Synthesis of Waterborne Polyurethane Using Phosphorus-Modified Rigid Polyol and its Physical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of P-Polyol
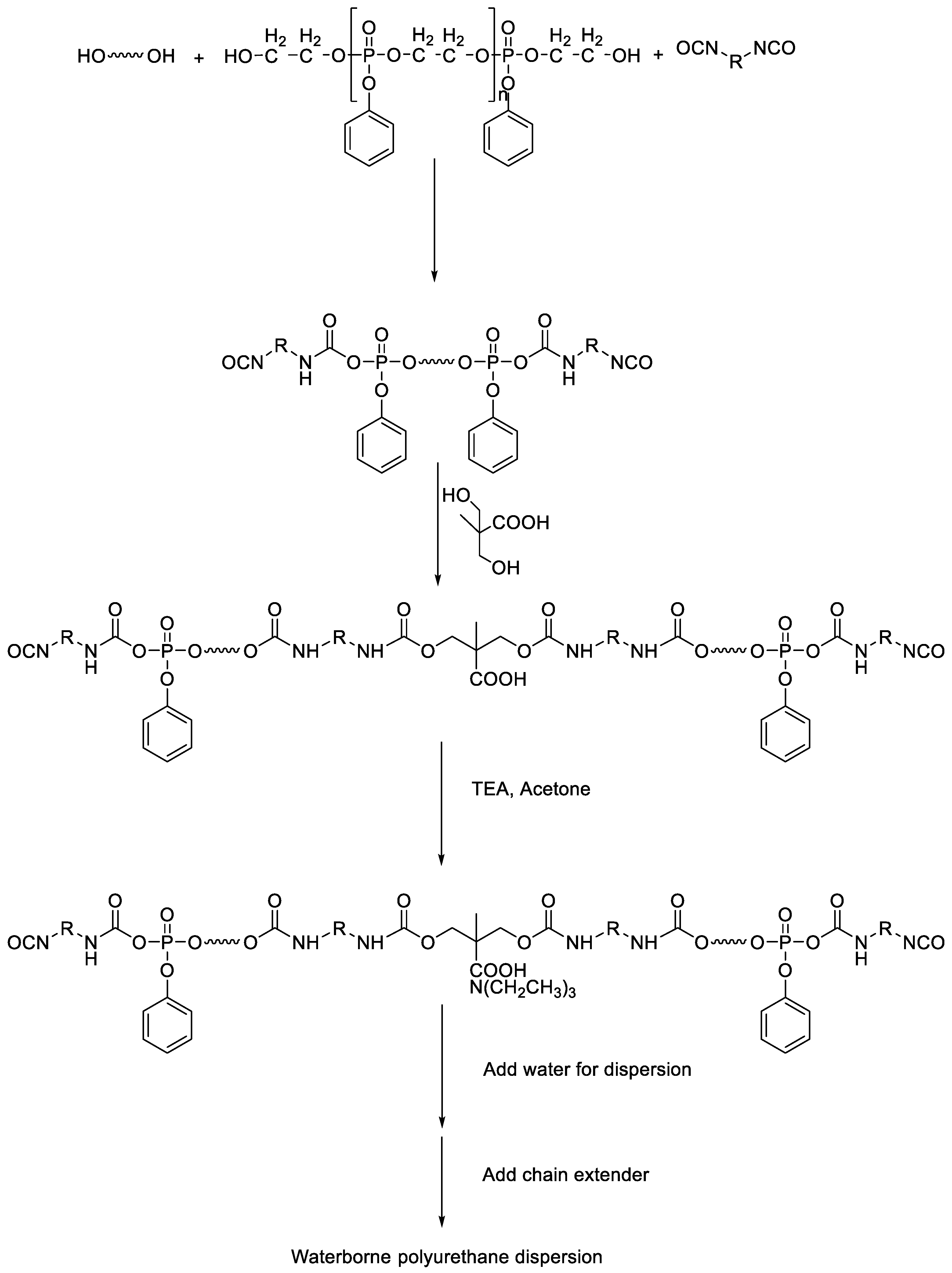
2.3. Synthesis of WPUs
2.4. Preparation of WPU Films
2.5. Characterization
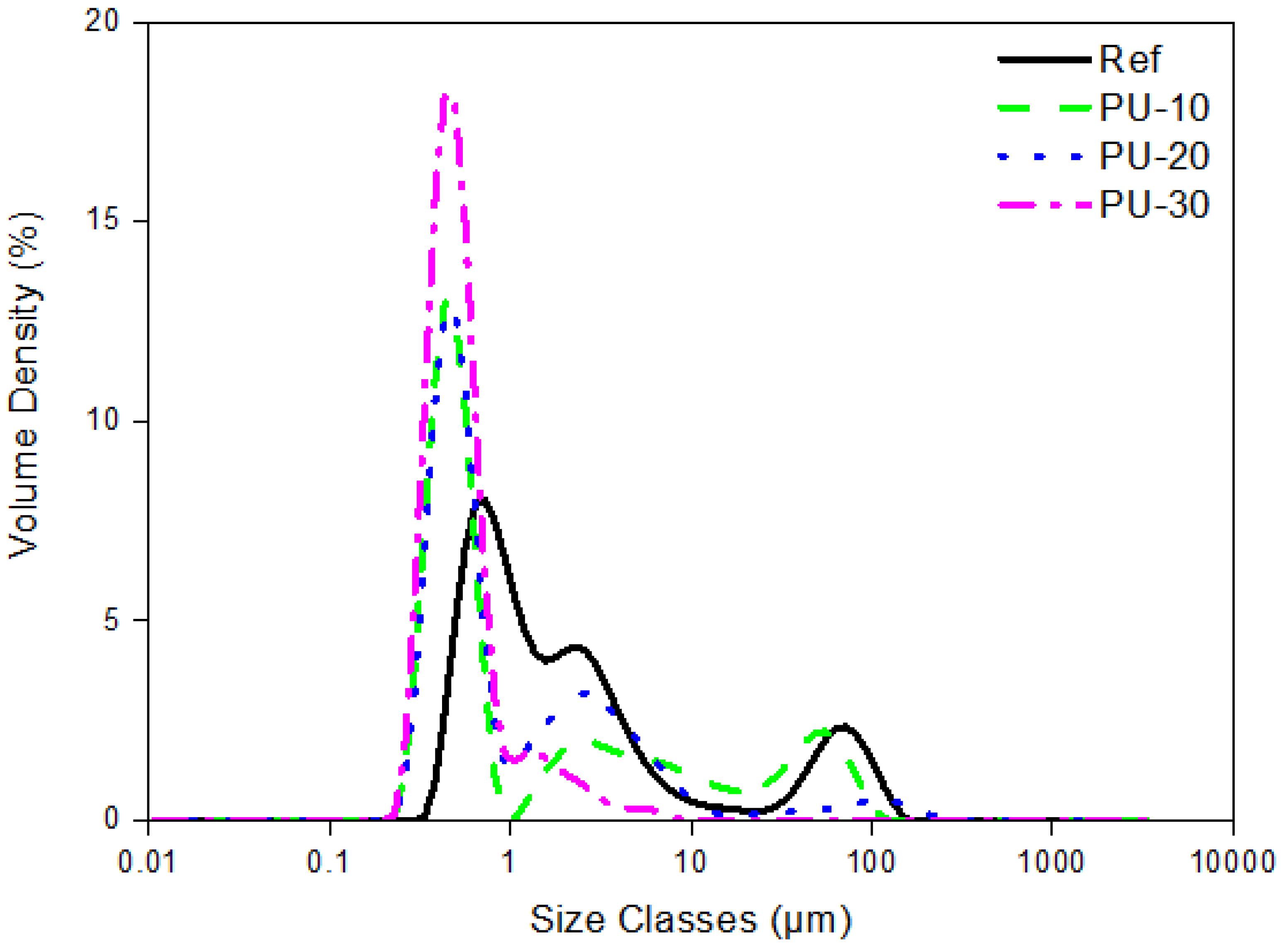
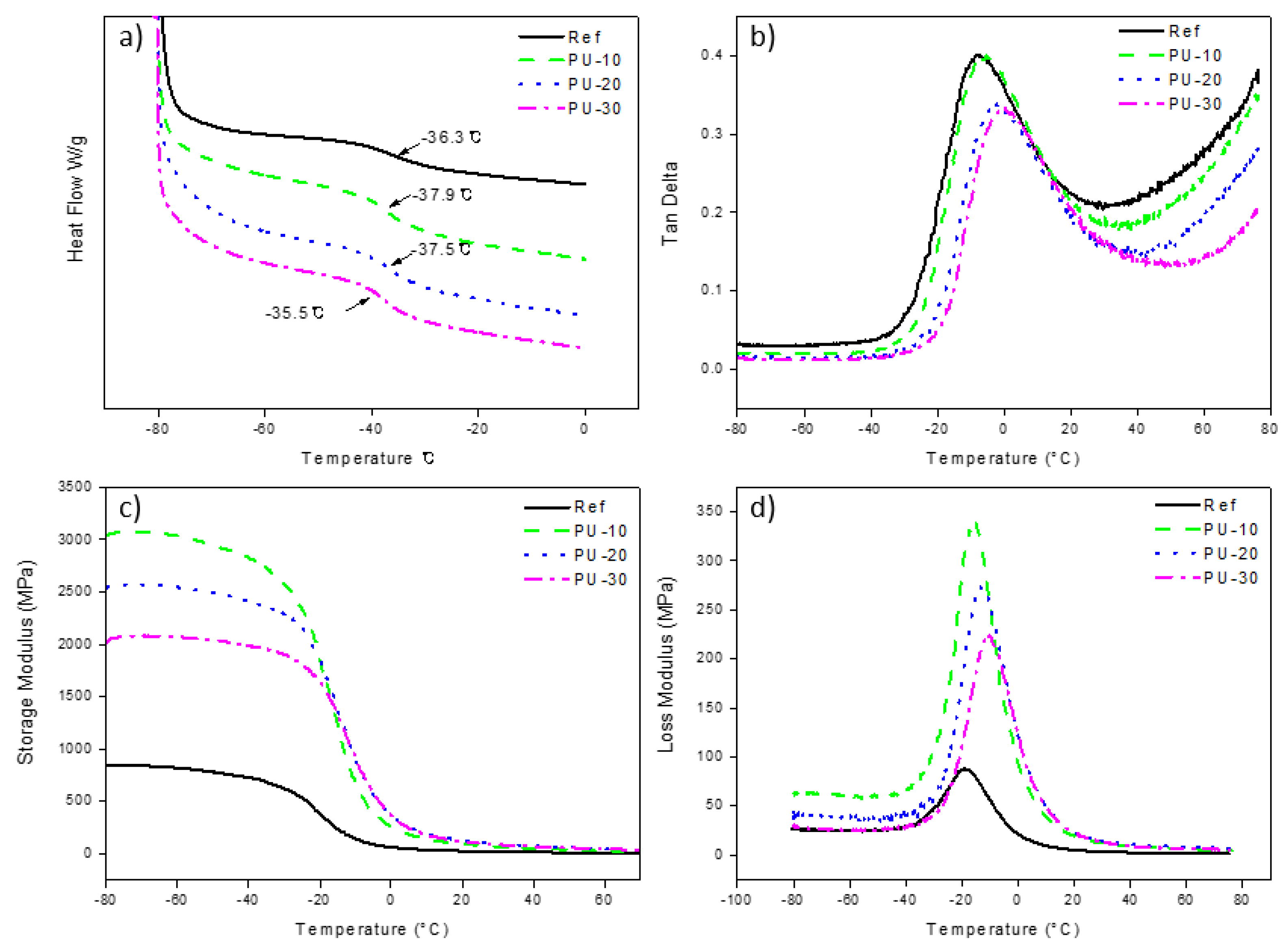
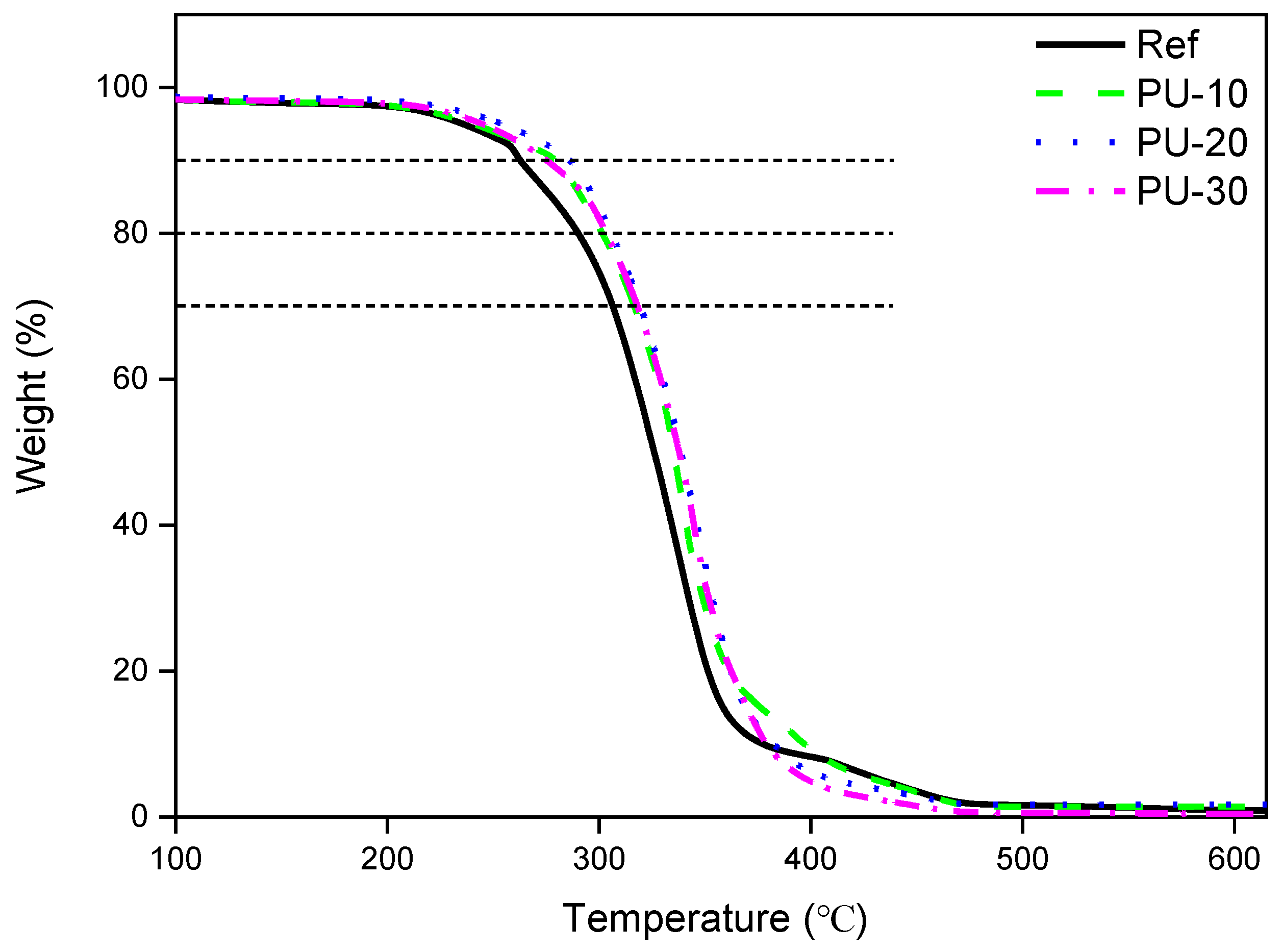
3. Results and Discussion
Characterization of WPUs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-Based Polymer Nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Xing, W.; Lu, H. In Situ Polymerization of Graphenenanosheets and Polyurethane with Enhanced Mechanical and Thermal Properties. J. Mater. Chem. 2011, 21, 4222. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, H. Constructing Sacrificial Bonds and Hidden Lengths for Ductile Graphene/Polyurethane Elastomers with Improved Strength and Toughness. J. Mater. Chem. 2012, 22, 12479. [Google Scholar] [CrossRef]
- Tsai, W.-T. Toxic Volatile Organic Compounds (VOCs) in the Atmospheric Environment: Regulatory Aspects and Monitoring in Japan and Korea. Environments 2016, 3, 23. [Google Scholar] [CrossRef]
- Fu, H.; Wang, Y.; Chen, W.; Zhou, W.; Xiao, J. A Novel Silanized CoFe2O4/fluorinated Waterborne Polyurethanepressure Sensitive Adhesive. Appl. Surf. Sci. 2015, 351, 1204–1212. [Google Scholar] [CrossRef]
- Lei, L.; Zhong, L.; Lin, X.; Li, Y.; Xia, Z. Synthesis and Characterization of Waterborne Polyurethane Dispersions with Different Chain Extenders for Potential Application in Waterborne Ink. Chem. Eng. J. 2014, 253, 518–525. [Google Scholar] [CrossRef]
- Heck, C.A.; dos Santos, J.H.Z.; Wolf, C.R. Waterborne Polyurethane: The Effect of the Addition or In Situ Formation of Silica on Mechanical Properties and Adhesion. Int. J. Adhes. Adhes. 2015, 58, 13–20. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent Developments on Nanocellulose Reinforced Polymer Nanocomposites: A Review. Polymer 2017, 132, 368–393. [Google Scholar]
- Zhao, J.; Zhou, T.; Zhang, J.H.; Chen, H.M.; Yuan, C.Y.; Zhang, W.D.; Zhang, A.M. Synthesis of a Waterborne Polyurethane-Fluorinated Emulsion and Its Hydrophobic Properties of Coating Films. Ind. Eng. Chem. Res. 2014, 53, 19257–19264. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Saralegi, A.; Costa, M.R.P.F.N.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Synthesis of Waterborne Polyurethane-Urea Dispersions with Chain Extension Step in Homogeneous and Heterogeneous Media. J. Colloid Interface Sci. 2016, 476, 184–192. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.A.; Basri, M.; Jusoh, E.R. Waterborne Polyurethane Dispersions Synthesized from Jatropha Oil. Ind. Crop. Prod. 2015, 64, 194–200. [Google Scholar] [CrossRef]
- Muzaffar, S.; Bhatti, I.A.; Zuber, M.; Bhatti, H.N.; Shahid, M. Study of the UV Protective and Antibacterial Properties of Aqueous Polyurethane Dispersions Extended with Low Molecular Weight Chitosan. Int. J. Biol. Macromol. 2017, 94, 51–60. [Google Scholar] [CrossRef] [PubMed]
- García-Pacios, V.; Jofre-Reche, J.A.; Costa, V.; Colera, M.; Martín-Martínez, J.M. Coatings Prepared from Waterborne Polyurethane Dispersions Obtained with Polycarbonates of 1,6-Hexanediol of Different Molecular Weights. Prog. Org. Coat. 2013, 76, 1484–1493. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Cincović, M.M.; Nikolić, N.Č.; Nikolić, L.B.; Cvetinov, M.J. Synthesis and Properties Biobased Waterborne Polyurethanes from Glycolysis Product of PET Waste and Poly (Caprolactone) Diol. Prog. Org. Coat. 2017, 105, 111–122. [Google Scholar] [CrossRef]
- Vogt-Birnbrich, B.V. Novel Synthesis of Low VOC Polymeric Dispersions and Their Application in Waterborne Coatings. Prog. Org. Coat. 1996, 29, 31–38. [Google Scholar] [CrossRef]
- Park, C.; Wu, J.; Park, H.J. Syntheses and characterizations of two-component polyurethane flame retardant coatings using 2, 4-dichloro modified polyesters. Coat. Technol. 1997, 69, 41. [Google Scholar] [CrossRef]
- Kim, B.K.; Seo, J.W.; Jeong, H.M. Morphology and Properties of Waterborne Polyurethane/Clay Nanocomposites. Eur. Polym. J. 2003, 39, 85–91. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Tian, X.; Zheng, K.; Cheng, Q. Effect of 3-aminopropyltriethox-ysilane on Polycarbonate Based Waterborne Polyurethane Transparent Coatings. Prog. Org. Coat. 2014, 77, 1073–1078. [Google Scholar] [CrossRef]
- Kale, M.B.; Divakaran, N.; Mubarak, S.; Dhamodharan, D.; Senthil, T.; Wu, L. Waterborne Polyurethane Nanocomposite Reinforced with Amine Intercalated α-Zirconium Phosphate—Study of Thermal and Mechanical Properties. Polymer 2020, 186, 122008. [Google Scholar]
- Kim, H.J.; Han, J.; Son, Y. Effect of a Monomer Composition on the Mechanical Properties and Glass Transition Temperature of a Waterborne Polyurethane/Graphene Oxide and Waterborne Polyurethane/MWCNT Nanocomposite. Polymers 2020, 12, 2013. [Google Scholar] [CrossRef]
- Lee, D.I.; Kim, S.H.; Lee, D.S. Synthesis of Self-Healing Waterborne Polyurethane Systems Chain Extended with Chitosan. Polymers 2019, 11, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.B.; Kim, T.H.; Kim, T.; Kim, H.J.; Seo, B.; Lim, C.S.; Lee, W. Modified Epoxy Resin Synthesis from Phosphorus—Containing Polyol and Physical Changes Studies in the Synthesized Products. Polymers 2019, 11, 2116. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, T.; Zhao, W.; Li, P.; Wu, Y. Synthesis and Characterization of Waterborne Polyurethane Emulsions Based on Poly(Butyleneitaconate) ester. Designed Monomers Polym. 2016, 19, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Ma, Y.; Zhang, Z.; Yang, X.; Huang, M.; Chai, C. The Relationship between Solid Content and Particle Size Ratio of Waterborne Polyurethane. Coatings 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Puri, G.; Berzins, D.W.; Dhuru, V.B.; Raj, P.A.; Rambhia, S.K.; Dhir, G.; Dentino, A.R. Effect of Phosphate Group Addition on the Properties of Denture Base Resins. J. Prosthet. Dent. 2008, 100, 302–308. [Google Scholar] [CrossRef] [Green Version]


| Sample | PCD (g) | P-Polyol (g) | DMPA (g) | TEA (g) | ED (g) | Water (g) |
|---|---|---|---|---|---|---|
| Ref | 75 | 0 | 5.7 | 4.30 | 2.49 | 200 |
| PU-10 | 75 | 1.7 | 5.7 | 4.30 | 2.26 | 200 |
| PU-20 | 75 | 3.4 | 5.7 | 4.30 | 2.03 | 200 |
| PU-30 | 75 | 5.7 | 5.7 | 4.30 | 1.81 | 200 |
| Sample | Mn (g/mol) | Mw (g/mol) | Ð |
|---|---|---|---|
| Ref | 17,540 | 37,427 | 2.13 |
| PU-10 | 18,397 | 37,360 | 2.03 |
| PU-20 | 20,095 | 40,778 | 2.03 |
| PU-30 | 17,915 | 37,077 | 2.07 |
| Sample | Tan δ (°C) | Storage Modulus (MPa) | Loss Modulus (°C) |
|---|---|---|---|
| Ref | −8.0 | 832 | −19.1 |
| PU-10 | −5.4 | 3042 | −15.3 |
| PU-20 | −3.0 | 2532 | −13.4 |
| PU-30 | −0.8 | 2019 | −10.2 |
| Sample | Ref | PU-10 | PU-20 | PU-30 |
|---|---|---|---|---|
| Td at 10% weight loss (°C) | 252 | 279 | 286 | 275 |
| Td at 20% weight loss (°C) | 290 | 301 | 305 | 303 |
| Td at 30% weight loss (°C) | 325 | 335 | 338 | 337 |
| Char residue at 615 °C | 0.8 | 1.4 | 1.7 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, T.; Kim, H.J.; Jang, J.B.; Kim, T.H.; Lee, W.; Seo, B.; Ko, W.B.; Lim, C.-S. Synthesis of Waterborne Polyurethane Using Phosphorus-Modified Rigid Polyol and its Physical Properties. Polymers 2021, 13, 432. https://doi.org/10.3390/polym13030432
Jang T, Kim HJ, Jang JB, Kim TH, Lee W, Seo B, Ko WB, Lim C-S. Synthesis of Waterborne Polyurethane Using Phosphorus-Modified Rigid Polyol and its Physical Properties. Polymers. 2021; 13(3):432. https://doi.org/10.3390/polym13030432
Chicago/Turabian StyleJang, Taewoo, Hye Jin Kim, Jeong Beom Jang, Tae Hee Kim, Wonjoo Lee, Bongkuk Seo, Weon Bae Ko, and Choong-Sun Lim. 2021. "Synthesis of Waterborne Polyurethane Using Phosphorus-Modified Rigid Polyol and its Physical Properties" Polymers 13, no. 3: 432. https://doi.org/10.3390/polym13030432







