Conception and Theoretical Study of a New Copolymer Based on MEH-PPV and P3HT: Enhancement of the Optoelectronic Properties for Organic Photovoltaic Cells
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
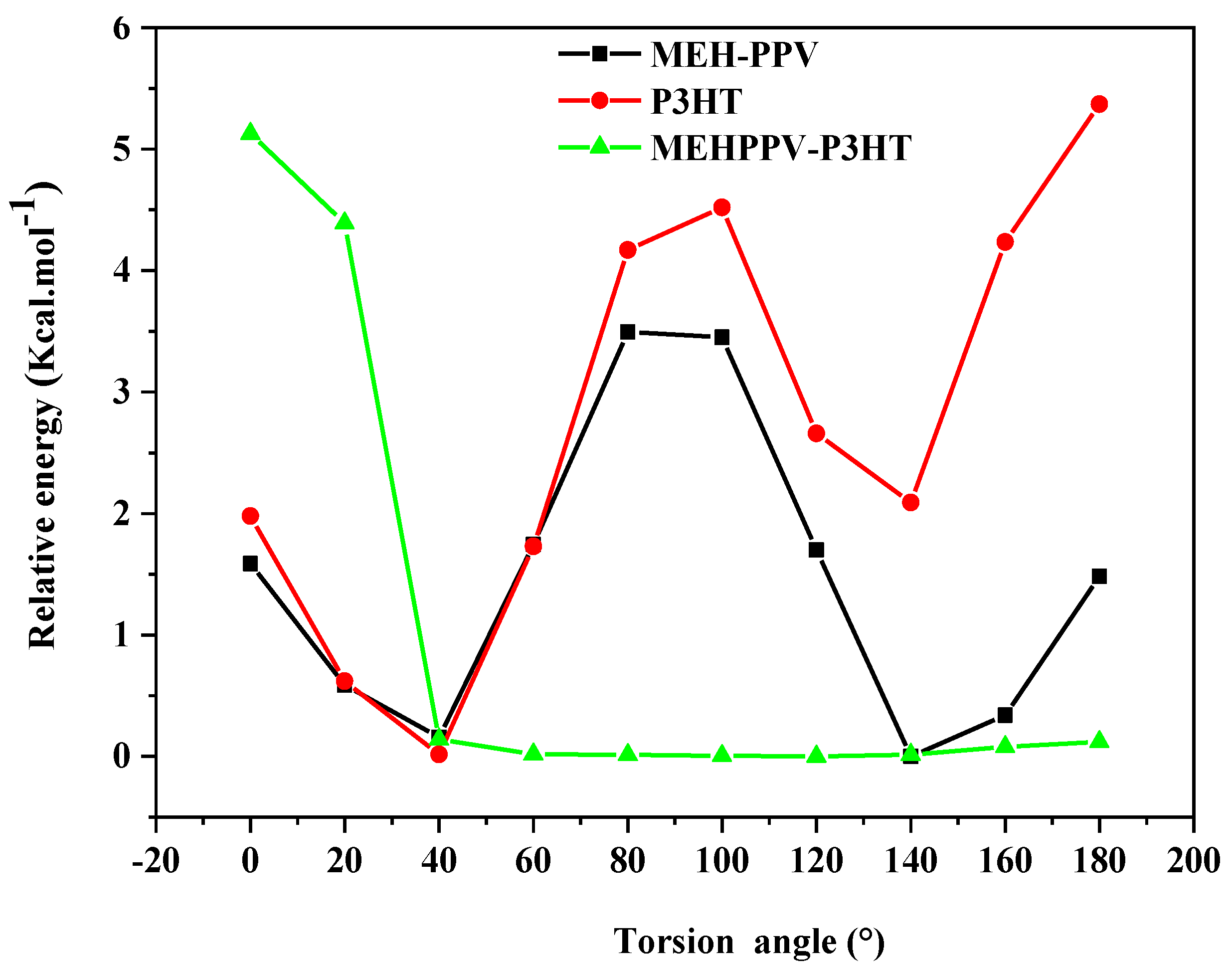
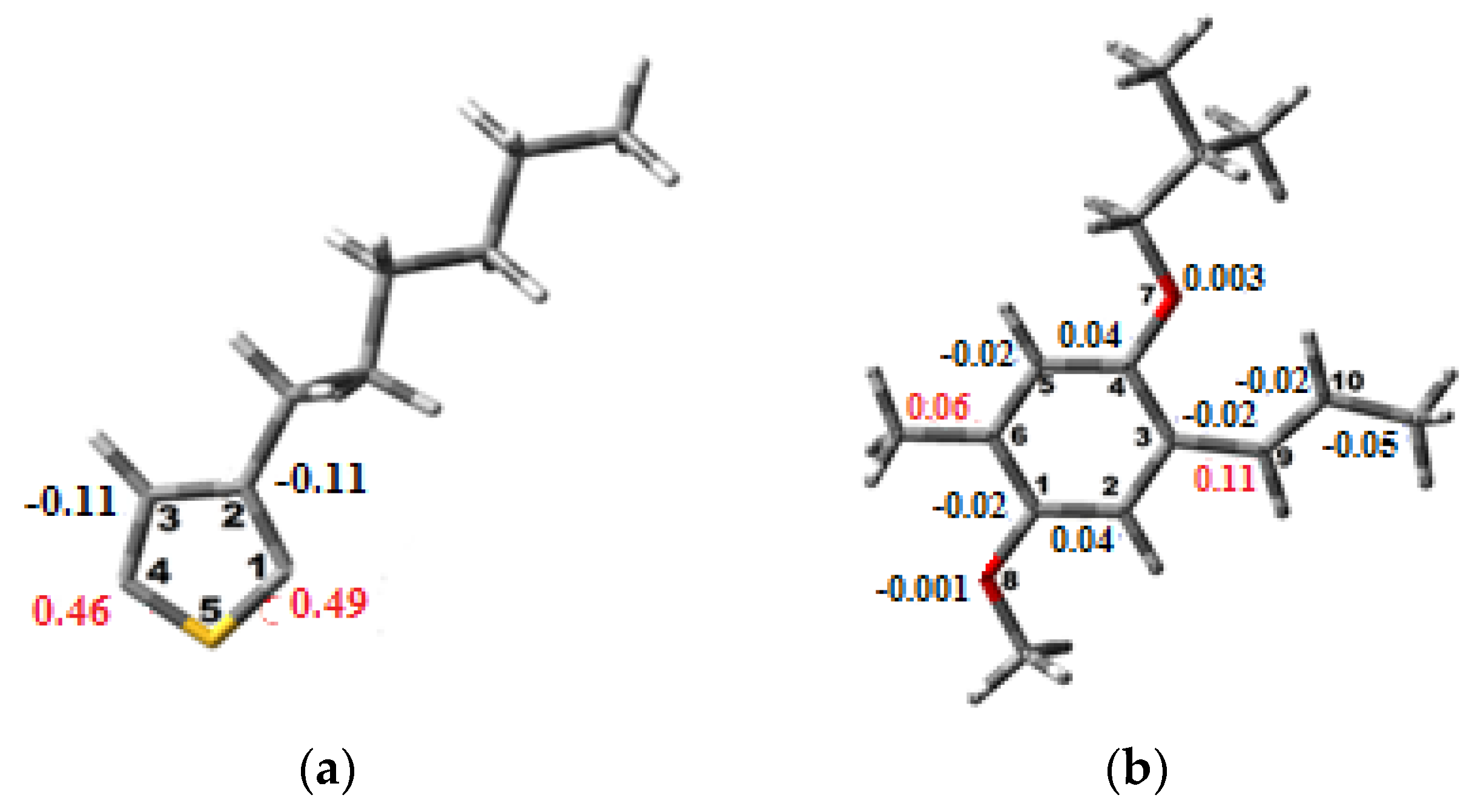
3.1. Conformation Studies of MEH-PPV and P3HT
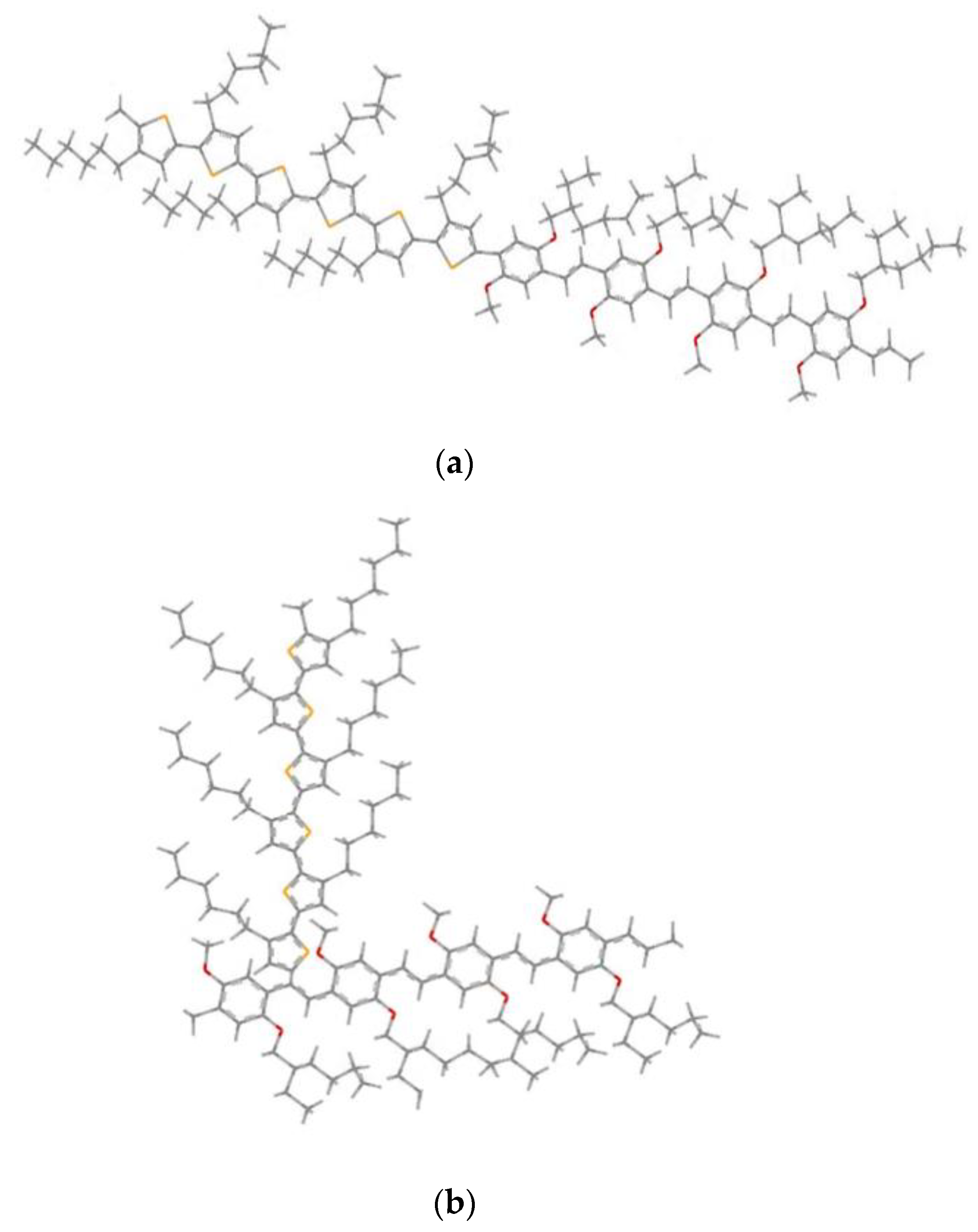
3.2. Conformation Studies of Copolymers 4MEH-PPV-6P3HT
3.3. Vibrational Properties
3.4. Electronic Properties
3.5. Optical Properties
3.5.1. Absorption Spectrum
3.5.2. Photoluminescence Spectrum
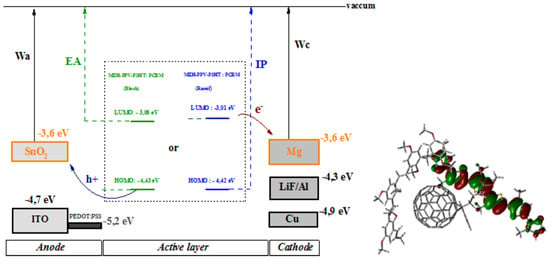
3.6. MEH-PPV-P3HT: PCBM Active Layer Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Liao, M.; Lou, H.; Hu, Y.; Sun, X.; Peng, H. Conjugated Polymers for Flexible Energy Harvesting and Storage. Adv. Mater. 2018, 30, 1704261. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Liu, S.; You, P.; Li, J.; Li, J.; Lee, C.-S.; Ong, B.S.; Yan, F. Enhanced efficiency of polymer solar cells by adding a high-mobility conjugated polymer. Energy Environ. Sci. 2015, 8, 1463–1470. [Google Scholar] [CrossRef]
- Sun, N.; Jiang, C.; Li, Q.; Tan, D.; Bi, S.; Song, J. Performance of OLED under mechanical strain: A review. J. Mater. Sci.: Mater. Electron. 2020, 31, 20688–20729. [Google Scholar] [CrossRef]
- Ltaief, A.; Bouazizi, A.; Davenas, J.; Chaabane, R.B.; Ouada, H.B. Electrical and optical properties of thin films based on MEH-PPV/fullerene blends. Synth. Met. 2004, 147, 261–266. [Google Scholar] [CrossRef]
- Yoon, S.E.; Han, J.M.; Seo, B.E.; Kim, S.W.; Kwon, O.P.; Kim, B.G.; Kim, J.H. Optimized selection of dopant solvents for improving the sequential doping efficiency of conjugated polymers. Org. Electron. 2021, 90, 106061. [Google Scholar] [CrossRef]
- Mbarek, M.; Almoneef, M.M.; ben Saleh, Y.; Alimi, K. Organic optoelectronic copolymer involving PVK and F8T2: Synthesis and characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119509. [Google Scholar] [CrossRef]
- Kajiya, D.; Koganezawa, T.; Saitow, K.I. Hole mobility enhancement of MEH-PPV film by heat treatment at T g. AIP Adv. 2015, 5, 127130. [Google Scholar] [CrossRef] [Green Version]
- Shrotriya, V.; Ouyang, J.; Tseng, R.J.; Li, G.; Yang, Y. Absorption spectra modification in poly(3-hexylthiophene):methanofullerene blend thin films. Chem. Phys. Lett. 2005, 411, 138–143. [Google Scholar] [CrossRef]
- Song, P.; Li, Y.; Ma, F.; Pullerits, T.; Sun, M. External Electric Field-Dependent Photoinduced Charge Transfer in a Donor–Acceptor System for an Organic Solar Cell. J. Phys. Chem. C 2013, 117, 15879–15889. [Google Scholar] [CrossRef]
- Liu, T.; Troisi, A. Absolute Rate of Charge Separation and Recombination in a Molecular Model of the P3HT/PCBM Interface. J. Phys. Chem. C 2011, 115, 2406–2415. [Google Scholar] [CrossRef]
- Rana, D.; Materny, A. Effect of static external electric field on bulk and interfaces in organic solar cell systems: A density-functional-theory-based study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119565. [Google Scholar] [CrossRef] [PubMed]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alhandel, R.H.; Alsaigh, R.A. TD-DFT Simulation and Experimental Studies of a Mirrorless Lasing of Poly [(9, 9-dioctylfluorenyl-2, 7-diyl)-co-(1, 4-diphenylene-vinylene-2-methoxy-5-{2-ethylhexyloxy}-benzene)]. Polymers 2021, 13, 1430. [Google Scholar] [CrossRef] [PubMed]
- Mbarek, M.; Almoneef, M.M.; ben Saleh, Y.; Alimi, K. Structural and photophysical properties of PVK-F8BT copolymer thin films, with single walled carbon nanotubes: Synthesis, characterization and modeling. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118502. [Google Scholar] [CrossRef]
- Roldao, J.C.; Batagin-Neto, A.; Lavarda, F.C.; Sato, F. Effects of Mechanical Stretching on the Properties of Conjugated Polymers: Case Study for MEH--PPV and P3HT Oligomers. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1413–1426. [Google Scholar] [CrossRef]
- Abbassi, F.; Mbarek, M.; Almoneef, M.; Alimi, K. Photophysical Properties of the PVK-MEH-PPV/PCBM Composite for Organic Solar Cells Application: Synthesis, Characterization and Computational Study. Polymers 2021, 13, 2902. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Franco Jr, F.C.; Padama, A.A.B. DFT and TD-DFT study on the structural and optoelectronic characteristics of chemically modified donor-acceptor conjugated oligomers for organic polymer solar cells. Polymer 2016, 97, 55–62. [Google Scholar] [CrossRef]
- Zade, S.S.; Zamoshchik, N.; Bendikov, M. From short conjugated oligomers to conjugated polymers. Lessons Stud. Long Conjug. Oligomers. Acc. Chem. Res. 2011, 44, 14–24. [Google Scholar]
- Batagin-Neto, A.; Oliveira, E.F.; Graeff, C.F.O.; Lavarda, F.C. Modelling polymers with side chains: MEH-PPV and P3HT. Mol. Simul. 2013, 39, 309–321. [Google Scholar] [CrossRef]
- Reynolds, J.R.; Ruiz, J.P.; Child, A.D.; Nayak, K.; Marynick, D.S. Electrically conducting polymers containing alternating substituted phenylenes and bithiophene repeat units. Macromolecules 1991, 24, 678–687. [Google Scholar] [CrossRef]
- Lu, B.; Zhen, S.; Zhang, S.; Xu, J.; Zhao, G. Highly stable hybrid selenophene-3, 4-ethylenedioxythiophene as electrically conducting and electrochromic polymers. Polym. Chem. 2014, 5, 4896–4908. [Google Scholar] [CrossRef]
- Heth, C.L.; Tallman, D.E.; Rasmussen, S.C. Electrochemical study of 3-(N-alkylamino) thiophenes: Experimental and theoretical insights into a unique mechanism of oxidative polymerization. J. Phys. Chem. B 2010, 114, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Halsey-Moore, C.; Jena, P.; McLeskey Jr, J.T. Tuning range-separated DFT functionals for modeling the peak absorption of MEH-PPV polymer in various solvents. Comput. Theor. Chem. 2019, 1162, 112506. [Google Scholar] [CrossRef]
- Bjorgaard, J.A.; Kose, M.E. Theoretical study of torsional disorder in poly (3-alkylthiophene) single chains: Intramolecular charge-transfer character and implications for photovoltaic properties. J. Phys. Chem. A 2013, 117, 3869–3876. [Google Scholar] [CrossRef]
- Mbarek, M.; Abbassi, F.; Alimi, K. Complementary study based on DFT to describe the structure and properties relationship of diblock copolymer based on PVK and PPV. Phys. B: Condens. Matter 2016, 497, 45–50. [Google Scholar] [CrossRef]
- Gorelsky, S.I. SWizard Program; University of Ottawa: Ottawa, ON, Canada, 2010. [Google Scholar]
- Abbassi, F.; Almoneef, M.M.; Mbarek, M.; Kreher, D.; Alimi, K. Optical and structural proprieties of the new triblock copolymer (ABC) based on p-terphenyl, PVK and MEH-PPV for organic electronics: Experimental and theoretical study. Mater. Res. Express 2021, 8, 075304. [Google Scholar] [CrossRef]
- Sharma, G.; Kattayat, S.; Naqvi, S.F.; Hashmi, S.Z.; Alvi, P.A. Role of MEH: PPV polymer in single layer OLEDs with its optoelectronic characteristics. Mater. Today Proc. 2021, 42, 1678–1681. [Google Scholar] [CrossRef]
- Jacobs, I.E.; Wang, F.; Hafezi, N.; Medina-Plaza, C.; Harrelson, T.F.; Li, J.; Augustine, M.P.; Mascal, M.; Moulé, A.J. Quantitative dedoping of conductive polymers. Chem. Mater. 2017, 29, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Zubair, M.; Mustafa, M.; Ali, A.; Doh, Y.H.; Choi, K.H. Improvement of solution based conjugate polymer organic light emitting diode by ZnO–graphene quantum dots. J. Mater. Sci. Mater. Electron. 2015, 26, 3344–3351. [Google Scholar] [CrossRef]
- Koo, B.; Sletten, E.M.; Swager, T.M. Functionalized poly (3-hexylthiophene) s via lithium–bromine exchange. Macromolecules 2015, 48, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.S.; Sharma, K.; Sharma, G.D. Efficient bulk heterojunction photovoltaic devices based on modified PCBM. Nanotechnol. Rev. 2015, 4, 419–428. [Google Scholar] [CrossRef]
- Ben Salah, Y.; Altowyan, A.S.; Mbarek, M.; Alimi, K. Complementary Study Based on DFT of Optical and Electronic Properties of New Copolymer PVK-F8T2. Polymers 2021, 13, 1805. [Google Scholar] [CrossRef]
- Inagi, S.; Fuchigami, T. Electronic property and reactivity of novel fused thiophene. Synth. Met. 2008, 158, 782–784. [Google Scholar] [CrossRef]
- Moon, H.S.; Park, J.K. Synthesis and spectroscopic characterization of the copolymers of aniline and aniline derivatives with poly (ethylene oxide) chains at the 3-position. Macromolecules 1998, 31, 6461–6468. [Google Scholar] [CrossRef]
- Farouil, L.; Alary, F.; Bedel-Pereira, E.; Heully, J.-L. Revisiting the Vibrational and Optical Properties of P3HT: A Combined Experimental and Theoretical Study. J. Phys. Chem. A 2018, 122, 6532–6545. [Google Scholar] [CrossRef]
- Brambilla, L.; Tommasini, M.; Botiz, I.; Rahimi, K.; Agumba, J.O.; Stingelin, N.; Zerbi, G. Regio-Regular Oligo and Poly(3-hexyl thiophene): Precise Structural Markers from the Vibrational Spectra of Oligomer Single Crystals. Macromolecules 2014, 47, 6730–6739. [Google Scholar] [CrossRef]
- Mombrú, D.; Romero, M.; Faccio, R.; Mombrú, Á.W. Unraveling the Lithium Bis(trifluoromethanesulfonyl)imide (LiTFSI) Doping Mechanism of Regioregular Poly(3-hexylthiophene): Experimental and Theoretical Study. J. Phys. Chem. C 2020, 124, 7061–7070. [Google Scholar] [CrossRef]
- Bellacanzone, C.; Roscini, C.; del Carmen Ruiz Delgado, M.; Ponce Ortiz, R.; Ruiz-Molina, D. Sonochemical Synthesis of Optically Tuneable Conjugated Polymer Nanoparticles. Part. Part. Syst. Charact. 2018, 35, 1700322. [Google Scholar] [CrossRef]
- Piacenza, M.; Comoretto, D.; Burger, M.; Morandi, V.; Marabelli, F.; Martinelli, C.; Della Sala, F. Raman Spectra of Poly(p-phenylenevinylene)s with Fluorinated Vinylene Units: Evidence of Inter-ring Distortion. ChemPhysChem 2009, 10, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Mbarek, M.; Abbassi, F.; Alimi, K. New polymer-matrix nanocomposites based on SWCNTs and PVK-PPV copolymer: Synthesis, functionalization and characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, C.-Y.; Chen, J.-K.; Hsu, M.-H. Ce-doped ZnO nanorods based low operation temperature NO2 gas sensors. Ceram. Int. 2014, 40, 10867–10875. [Google Scholar] [CrossRef]
- Giri, S.; Moore, C.H.; Mcleskey, J.T.; Jena, P. Origin of Red Shift in the Photoabsorption Peak in MEH–PPV Polymer. J. Phys. Chem. C 2014, 118, 13444–13450. [Google Scholar] [CrossRef]
- Khlaifia, D.; Ewels, C.P.; Massuyeau, F.; Chemek, M.; Faulques, E.; Duvail, J.L.; Alimi, K. Unraveling the real structures of solution-based and surface-bound poly (3-hexylthiophene) (P3HT) oligomers: A combined theoretical and experimental study. RSC Adv. 2016, 6, 56174–56182. [Google Scholar] [CrossRef]
- Mbarek, M.; Almoneef, M.M.; Alimi, K. Elaboration and study of the new copolymer based on vinylcarbazole and Stilbene (VK-Stilbene): Correlation structure-proprieties. J. Mol. Struct. 2020, 1217, 128384. [Google Scholar] [CrossRef]
- Mbarek, M.; Sagaama, L.; Alimi, K. New copolymer involving PVK and F8BT for organic solar cells applications: Design, synthesis, characterization and theoretical studies. Opt. Mater. 2019, 91, 447–454. [Google Scholar] [CrossRef]




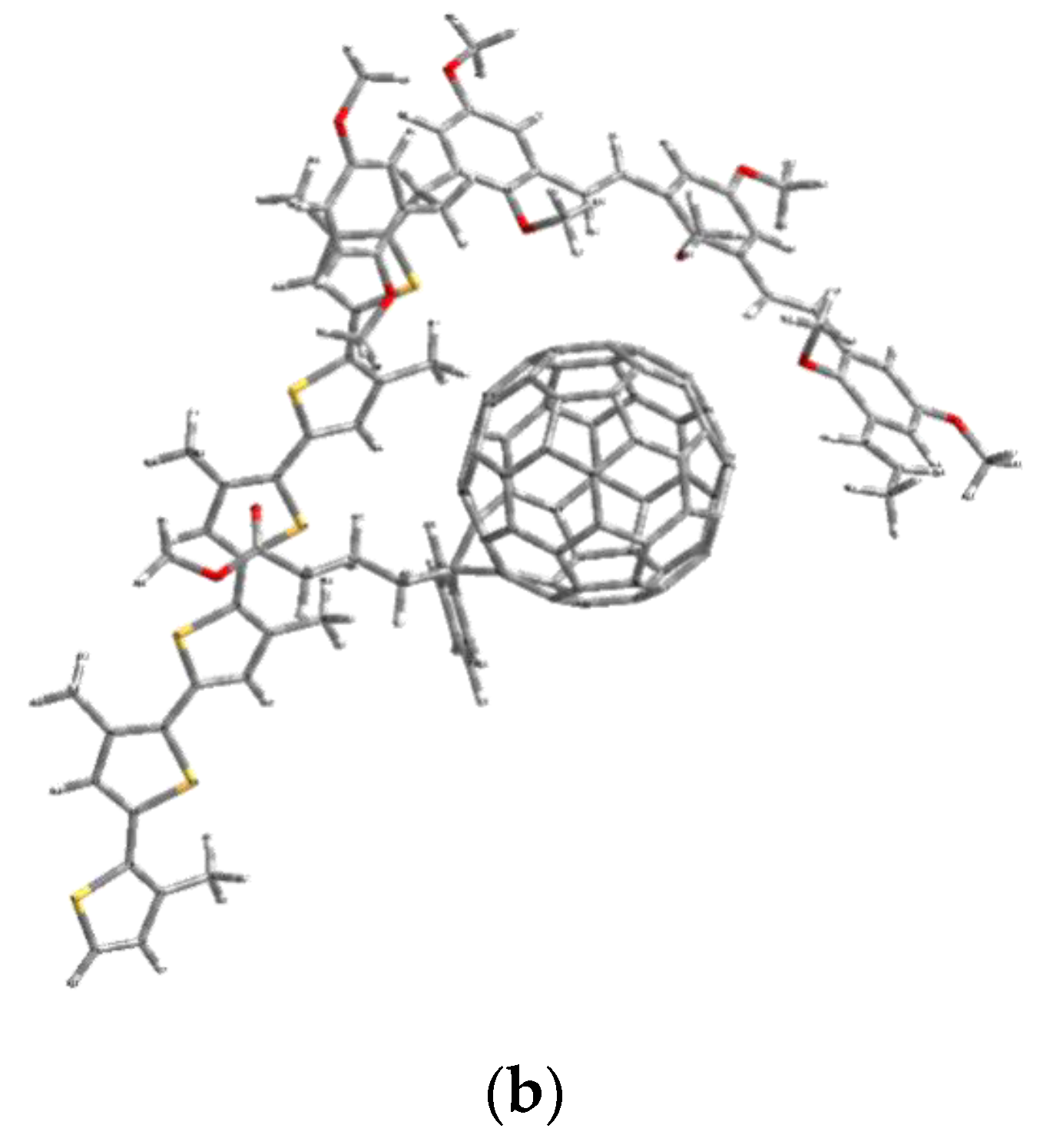

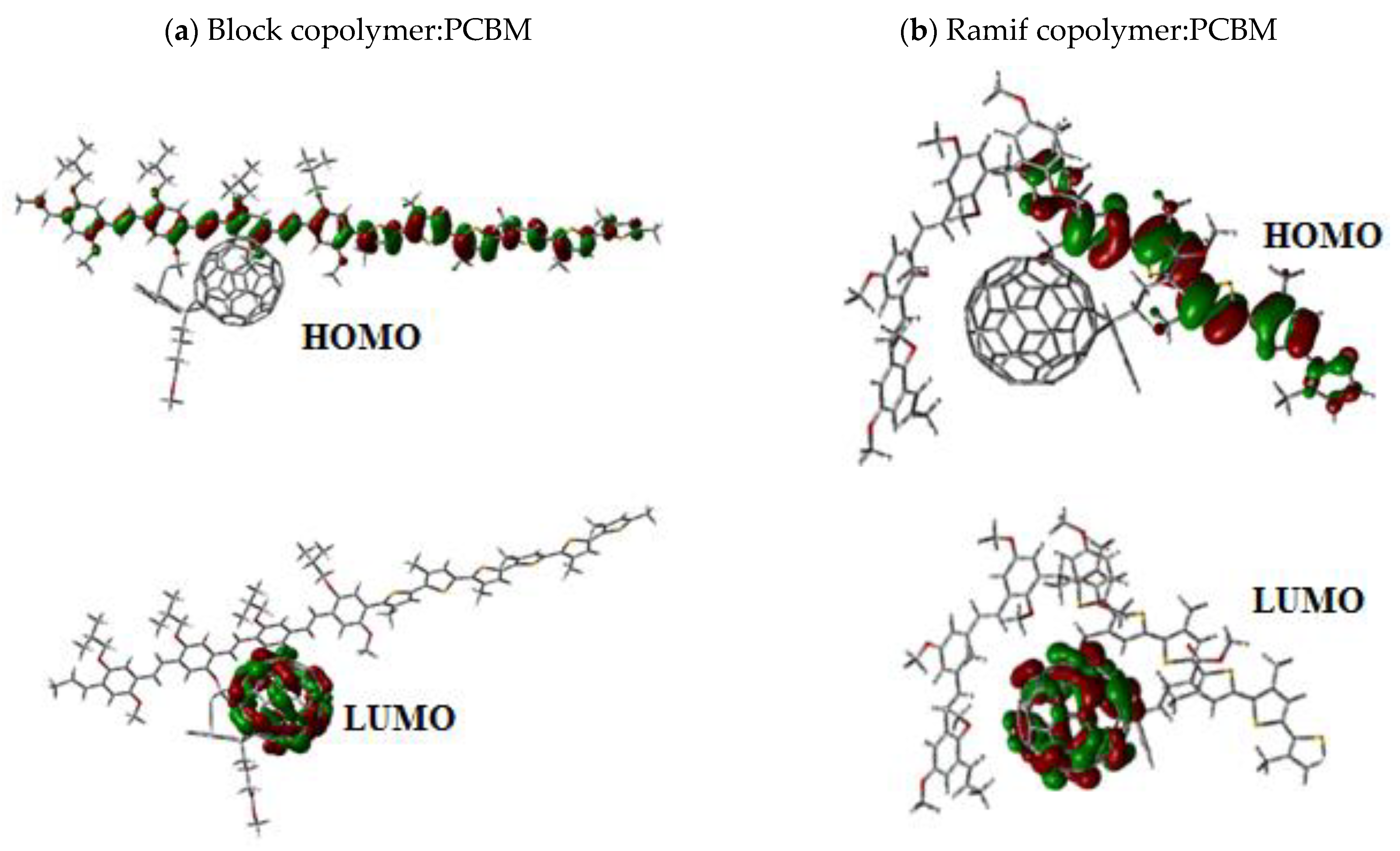

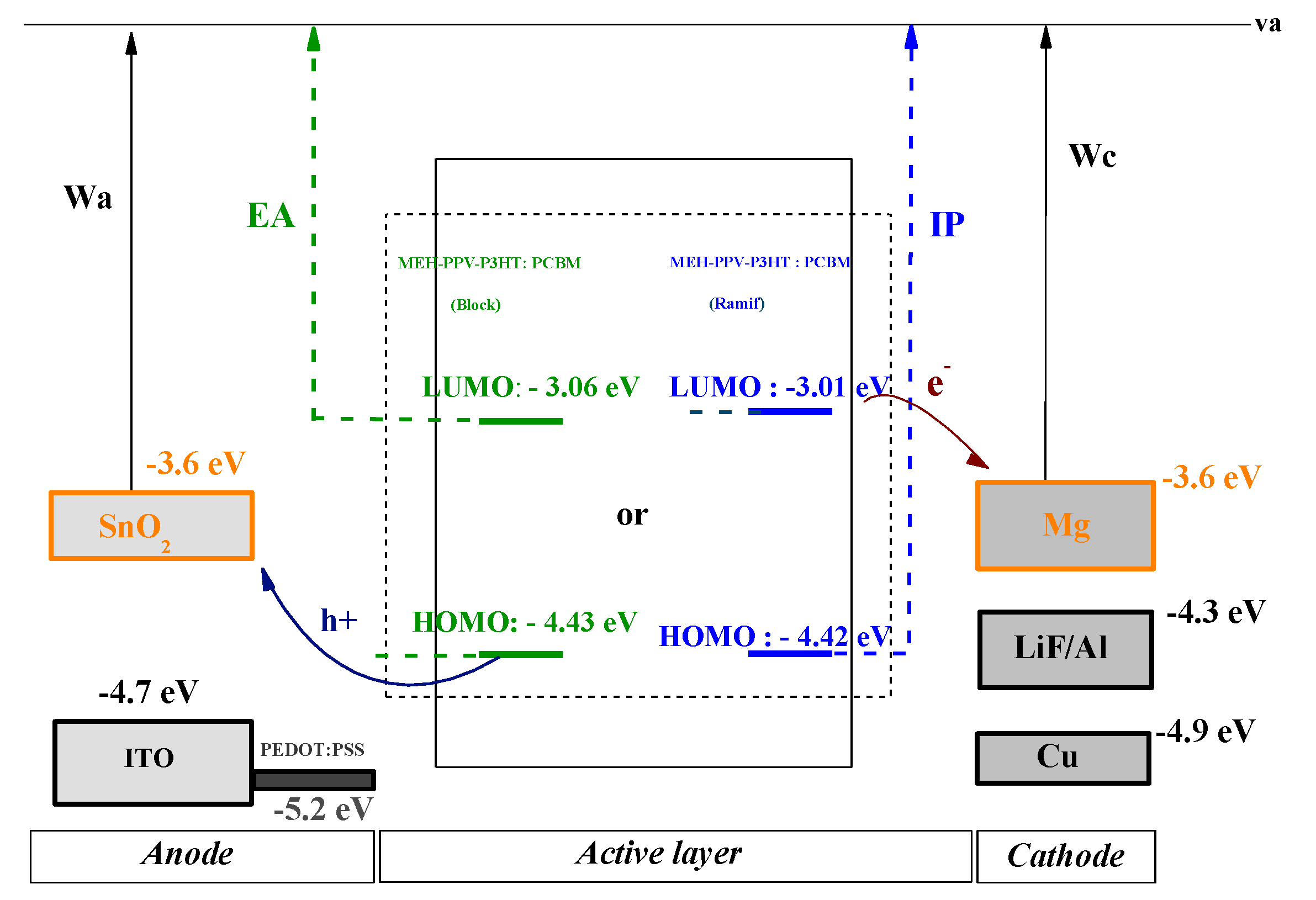
| HOMO (eV) | LUMO (eV) | ||||
|---|---|---|---|---|---|
| TD-DFT | ZINDO | ||||
| 1MEH-PPV | 4.99 | 0.39 | 4.6 | 278 | 317 |
| 2MEH-PPV | 4.59 | 1.10 | 3.49 | 352 | 387 |
| 3MEH-PPV | 4.39 | 1.38 | 3.01 | 398 | 424 |
| 4MEH-PPV | 4.29 | 1.52 | 2.77 | 425 | 443 |
| HOMO (eV) | LUMO (eV) | ||||
|---|---|---|---|---|---|
| TD-DFT | ZINDO | ||||
| 1P3HT | 5.64 | 0.069 | 5.57 | 225 | 299 |
| 2P3HT | 5.12 | 0.85 | 4.27 | 300 | 395 |
| 3P3HT | 4.71 | 1.39 | 3.32 | 377 | 500 |
| 4P3HT | 4.58 | 1.63 | 2.95 | 421 | 557 |
| 5P3HT | 4.57 | 1.83 | 2.74 | 448 | 595 |
| 6P3HT | 4.50 | 1.93 | 2.57 | 470 | 625 |
| IR Ramif | |
| Wavenumber (cm−1) | Vibration Mode |
| 846 | C=C and C–H wagging in P3HT |
| 1060 | CH–CH2 twisting in P3HT |
| 1094 | C–H scissoring in PPV |
| 1267 | C–H rocking of P3HT and MEH-PPV |
| 1333 | C–H twisting of vinyl group + scissoring of phenyl group |
| 1514 | C–H rocking and twisting of phenyl group |
| 1562 | C=C asymmetric stretching in P3HT |
| 1645 | Asymmetric stretching of phenyl group |
| IR Block | |
| Wavenumber (cm−1) | Vibration mode |
| 1016 | C–H twisting in vinyl group |
| 1080 | Out-of-plane rocking of phenyl group |
| 1248 | C–H symmetrical stretching in-plane of PPV |
| 1273 | C–H rocking in vinyl group |
| 1377 | Asymmetric stretching of phenyl + C–H rocking in vinyl |
| 1427 | CH3 symmetric stretching in P3HT |
| 1458 | Phenyl and C–H symmetric stretching in MEH-PPV |
| 1551 | C= scissoring in phenyl + C–H rocking in phenyl group |
| 1559 | C=C symmetric stretching in phenyl and thiophene |
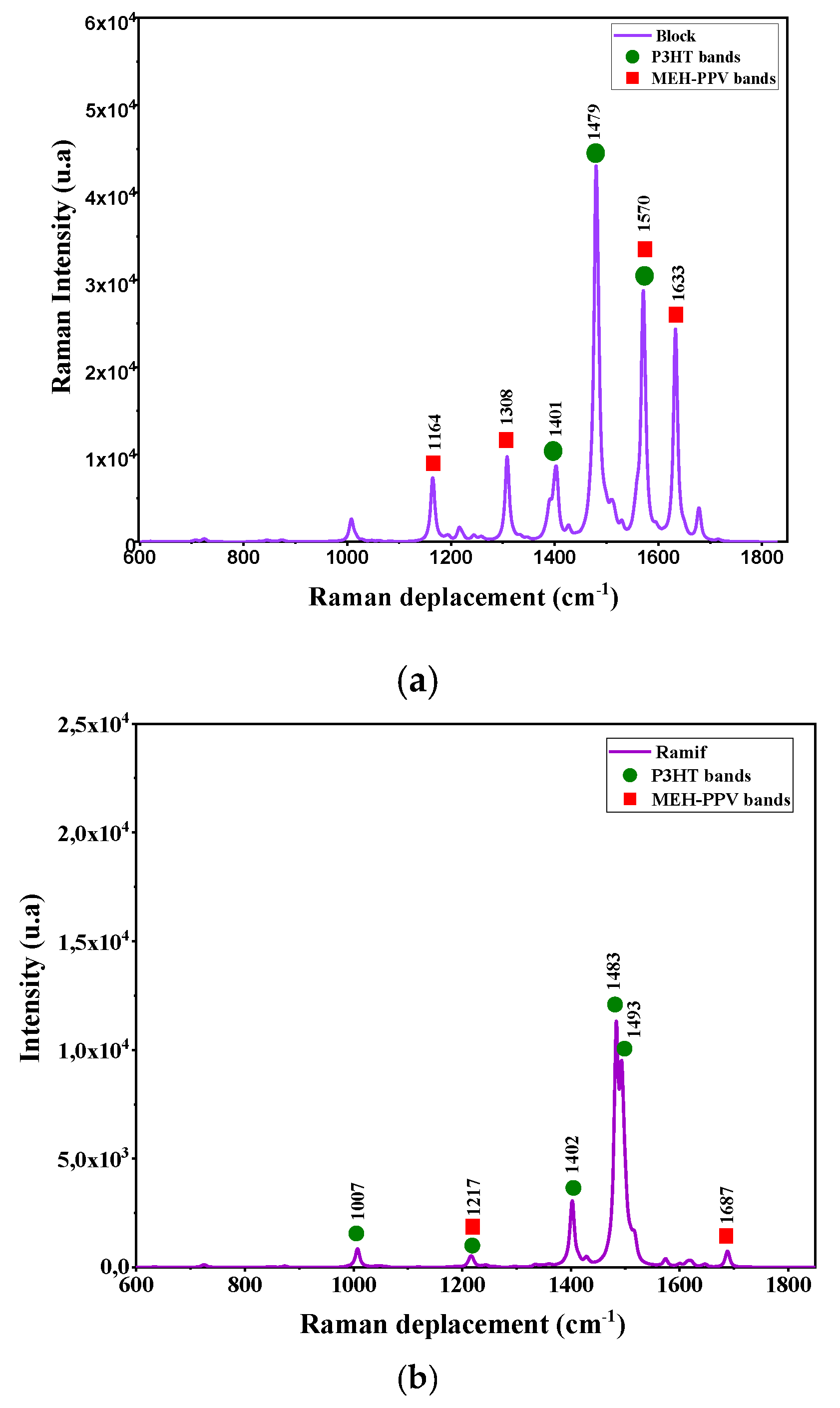
| Raman Ramif | |
| Wavenumber (cm−1) | Vibration Mode |
| 1164 | C–H asymmetric stretching in PPV |
| 1308 | Scissoring in-plane of phenyl in MEH-PPV |
| 1401 | P3HT deformation |
| 1479 | Thiophene scissoring |
| 1570 | Twisting of PPV and thiophene |
| 1633 | Out-of-plane rocking of PPV |
| Raman Block | |
| Wavenumber (cm−1) | Vibration mode |
| 1007 | CH–CH2 asymmetric stretching in P3HT |
| 1217 | C–H rocking in P3HT and MEH-PPV |
| 1402 | C=C scissoring in polythiophene PT |
| 1483 | Symmetric stretching of thiophene |
| 1493 | C=C asymmetric stretching in thiophene |
| 1687 | C=C scissoring in vinylene group in MEH-PPV |
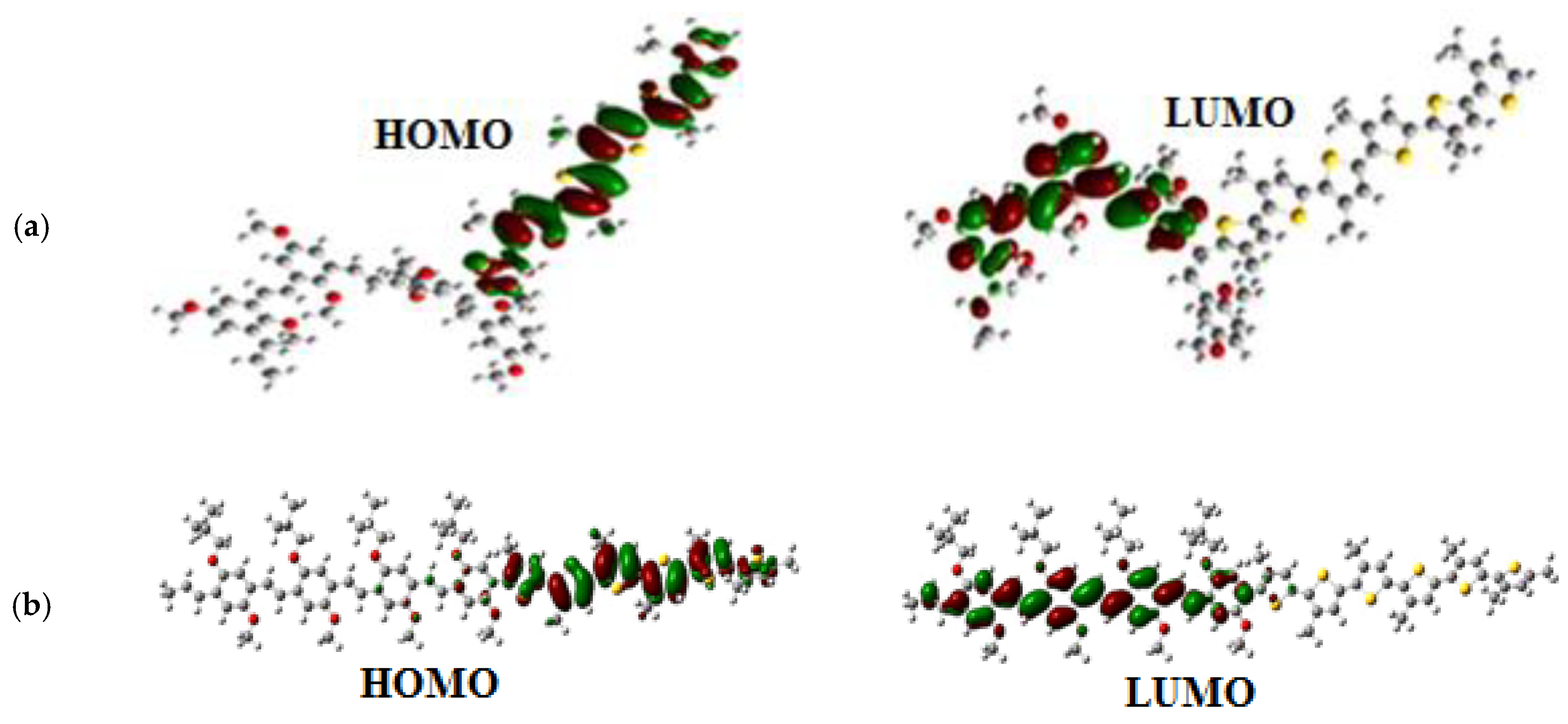
| Polymer | HOMO (eV) | LUMO (eV) | |
|---|---|---|---|
| 4MEH-PPV | 4.29 | 1.52 | 2.77 |
| 6P3HT | 4.50 | 1.93 | 2.57 |
| 4MEH-PPV-6P3HT (Ramif) | 4.54 | 1.82 | 2.72 |
| 4MEH-PPV-6P3HT (Block) | 4.31 | 1.85 | 2.46 |
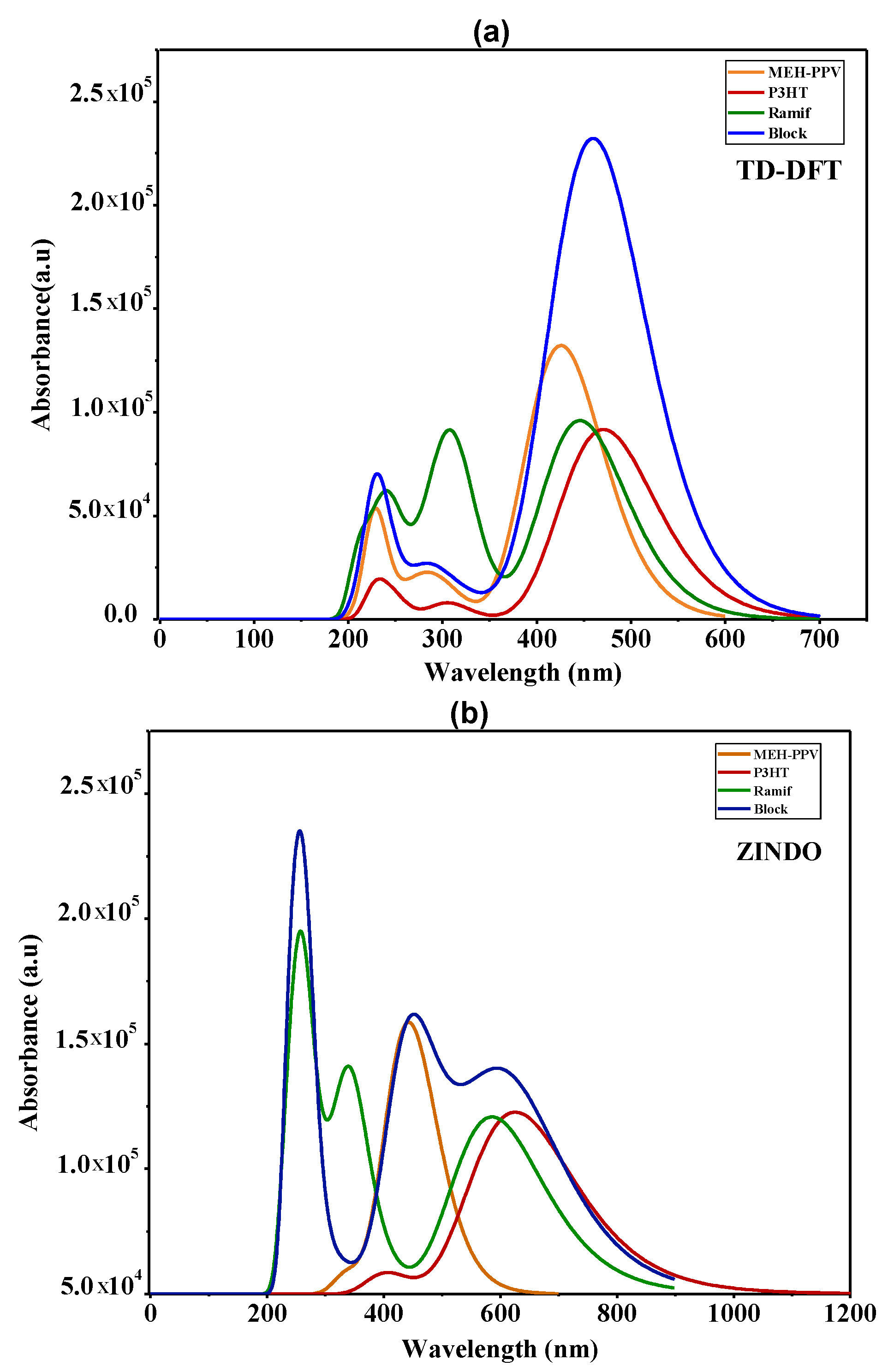
| Polymer | Transition | E (eV) | Assignment; H = HOMO, L = LUMO | |||
|---|---|---|---|---|---|---|
| TD-DFT | 4MEH-PPV | 425.7 | 2.91 | 3.2642 | H0->L+0(+85%) H1->L+1(+9%) | |
| 6P3HT | 470.7 | 2.63 | 2.2636 | H0->L+0(+88%) H1->L+1(7%) | ||
| Ramif | 445.9 | 2.78 | 2.3700 | H0->L+0(+86%) H1->L+2(7%) | ||
| Block | 469.5 | 2.64 | 4.4501 | H0->L+0(+44%)H1-> L+0(+29%) H1->L+1(11%) | ||
| ZINDO | 4MEH-PPV | 443.1 | 2.80 | 2.6780 | H0->L+0(+75%) H1-> L+1(+14%) | |
| 6P3HT | 625.6 | 1.98 | 1.7946 | H0->L+0(+81%) H1-> L+1(11%) | ||
| Ramif | 585.9 | 2.12 | 1.7458 | H0->L+0(+77%) H1-> L+1(10%) | ||
| Block | 610 | 2.03 | 2.0843 | H0->L+0(+75%) H2-> L+2(+5%) |
| Polymer | |||
|---|---|---|---|
| MEH-PPV | 2.33 | 2.19 | 2.2 [44] |
| P3HT | 2.03 | 1.4 | 1.9–2.1 [45] |
| Block | 2 | 1.49 | – |
| Ramif | 2.64 | 2.17 | – |
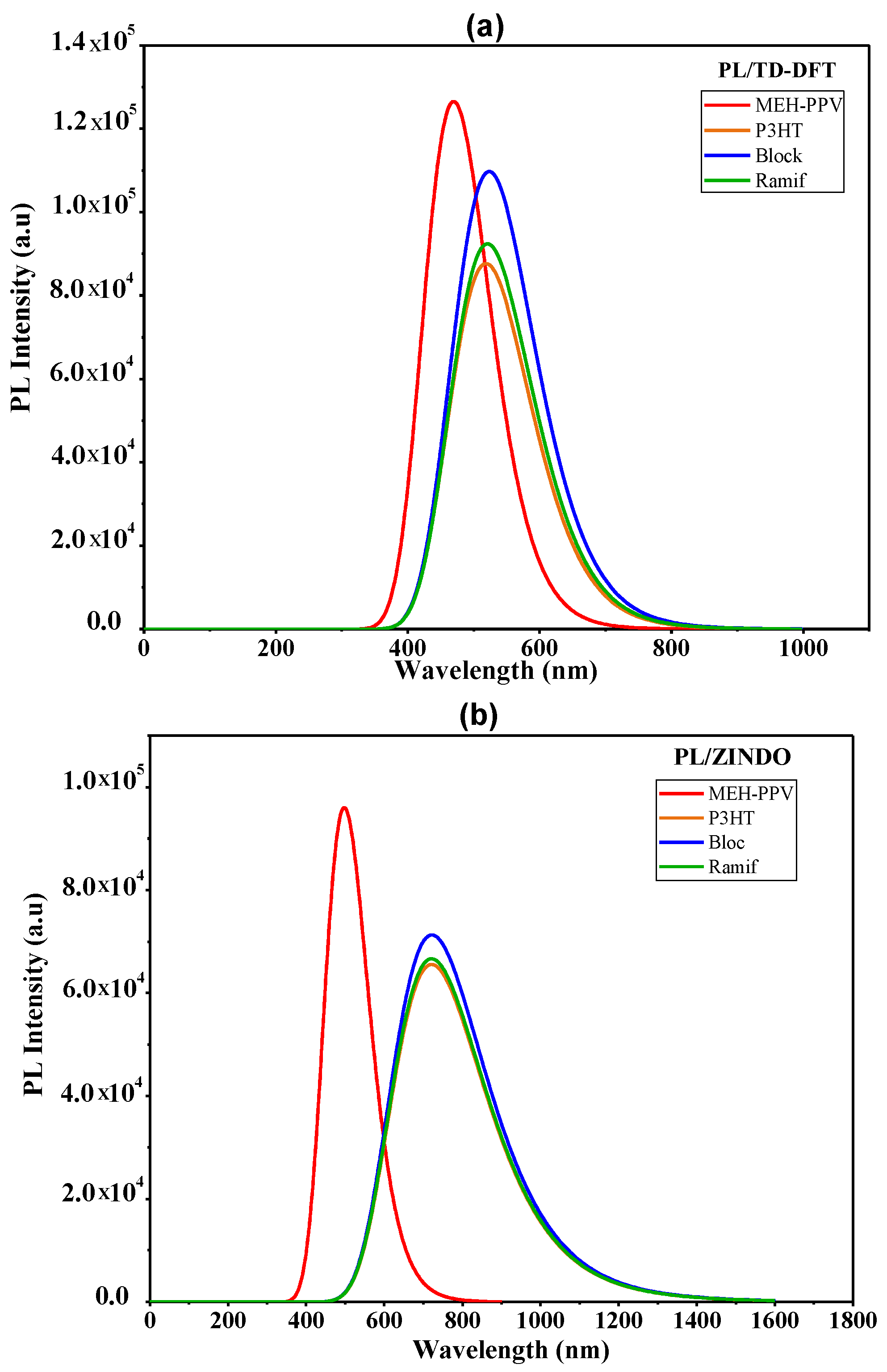
| Polymer | ||
|---|---|---|
| MEH-PPV | 469.5 | 497.6 |
| P3HT | 518.5 | 721.3 |
| MEH-PPV-P3HT (Block) | 523.4 | 721.8 |
| MEH-PPV-P3HT (Ramif) | 520.7 | 721.1 |
| Polymer | Transition | E (eV) | Assignment; H = HOMO, L = LUMO | ||||
|---|---|---|---|---|---|---|---|
| TD-DFT | MEH-PPV | 469.5 | 2.64 | 3.12 | 1.39 | H0->L+0(+91%) H-1-> L+1(+5%) | |
| P3HT | 518.6 | 2.39 | 2.16 | 2.23 | H0->L+0(+93%) | ||
| Ramif | 520.7 | 2.38 | 2.2819 | 2.12 | H0->L+0(+92%) | ||
| Block | 523.4 | 2.37 | 2.7115 | 1.79 | H0->L+0(+90%) | ||
| ZINDO | MEH-PPV | 497.6 | 2.49 | 2.37 | 1.95 | H0->L+0(+84%) H-1-> L+1(+7%) | |
| P3HT | 721.3 | 1.72 | 1.61 | 4.16 | H0->L+0(+87%) H-1-> L+1(6%) | ||
| Ramif | 721.1 | 1.72 | 1.6460 | 4.08 | H0->L+0(+86%) H-1-> L+1(+5%) | ||
| Block | 721.8 | 1.72 | 1.7651 | 3.80 | H0->L+0(+86%) |
| Composite | HOMO (eV) | LUMO (eV) | |
|---|---|---|---|
| MEH-PPV-P3HT (Block): PCBM | 4.43 | 3.06 | 1.37 |
| MEH-PPV-P3HT (Ramif): PCBM | 4.42 | 3.01 | 1.41 |
| MEH-PPV-P3HT (Block) | 4.31 | 1.85 | 2.46 |
| MEH-PPV-P3HT (Ramif) | 4.54 | 1.82 | 2.72 |
| PCBM | 5.8 | 3.09 | 2.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ltayef, M.; Almoneef, M.M.; Taouali, W.; Mbarek, M.; Alimi, K. Conception and Theoretical Study of a New Copolymer Based on MEH-PPV and P3HT: Enhancement of the Optoelectronic Properties for Organic Photovoltaic Cells. Polymers 2022, 14, 513. https://doi.org/10.3390/polym14030513
Ltayef M, Almoneef MM, Taouali W, Mbarek M, Alimi K. Conception and Theoretical Study of a New Copolymer Based on MEH-PPV and P3HT: Enhancement of the Optoelectronic Properties for Organic Photovoltaic Cells. Polymers. 2022; 14(3):513. https://doi.org/10.3390/polym14030513
Chicago/Turabian StyleLtayef, Mariem, Maha M. Almoneef, Walid Taouali, Mohamed Mbarek, and Kamel Alimi. 2022. "Conception and Theoretical Study of a New Copolymer Based on MEH-PPV and P3HT: Enhancement of the Optoelectronic Properties for Organic Photovoltaic Cells" Polymers 14, no. 3: 513. https://doi.org/10.3390/polym14030513
APA StyleLtayef, M., Almoneef, M. M., Taouali, W., Mbarek, M., & Alimi, K. (2022). Conception and Theoretical Study of a New Copolymer Based on MEH-PPV and P3HT: Enhancement of the Optoelectronic Properties for Organic Photovoltaic Cells. Polymers, 14(3), 513. https://doi.org/10.3390/polym14030513