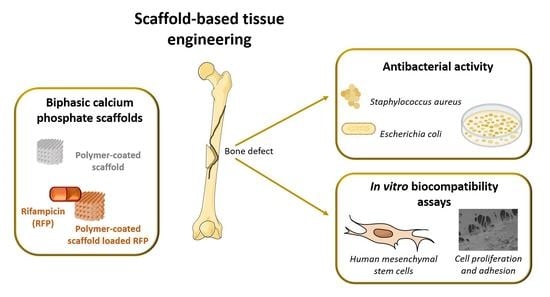
Highly Porous Composite Scaffolds Endowed with Antibacterial Activity for Multifunctional Grafts in Bone Repair
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BCP Scaffolds
2.2. Preparation of Polymeric Coated Scaffolds Loaded with RFP
2.3. Characterization of the Obtained Scaffolds
2.4. In Vitro RFP Release Study
2.5. Antibacterial Activity Assay
2.6. Isolation and Culture of Human Mesenchymal Stem Cells from Umbilical Cord Matrix
2.7. In Vitro Cytocompatibility Assays
2.8. Cell Viability and Proliferation
2.9. Cell Attachment
2.10. Statistical Analysis
3. Results and Discussion
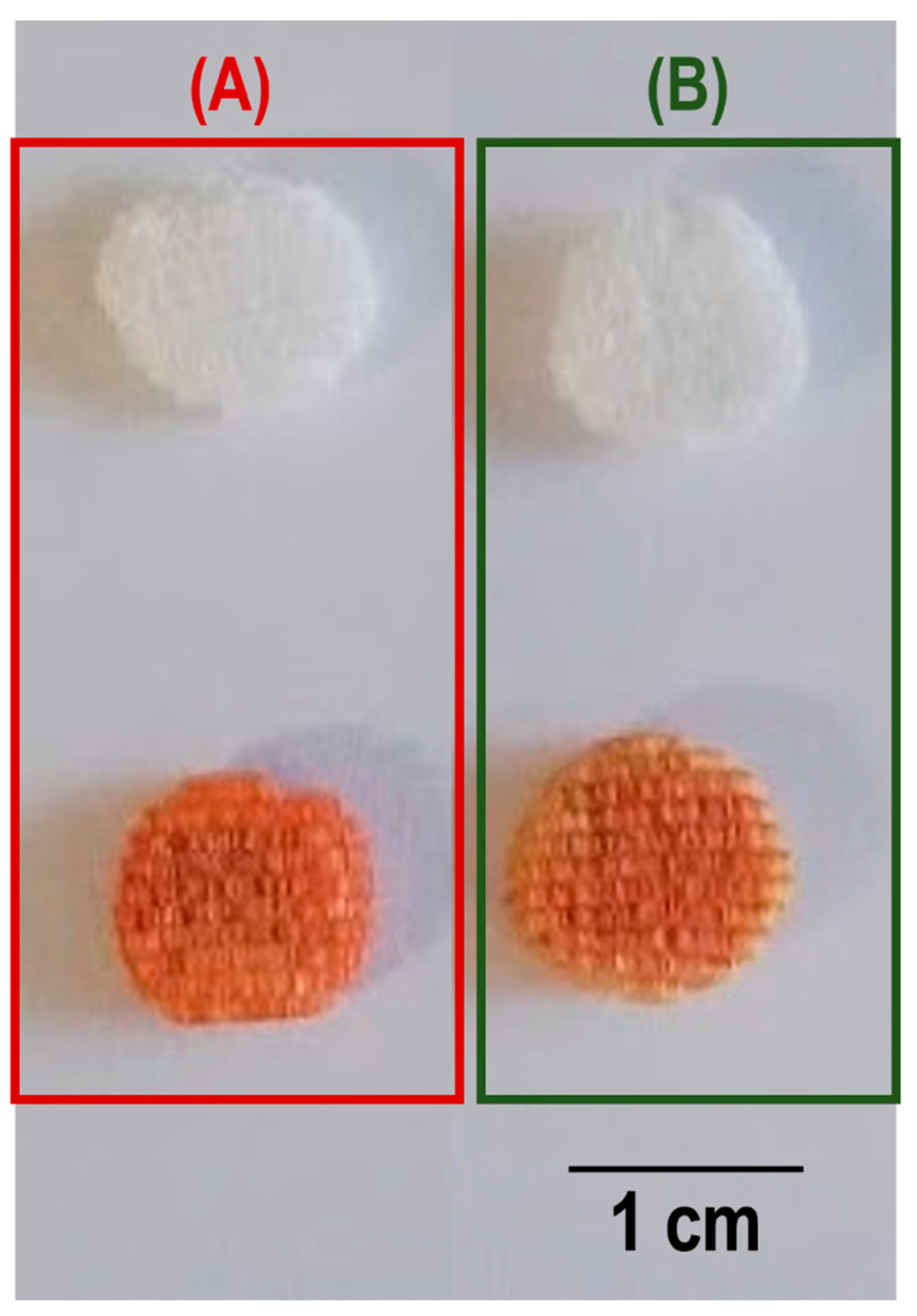
3.1. Characterization of the Scaffolds
3.2. In Vitro RFP Release Study
3.3. Antibacterial Activity Assays
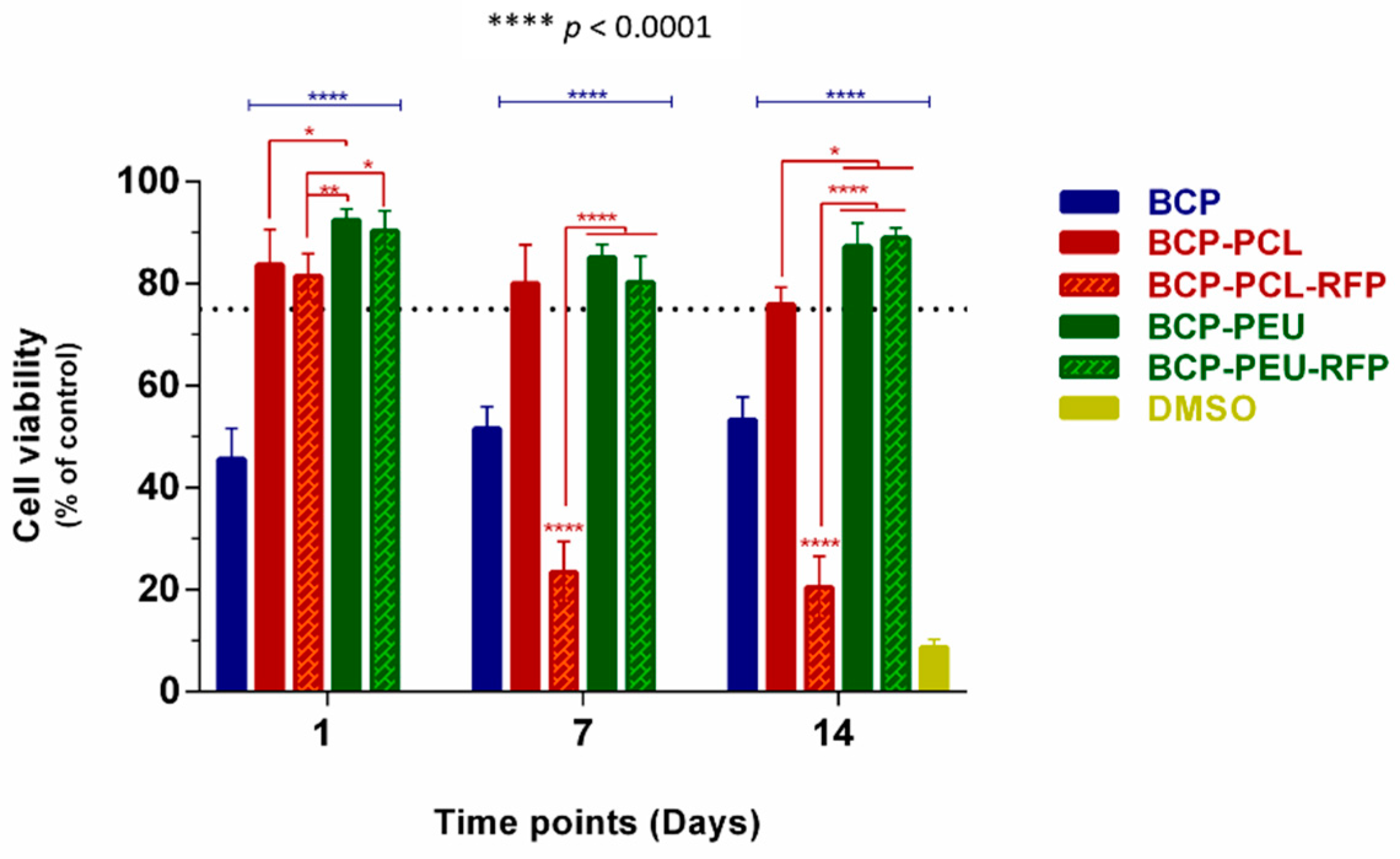
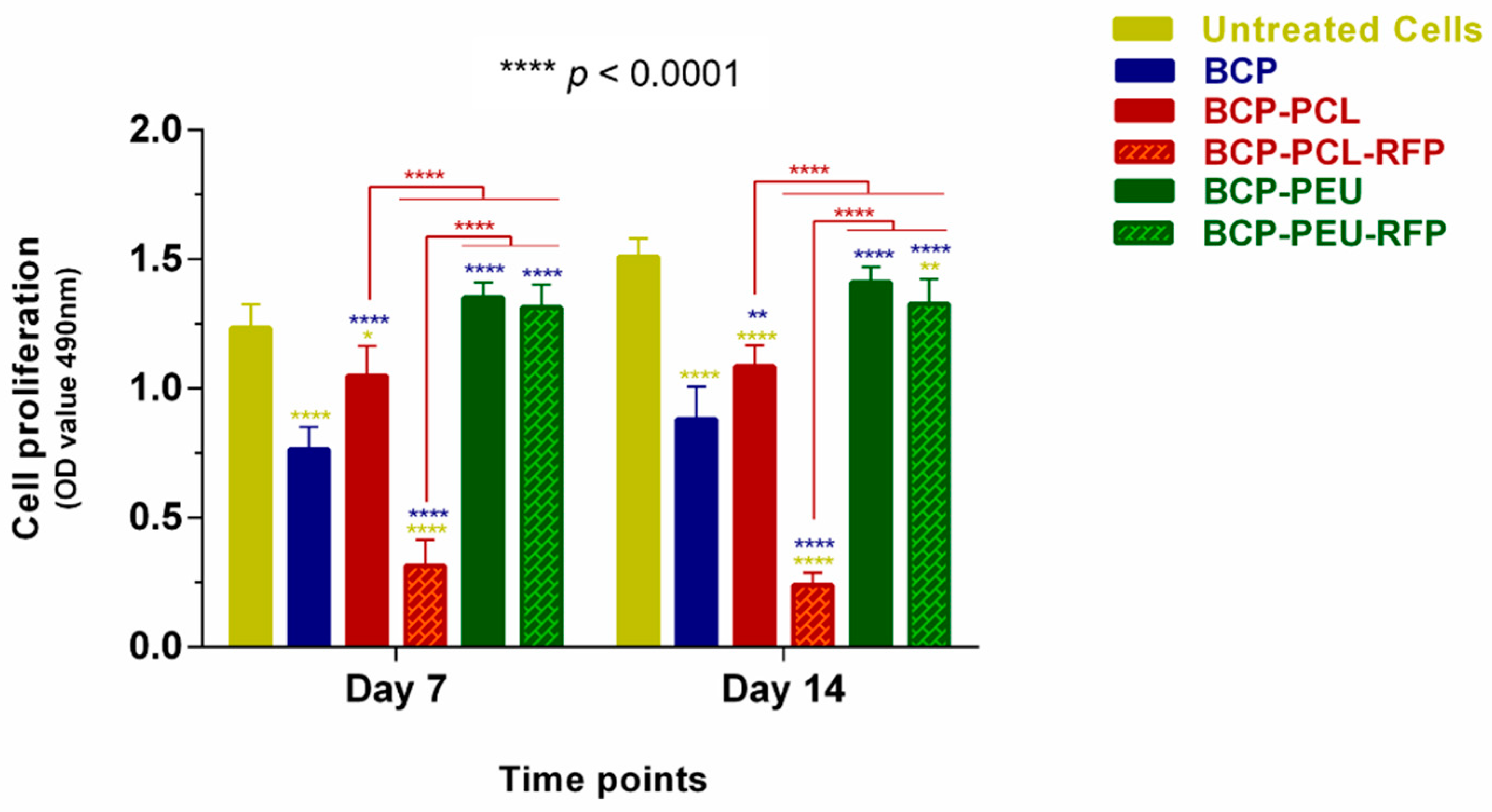
3.4. Viability and Proliferation of Human Mesenchymal Stem Cells
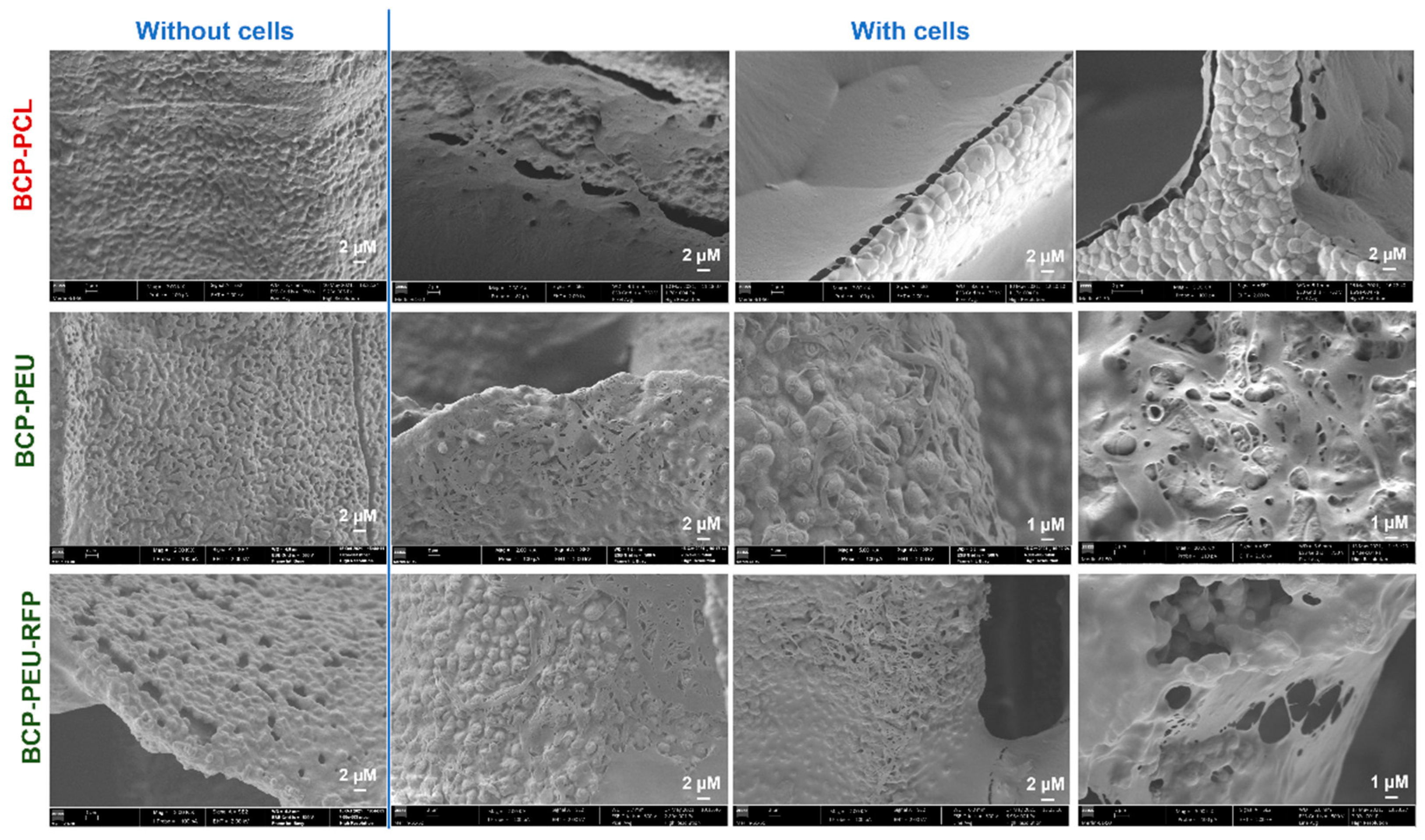
3.5. Cell Attachment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.E.; Arinzeh, T.L. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Motealleh, A.; Eqtesadi, S.; Pajares, A.; Miranda, P. Enhancing the mechanical and in vitro performance of robocast bioglass scaffolds by polymeric coatings: Effect of polymer composition. J. Mech. Behav. Biomed. Mater. 2018, 84, 35–45. [Google Scholar] [CrossRef]
- Cortizo, M.S.; Belluzo, M.S. Biodegradable Polymers for Bone Tissue Engineering. In Industrial Applications of Renewable Biomass Products; Goyanes, S.N., D’Accorso, N.B., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–74. [Google Scholar]
- Araújo, M.; Viveiros, R.; Philippart, A.; Miola, M.; Doumett, S.; Baldi, G.; Perez, J.; Boccaccini, A.R.; Aguiar-Ricardo, A.; Verné, E. Bioactivity, mechanical properties and drug delivery ability of bioactive glass-ceramic scaffolds coated with a natural-derived polymer. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 342–351. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Sarkar, N.; Banerjee, D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Mater. Today Chem. 2018, 8, 110–120. [Google Scholar] [CrossRef]
- Al-Sokanee, Z.N.; Toabi, A.A.H.; Al-Assadi, M.J.; Alassadi, E.A.S. The drug release study of ceftriaxone from porous hydroxyapatite scaffolds. AAPS PharmSciTech 2009, 10, 772–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, F.; Hornez, J.C.; Blanchemain, N.; Neut, C.; Descamps, M.; Hildebrand, H.F. Antibacterial activation of hydroxyapatite (HA) with controlled porosity by different antibiotics. Biomol. Eng. 2007, 24, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- KKundu, B.; Soundrapandian, C.; Nandi, S.K.; Mukherjee, P.; Dandapat, N.; Roy, S.; Datta, B.K.; Mandal, T.K.; Basu, D.; Bhattacharya, R.N. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: A systematic approach for in vitro and in vivo animal trial. Pharm. Res. 2010, 27, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Birchall, J.D.; Thomas, N.L. On the architecture and function of cuttlefish bone. J. Mater. Sci. 1983, 18, 2081–2086. [Google Scholar] [CrossRef]
- Neto, A.S.; Fonseca, A.C.; Abrantes, J.C.C.; Coelho, J.F.J.; Ferreira, J.M.F. Surface functionalization of cuttlefish bone-derived biphasic calcium phosphate scaffolds with polymeric coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110014. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kang, H.J.; Lee, J. Improvement of the compressive strength of a cuttlefish bone-derived porous hydroxyapatite scaffold via polycaprolactone coating. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1302–1309. [Google Scholar] [CrossRef]
- Milovac, D.; Gallego Ferrer, G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 437–445. [Google Scholar] [CrossRef]
- Rogina, A.; Antunovic, M.; Milovac, D. Biomimetic design of bone substitutes based on cuttlefish bone-derived hydroxyapatite and biodegradable polymers. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 107, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Saranya, N.; Saravanan, S.; Moorthi, A.; Ramyakrishna, B.; Selvamurugan, N. Enhanced Osteoblast Adhesion on Polymeric Nano-Scaffolds for Bone Tissue Engineering. J. Biomed. Nanotechnol. 2011, 7, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.; Gil, M.H.; Simoes, P.N. Biodegradable poly(ester amide)s—A remarkable opportunity for the biomedical area: Review on the synthesis, characterization and applications. Prog. Polym. Sci. 2014, 39, 1291–1311. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Yu, J.; Becker, M.L. Enhanced osteogenic activity of poly(ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials 2017, 141, 176–187. [Google Scholar] [CrossRef]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Sandeep Kranthi Kiran, A.; Kizhakeyil, A.; Ramalingam, R.; Verma, N.K.; Lakshminarayanan, R.; Kumar, T.S.; Doble, M.; Ramakrishna, S. Drug loaded electrospun polymer/ceramic composite nanofibrous coatings on titanium for implant related infections. Ceram. Int. 2019, 45, 18710–18720. [Google Scholar] [CrossRef]
- Aragón, J.; Feoli, S.; Irusta, S.; Mendoza, G. Composite scaffold obtained by electro-hydrodynamic technique for infection prevention and treatment in bone repair. Int. J. Pharm. 2019, 557, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruckh, T.T.; Oldinski, R.A.; Carroll, D.A.; Mikhova, K.; Bryers, J.D.; Popat, K.C. Antimicrobial effects of nanofiber poly(caprolactone) tissue scaffolds releasing rifampicin. J. Mater. Sci. Mater. Med. 2012, 23, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian ans non-Fikian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Pati, R.; Sahu, R.; Panda, J.; Sonawane, A. Encapsulation of zinc-rifampicin complex into transferrin-conjugated silver quantum-dots improves its antimycobacterial activity and stability and facilitates drug delivery into macrophages. Sci. Rep. 2016, 6, 24184. [Google Scholar] [CrossRef] [Green Version]
- Bianco, P.; Sacchetti, B.; Riminucci, M. Stem cells in skeletal physiology and endocrine diseases of bone. Endocr. Dev. 2011, 21, 91–101. [Google Scholar] [PubMed]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, B.; Han, C.; Lu, X.; Sun, W.; Wang, D.; Lu, J.; Zhao, J.; Zhang, C.; Xie, Y. In vitro comparison of three rifampicin loading methods in a reinforced porous β-tricalcium phosphate scaffold. J. Mater. Sci. Mater. Med. 2015, 26, 174. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Chandrasekaran, S.K.; Paul, D.R. Dissolution-Controlled transport from dispersed matrixes. J. Pharm. Sci. 1982, 71, 1399–1402. [Google Scholar] [CrossRef]
- Wherli, W. Rifampin: Mechanisms of Action and Resistance. Rev. Infect. Dis. 1983, 5 (Suppl. S3), S407–S411. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Yang, S.S.; Lee, J. Comparison of in vitro and in vivo bioactivity: Cuttlefish-bone-derived hydroxyapatite and synthetic hydroxyapatite granules as a bone graft substitute. Biomed. Mater. 2014, 9, 025004. [Google Scholar] [CrossRef] [PubMed]
- Hongmin, L.; Wei, Z.; Xingrong, Y.; Jing, W.; Wenxin, G.; Jihong, C.; Xin, X.; Fulin, C. Osteoinductive nanohydroxyapatite bone substitute prepared via in situ hydrothermal transformation of cuttlefish bone. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 816–824. [Google Scholar] [CrossRef]
- Milovac, D.; Gamboa-Martínez, T.C.; Ivankovic, M.; Ferrer, G.G.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: In vitro cell culture studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.A.; Manzoor, F.; Jamal, A.; Tariq, M.; Ahmad, R.; Kamran, M.; Chaudhry, A.; Rehman, I.U. Mesenchymal stem cell (MSC) viability on PVA and PCL polymer coated hydroxyapatite scaffolds derived from cuttlefish. RSC Adv. 2016, 6, 32897–32904. [Google Scholar] [CrossRef]


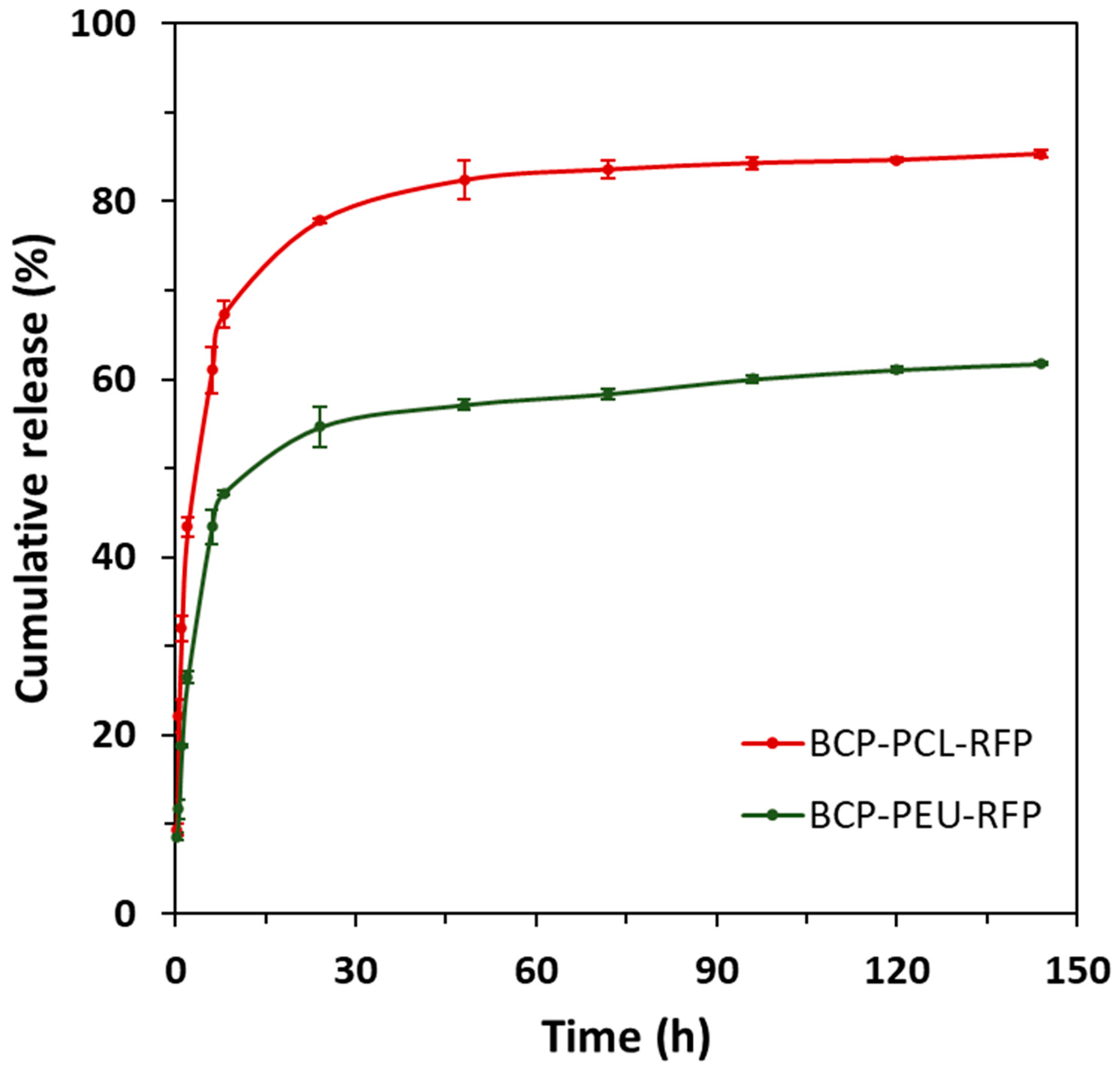

| Scaffold | n | k | R2 |
|---|---|---|---|
| BCP-PCL-RFP | 0.6215 | 0.3058 | 0.9807 |
| BCP-PEU-RFP | 0.4640 | 0.1841 | 0.9890 |
| E. coli | ||||
| Scaffold | 24 h | 48 h | 72 h | Attached Cells |
| BCP-PCL | >3 × 103 | |||
| BCP-PCL-RFP | 4.25 ± 0.25 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.00 ± 6.00 |
| BCP-PEU | >3 × 103 | |||
| BCP-PEU-RFP | >3 × 103 | >3 × 103 | >3 × 103 | >3 × 103 |
| S. aureus | ||||
| Scaffold | 24 h | 48 h | 72 h | Attached Cells |
| BCP-PCL | >3 × 103 | |||
| BCP-PCL-RFP | 0.00 ± 0.00 | 0.17 ± 0.24 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| BCP-PEU | >3 × 103 | |||
| BCP-PEU-RFP | 0.17 ± 0.24 | 0.00 ± 0.00 | 0.33 ± 0.47 | 10.00 ± 10.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neto, A.S.; Pereira, P.; Fonseca, A.C.; Dias, C.; Almeida, M.C.; Barros, I.; Miranda, C.O.; de Almeida, L.P.; Morais, P.V.; Coelho, J.F.J.; et al. Highly Porous Composite Scaffolds Endowed with Antibacterial Activity for Multifunctional Grafts in Bone Repair. Polymers 2021, 13, 4378. https://doi.org/10.3390/polym13244378
Neto AS, Pereira P, Fonseca AC, Dias C, Almeida MC, Barros I, Miranda CO, de Almeida LP, Morais PV, Coelho JFJ, et al. Highly Porous Composite Scaffolds Endowed with Antibacterial Activity for Multifunctional Grafts in Bone Repair. Polymers. 2021; 13(24):4378. https://doi.org/10.3390/polym13244378
Chicago/Turabian StyleNeto, Ana S., Patrícia Pereira, Ana C. Fonseca, Carla Dias, Mariana C. Almeida, Inês Barros, Catarina O. Miranda, Luís P. de Almeida, Paula V. Morais, Jorge F. J. Coelho, and et al. 2021. "Highly Porous Composite Scaffolds Endowed with Antibacterial Activity for Multifunctional Grafts in Bone Repair" Polymers 13, no. 24: 4378. https://doi.org/10.3390/polym13244378