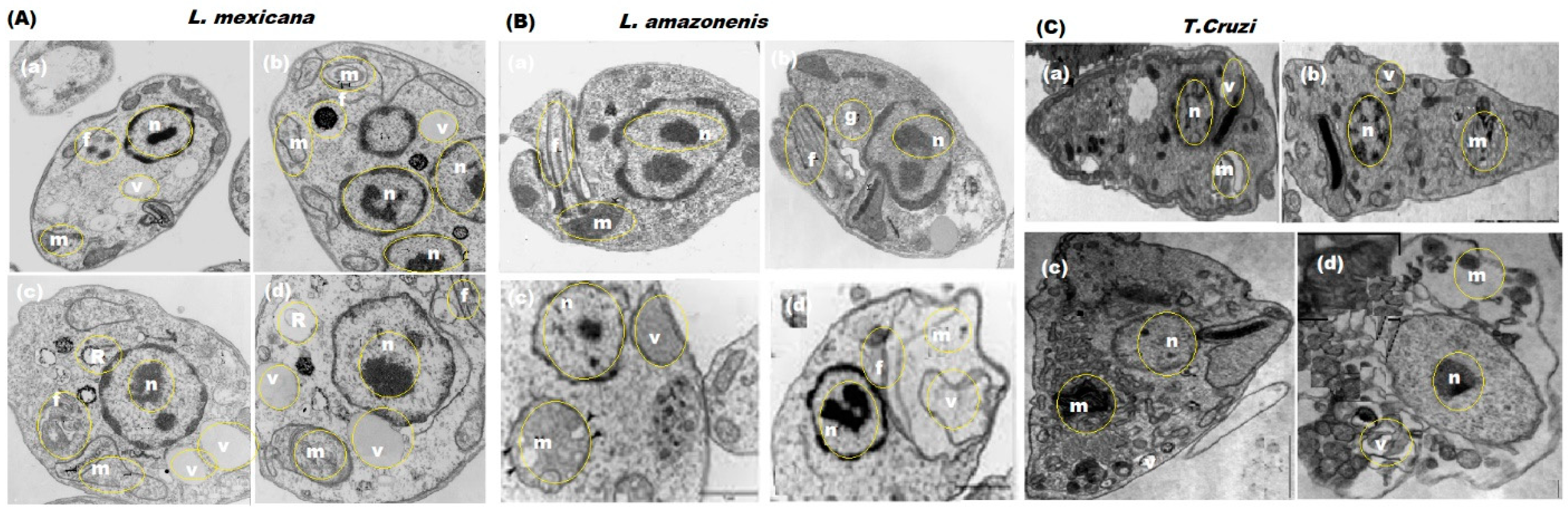
3.7. Morphological Changes in L. mexicana, L. amazonensis, and T. cruzi
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Condens. Matter Phys. 2017, 29, 203002. Available online: https://iopscience.iop.org/article/10.1088/1361-648X/aa60f3 (accessed on 15 December 2020). [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Wu, R.; Peng, H.; Zhu, J.J.; Jiang, L.P.; Liu, J. Attaching DNA to gold nanoparticles with a protein corona. Front. Chem. 2020, 8, 121. [Google Scholar] [CrossRef] [Green Version]
- Pan, R.; Jiang, Y.; Sun, L.; Wang, R.; Zhuang, K.; Zhao, Y.; Wang, H.; Ali, M.A.; Xu, H.; Man, C. Gold nanoparticle-based enhanced lateral flow immunoassay for detection of Cronobacter sakazakii in powdered infant formula. Int. J. Dairy Sci. 2018, 101, 3835–3843. [Google Scholar] [CrossRef] [Green Version]
- Laksee, S.; Sansanaphongpricha, K.; Puthong, S.; Sangphech, N.; Palaga, T.; Muangsin, N. New organic/inorganic nanohybrids of targeted pullulan derivative/gold nanoparticles for effective drug delivery systems. Int. J. Biol. Macromol. 2020, 162, 561–577. [Google Scholar] [CrossRef]
- Ali, S.; Ali, H.; Siddique, M.; Gulab, H.; Haleem, M.A.; Ali, J. Exploring the biosynthesized gold nanoparticles for their antibacterial potential and photocatalytic degradation of the toxic water wastes under solar light illumination. J. Mol. Struct. 2020, 1215, 128259. [Google Scholar] [CrossRef]
- Haddada, M.B.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Spadavecchia, J.; Morel, A.L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloid Surf. B Biointerfaces 2020, 189, 110855. [Google Scholar] [CrossRef]
- Pham, K.; Temerov, F.; Saarinen, J.J. Multicompound inverse opal structures with gold nanoparticles for visible light photocatalytic activity. Mater. Des. 2020, 194, 108886. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Mobaraki, F.; Momeni, M.; Yazdi, M.E.T.; Meshkat, Z.; Toosi, M.S.; Hosseini, S.M. Plant-derived synthesis and characterization of gold nanoparticles: Investigation of its antioxidant and anticancer activity against human testicular embryonic carcinoma stem cells. Proc. Biochem. 2021, 111, 167–177. [Google Scholar] [CrossRef]
- Liu, L.; Choi, S. Enhanced biophotoelectricity generation in cyanobacterial biophotovoltaics with intracellularly biosynthesized gold nanoparticles. J. Power Sources 2021, 506, 230251. [Google Scholar] [CrossRef]
- Souri, Z.; Alizadeh, S.; Nematollahi, D.; Mazloum-Ardakani, M.; Karami, A. A green and template-free electropolymerization of imipramine. The decoration of sponge-like polymer film with gold nanoparticles. J. Electroanal. Chem. 2021, 894, 115340. [Google Scholar] [CrossRef]
- Kausar, A. A review of high-performance polymer nanocomposites for packaging applications in electronics and food industries. J. Plast. Film Sheeting 2020, 36, 94–112. [Google Scholar] [CrossRef]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Orabi, H.E.; Bukhari, S.I.; Mahmoud, A.Z. Exploiting the potential of Moringa oleifera oil/polyvinyl chloride polymeric bionanocomposite film enriched with silver nanoparticles for antimicrobial activity. Int. J. Polym. Sci. 2019, 2019, 5678149. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; Amina, M.; Al Musayeib, N.M.; El-Tohamy, M.F.; Oraby, H.F.; Bukhari, S.I.; Moubayed, N.M.S. Prospective of green synthesized Oleum cumini oil/PVP/MgO bionanocomposite film for its antimicrobial, antioxidant and anticancer applications. J. Polym. Environ. 2020, 28, 2108–2124. [Google Scholar] [CrossRef]
- Oshodi, H.N.; Ogungbenle, M.O.; Oladimeji, A.A. Chemical composition, nutritionally valuable minerals and functional properties of benniseed (Sesamum radiatum), pearl millet (Pennisetum typhoides) and quinoa (Chenopodium quinoa) flours. Int. J. Food Sci. Nutr. 1999, 50, 325–331. [Google Scholar] [CrossRef]
- Quasem, J.M.; Mazahreh, A.S.; Abu-Alruz, K. Development of vegetable-based milk from decorticated sesame (Sesamum indicum). Am. J. Appl. Sci. 2009, 6, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, D.K.; Chavan, J.K.; Adsule, R.N.; Kadam, S.S. Sesame: World Oilseeds: History, Technology and Utilization; Van Nostrand Reinhold: New York, NY, USA, 2009; pp. 371–402. [Google Scholar]
- Xu-Yan, D.; Ping-Ping, L.; Fang, W.; Mu-lan, J.; Ying-Zhong, Z.; Guang-Ming, L.; Hong, C.; Yuan-Di, Z. The impact of processing on the profile of volatile compounds in sesame oil. Eur. J. Lipid Sci. Technol. 2012, 114, 277–286. [Google Scholar] [CrossRef]
- Shahidi, F.; Amarowicz, R.; Abou-Gharbia, H.A.; Shehata, A.A.Y. Endogenous antioxidants and stability of sesame oil as affected by processing and storage. J. Am. Oil Chem. Soc. 1997, 74, 143–148. [Google Scholar] [CrossRef]
- Alshahrani, S.; Al Sreaya, A.A.; Mashyakhi, M.Y.; Alqahtani, S.; Sivakumar, S.M.; Alhazmi, H.A.; Rehman, Z.; Alam, F. Chemical characterization and antibacterial efficacy of Saudi sesame oil against human pathogenic bacteria. Environ. Conserv. J. 2020, 21, 19–29. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Conto, D.; dos Santos, V.; Zattera, A.J.; Santana, R.M.C. Swelling of biodegradable polymers for the production of nanocapsules and films with the incorporation of essential oils. Polym. Bull. 2020, 78, 7261–7278. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Schenck, H.U.; Simak, P.; Haedicke, E. Structure of polyvinylpyrrolidone-iodine (povidone-iodine). J. Pharm. Sci. 1979, 68, 1505–1509. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Kyrychenko, A.; Korsun, O.M.; Gubin, I.I.; Kovalenko, S.M.; Kalugin, O.N. Atomistic simulations of coating of silver nanoparticles with poly (vinylpyrrolidone) oligomers: Effect of oligomer chain length. J. Phys. Chem. C 2015, 119, 7888–7899. [Google Scholar] [CrossRef]
- Vellaichamy, B.; Periakaruppan, P.; Paulmony, T. Evaluation of a new biosensor based on in situ synthesized PPy-Ag-PVP nanohybrid for selective detection of dopamine. J. Phys. Chem. B 2017, 121, 1118–1127. [Google Scholar] [CrossRef]
- Alfuraydi, A.A.; Devanesan, S.; Al-Ansari, M.; AlSalhi, M.S.; Ranjitsingh, A.J. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2019, 192, 83–89. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Taha, R.; Ghazy, E.W.; El-Sayed, Y.S. Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: Hematological, biochemical, and antioxidant studies. Can. J. Physiol. Pharmacol. 2016, 94, 81–88. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; Altamimi, S.A.; El-Tohamy, M.F.; Almahri, A.M. Enhanced SIA-chemiluminescence probes for angiotensin II receptor antagonist detection using silver and gold nanoparticles: Applications in pharmaceutical formulations. New J. Chem. 2018, 42, 3383–3393. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Nair, I.C.; Siengchin, S.; Parameswaranpillai, J.; Radhakrishnan, E.K. Lipopeptide and zinc oxide nanoparticles blended polyvinyl alcohol-based nanocomposite films as antimicrobial coating for biomedical applications. Process. Biochem. 2021, 102, 220–228. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Pandur, E.; Tamasi, K.; Pap, R.; Varga, E.; Miseta, A.; Sipos, K. Fractalkine induces hepcidin expression of BV-2 microglia and causes iron accumulation in SH-SY5Y cells. Cell. Mol. Neurobiol. 2019, 39, 985–1001. [Google Scholar] [CrossRef] [Green Version]
- Pandur, E.; Varga, E.; Tamasi, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of inflammatory mediators’ lipopolysaccharide and lipoteichoic acid on iron metabolism of differentiated SH-SY5Y cells alters in the presence of BV-2 microglia. Int. J. Mol. Sci. 2019, 20, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pap, R.; Montsko, G.; Janosa, G.; Sipos, K.; Kovacs, G.L.; Pandur, E. Fractalkine regulates HEC-1A/JEG-3 interaction by influencing the expression of implantation-related genes in an in vitro co-culture model. Int. J. Mol. Sci. 2020, 21, 3175. [Google Scholar] [CrossRef]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 23 September 1997).
- Zhang, L.X.; Song, J.W.; Ma, Y.D.; Wang, Y.C.; Cui, Z.H.; Long, Y.; Yuan, D.Z.; Zhang, J.H.; Hu, Y.; Yu, L.L.; et al. Expression of SGLT1 in the Mouse Endometrial Epithelium and its Role in Early Embryonic Development and Implantation. Reprod. Sci. 2021, 28, 1–15. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; Altamimi, S.A.; El-Tohamy, M.F.; Almahri, A.M. Exploitation of localized surface plasmon resonance of silver/gold nanoparticles for the fluorescence quantification of angiotensin II receptor antagonists in their tablets and bio-samples. New J. Chem. 2019, 43, 492–503. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, L.; Chen, W. Studies on the preparation and characterization of gold nanoparticles protected by dendrons. Mater. Lett. 2007, 61, 278–283. [Google Scholar] [CrossRef]
- Sun, K.; Qiu, J.; Liu, J.; Miao, Y. Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles. J. Mater. Sci. 2009, 44, 754–758. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Chen, S.; Wu, A. Green chemistry synthesis of gold nanoparticles using lactic acid as a reducing agent. Micro Nano Lett. 2010, 5, 270–273. [Google Scholar] [CrossRef]
- Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical properties of poly-vinyl polymers and their influence on ketoprofen amorphous solid dispersion performance: A polymer selection case study. Pharmaceutics 2020, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, B.I.; Popescu, M.C.; Simionescu, B.C. Thermal stability of bio-nanocomposite films based on poly (vinyl alcohol)/starch/cellulose nano-crystals. Rev. Roum. Chim. 2017, 62, 599–604. Available online: http://web.icf.ro/rrch (accessed on 15 December 2020).
- Bhatt, D.; Ghosh, S. Regulation of the NF-κB-mediated transcription of inflammatory genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264. 7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement. Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Cavalleri, R.; Becker, J.S.; Pavan, A.M.; Bianchetti, P.; Goettert, M.I.; Ethur, E.M.; Bustamante-Filho, I.C. Essential oils rich in monoterpenes are unsuitable as additives to boar semen extender. Andrologia 2018, 50, e13074. [Google Scholar] [CrossRef] [PubMed]
- Korshoj, L.E.; Shi, W.; Duan, B.; Kielian, T. The prospect of nanoparticle systems for modulating immune cell polarization during central nervous system infection. Front. Immunol. 2021, 12, 670931. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef]
- Sabogal-Guaqueta, A.M.; Osorio, E.; Cardona-Gomez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Lanza, M.; Casili, G.; Campolo, M.; Paterniti, I.; Colarossi, C.; Mare, M.; Giuffrida, R.; Caffo, M.; Esposito, E.; Cuzzocrea, S. Immunomodulatory effect of microglia-released cytokines in gliomas. Brain Sci. 2021, 11, 466. [Google Scholar] [CrossRef]
- Sun, J.; Tian, T.; Wang, Y.; Yan, W.; Zhang, B.; Wang, K.; Yang, H.; Huang, M. Paraquat-activated BV-2 microglia induce neuroinflammatory responses in the neuron model through NF-κB signaling pathway. Toxicol. Vitr. 2021, 72, 105076. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Zhu, H.; Chen, C.; Zhao, G. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-κB and p38 MAPK. Exp. Ther. Med. 2016, 12, 3607–3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulsen, V.P.; Frank, F.M.; Cazorla, S.I.; Anesini, C.A.; Malchiodi, E.L.; Freixa, B.; Vila, R.; Muschietti, L.V.; Martino, V.S. Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia Sprengel (Asteraceae). Antimicrob. Agents Chemother. 2008, 52, 2415–2419. [Google Scholar] [CrossRef] [Green Version]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly (lactic acid) and poly (butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef]
- Khumkomgool, A.; Saneluksana, T.; Harnkarnsujarit, N. Active meat packaging from thermoplastic cassava starch containing sappan and cinnamon herbal extracts via LLDPE blown-film extrusion. Food Packag. Shelf Life 2020, 26, 100557. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]




| Primer | |
|---|---|
| IL-6 forward | CTCTGCAAGAGACTTCCATCCA |
| IL-6 reverse | GACAGGTCTGTTGGGAGTGG |
| TNFα forward | GATCGGTCCCCAAAGGGATG |
| TNFα reverse | CCACTTGGTGGTTTGTGAGTG |
| β-actin forward | CTGTCGAGTCGCGTCCA |
| β-actin reverse | TCATCCATGGCGAACTGGTG |
| No. | Name of Compound | Rt | Peak * Area % | Molecular Formula | Molecular Mass | Nature |
|---|---|---|---|---|---|---|
| 1 | Carvacrol | 16.70 | 1.05 | C10H14O | 150.22 | Phenol |
| 2 | Sesamol | 17.44 | 4.52 | C7H6O3 | 138.12 | Phenol |
| 3 | Eicosanoic acid methyl ester | 24.67 | 0.21 | C21H42O2 | 326.56 | Fatty acid |
| 4 | 9-hydroxy pentadecanoic acid-methyl ester | 24.76 | 0.14 | C19H38O3 | 314.50 | Fatty acid |
| 5 | Docosanoic acid methyl ester | 29.41 | 0.12 | C23H46O2 | 354.61 | Fatty acid |
| 6 | Palmitic acid | 32.71 | 8.01 | C16H32 O2 | 256.43 | Fatty acid |
| 7 | Cis-9-hexadecenal | 36.71 | 35.2 | C16H30O | 238.41 | Fat |
| 8 | Oleic acid | 41.25 | 26.43 | C18H34O2 | 282.47 | Fatty acid |
| 9 | Palmitic acid-β- monoglyceride | 42.01 | 0.5 | C19H31ClO | 330.5 | Fat |
| 10 | Oleoyl chloride | 45.84 | 1.2 | C18H33ClO | 300.9 | Salt of fatty acid |
| 11 | Methyl ricinoleate | 51.26 | C19H36O2 | 312.5 | Salt of fatty acid | |
| 12 | (+)-Sesamin | 52.69 | 4.91 | C20H18O6 | 354.35 | Lignan |
| 13 | 6-Nitrocholest-5-en-3-yl acetate | 53.78 | 0.32 | C29H47NO4 | 473.7 | Steroid |
| 14 | Propylidenecholesterol | 54.89 | 0.21 | C30H50O | 480.6 | Steroid |
| 15 | Ergost-5-en-3β-ol | 54.92 | 1.95 | C28H48O | 400.7 | Steroid |
| IC50 (µg mL−1) | |||
|---|---|---|---|
| Sample | L. mexicana | L. amazonensis | T. cruzi |
| Sesame oil | 5.31 ± 1.22 | 8.84 ± 2.21 | 2.87 ± 1.28 |
| AuNPs | 1.71 ± 1.42 | 1.68 ± 0.75 | 0.57 ± 0.32 |
| Bionanocomposite | 1.12 ± 1.10 | 1.42 ± 0.69 | 0.05 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarfaj, N.A.; Amina, M.; Al Musayeib, N.M.; El-Tohamy, M.F.; Al-Hamoud, G.A. Immunomodulatory and Antiprotozoal Potential of Fabricated Sesamum radiatum Oil/Polyvinylpyrrolidone/Au Polymeric Bionanocomposite Film. Polymers 2021, 13, 4321. https://doi.org/10.3390/polym13244321
Alarfaj NA, Amina M, Al Musayeib NM, El-Tohamy MF, Al-Hamoud GA. Immunomodulatory and Antiprotozoal Potential of Fabricated Sesamum radiatum Oil/Polyvinylpyrrolidone/Au Polymeric Bionanocomposite Film. Polymers. 2021; 13(24):4321. https://doi.org/10.3390/polym13244321
Chicago/Turabian StyleAlarfaj, Nawal A., Musarat Amina, Nawal M. Al Musayeib, Maha F. El-Tohamy, and Gadah A. Al-Hamoud. 2021. "Immunomodulatory and Antiprotozoal Potential of Fabricated Sesamum radiatum Oil/Polyvinylpyrrolidone/Au Polymeric Bionanocomposite Film" Polymers 13, no. 24: 4321. https://doi.org/10.3390/polym13244321
APA StyleAlarfaj, N. A., Amina, M., Al Musayeib, N. M., El-Tohamy, M. F., & Al-Hamoud, G. A. (2021). Immunomodulatory and Antiprotozoal Potential of Fabricated Sesamum radiatum Oil/Polyvinylpyrrolidone/Au Polymeric Bionanocomposite Film. Polymers, 13(24), 4321. https://doi.org/10.3390/polym13244321






