Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Collagen Type I in Bone Tissue Engineering
3. Forms of Collagen Type I Biomaterials for Bone Tissue Engineering
3.1. Powders/Particles
3.2. Fibers and Tubes
3.3. Gels
3.4. Sponges
4. Crosslinking of Collagen Type I-Based Biomaterials
5. Biological Functionalization of Collagen Type I Biomaterials
5.1. Adsorption
5.2. Entrapment
5.3. Covalent Linkage
5.4. Specific Binding through Affinity Domains
6. Composite Materials with Collagen Type I
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.D.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Egol, K.A.; Nauth, A.; Lee, M.; Pape, H.-C.; Watson, J.T.; Borrelli, J. Bone Grafting. J. Orthop. Trauma 2015, 29, S10–S14. [Google Scholar] [CrossRef] [PubMed]
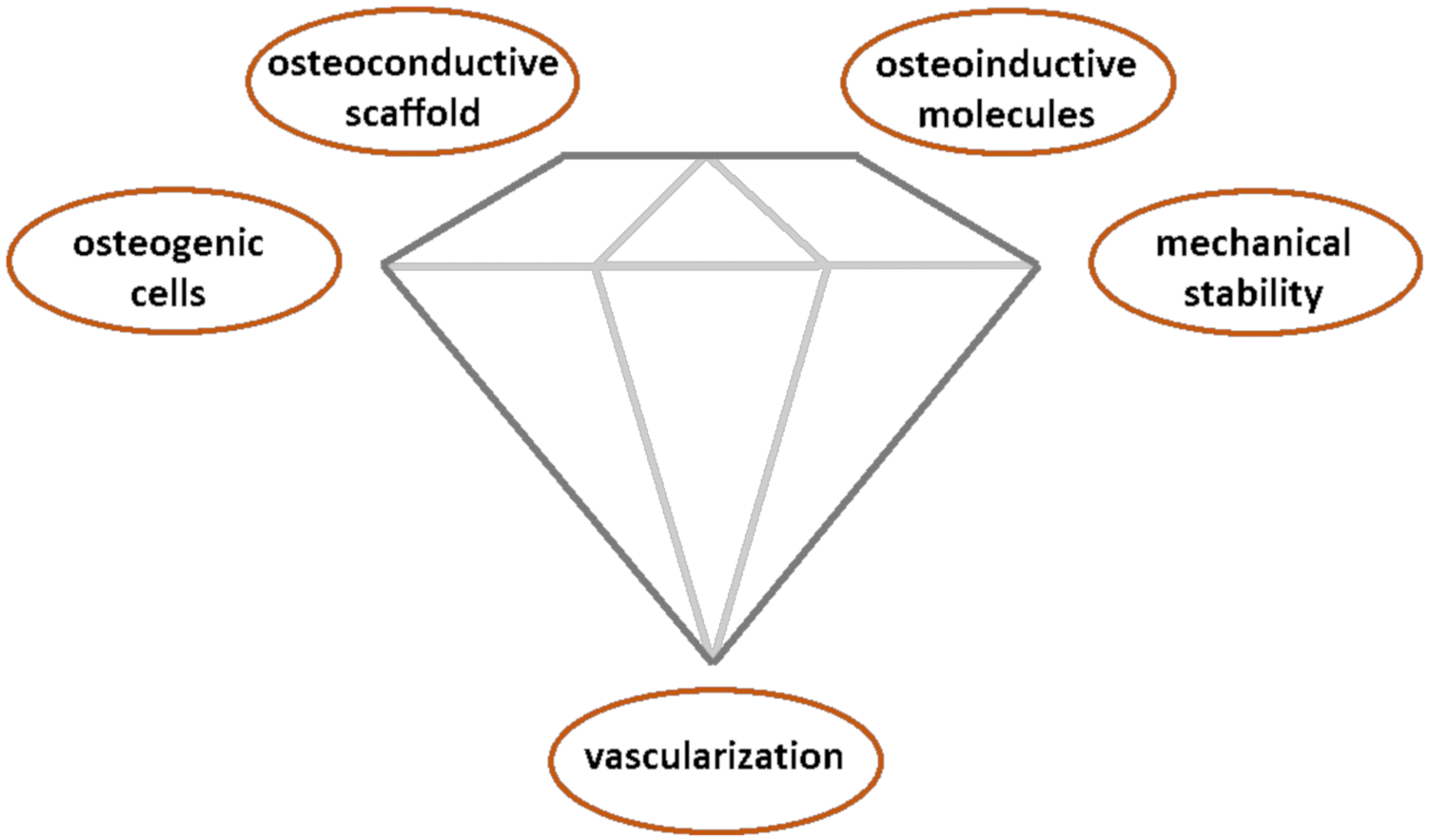
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Flierl, M.A.; Smith, W.R.; Mauffrey, C.; Irgit, K.; Williams, A.E.; Ross, E.; Peacher, G.; Hak, D.J.; Stahel, P.F. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: A retrospective cohort study in 182 patients. J. Orthop. Surg. Res. 2013, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Lobb, D.C.; DeGeorge, B.R.; Chhabra, A.B. Bone Graft Substitutes: Current Concepts and Future Expectations. J. Hand Surg. 2019, 44, 497–505. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric Scaffolds for Bone Tissue Engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Gelse, K. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.R.; Busra, M.F.M.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Idrus, R.B.H. Collagen Type I: A Versatile Biomaterial. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1077, pp. 389–414. [Google Scholar]
- Taubenberger, A.V.; Woodruff, M.A.; Bai, H.; Muller, D.J.; Hutmacher, D.W. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation. Biomaterials 2010, 31, 2827–2835. [Google Scholar] [CrossRef] [PubMed]
- Staatz, W.D.; Fok, K.F.; Zutter, M.M.; Adams, S.P.; Rodriguez, B.A.; Santoro, S.A. Identification of a tetrapeptide recognition sequence for the α2β1 integrin in collagen. J. Biol. Chem. 1991, 266, 7363–7367. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844. [Google Scholar] [CrossRef] [Green Version]
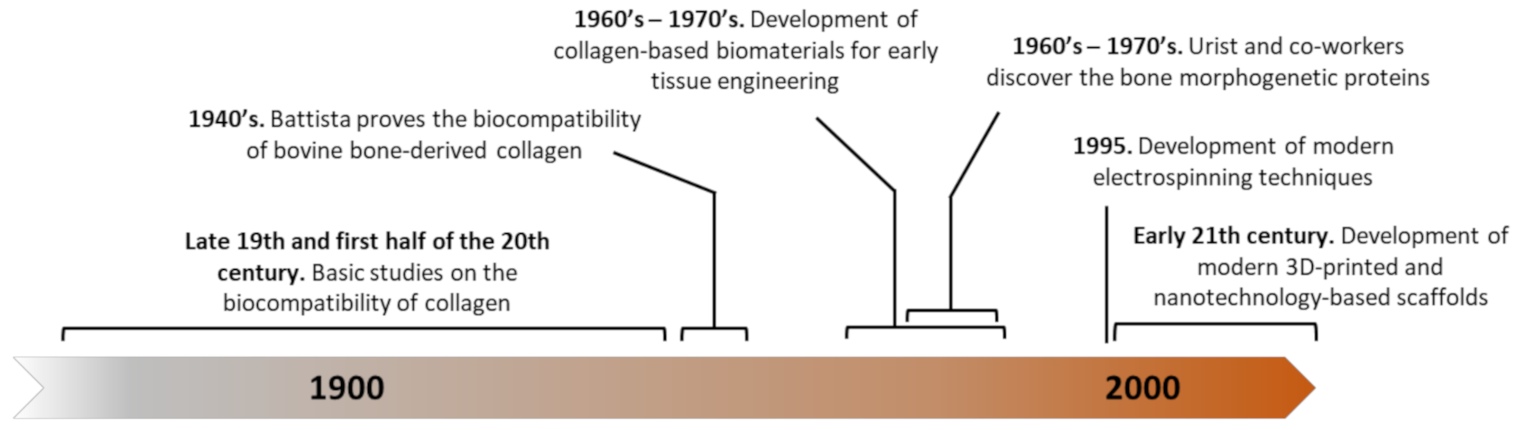
- Battista, A.F. The Reaction of Various Tissues to Implants of a Collagen Derivative. Can. J. Res. 1949, 27, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Mulberger, R.D.; Carmichael, P.L. Experimental Implants of Collagen Sponge Material in Rabbit Eyes. Am. J. Ophthalmol. 1962, 54, 19–20. [Google Scholar] [CrossRef]
- Dunn, M.W.; Stenzel, K.H.; Rubin, A.L. Collagen Implants in the Vitreous. Arch. Ophthalmol. 1969, 82, 840–844. [Google Scholar] [CrossRef]
- Bellucci, R.J.; Wolff, D. Experimental Stapedectomy with Collagen Sponge Implant. Laryngoscope 1964, 74, 668. [Google Scholar] [CrossRef]
- Friedman, E.A.; Meltzer, R.M. Collagen mesh prosthesis for repair of endopelvic fascial defects. Am. J. Obstet. Gynecol. 1970, 106, 430–433. [Google Scholar] [CrossRef]
- Bedacht, R. Tierexperimentelle Untersuchungen mit autologen und heterologen Implantaten in Röhrenknochen. Langenbeck Arch. Surg. 1971, 329, 1026–1027. [Google Scholar] [CrossRef]
- Cucin, R.L.; Goulian, D.; Stenzel, K.H.; Rubin, A.L. The effect of reconstituted collagen gels on the healing of experimental bony defects: A preliminary report. J. Surg. Res. 1972, 12, 318–321. [Google Scholar] [CrossRef]
- Speer, D.P.; Chvapil, M.; Vorz, R.G.; Holmes, M.D. Enhancement of Healing in Osteochondral Defects by Collagen Sponge Implants. Clin. Orthop. Relat. Res. 1979, 326–335. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Van De Putte, K.A.; Urist, M.R. Experimental Mineralization of Collagen Sponge and Decalcified Bone. Clin. Orthop. Relat. Res. 1965, 40, 48–56. [Google Scholar] [CrossRef]
- Urist, M.R.; Strates, B.S. Bone Morphogenetic Protein. J. Dent. Res. 1971, 50, 1392–1406. [Google Scholar] [CrossRef]
- Urist, M.R.; Iwata, H. Preservation and biodegradation of the morphogenetic property of bone matrix. J. Theor. Biol. 1973, 38, 155–167. [Google Scholar] [CrossRef]
- Urist, M.R.; Iwata, H.; Ceccotti, P.L.; Dorfman, R.L.; Boyd, S.D.; McDowell, R.M.; Chien, C. Bone Morphogenesis in Implants of Insoluble Bone Gelatin. Proc. Natl. Acad. Sci. USA 1973, 70, 3511–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreira, A.; Lojudice, F.; Halcsik, E.; Navarro, R.; Sogayar, M.; Granjeiro, J. Bone Morphogenetic Proteins. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- Saito, W.; Uchida, K.; Ueno, M.; Matsushita, O.; Inoue, G.; Nishi, N.; Ogura, T.; Hattori, S.; Fujimaki, H.; Tanaka, K.; et al. Acceleration of bone formation during fracture healing by injectable collagen powder and human basic fibroblast growth factor containing a collagen-binding domain fromClostridium histolyticumcollagenase. J. Biomed. Mater. Res. Part A 2013, 102, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, B.; Yin, Y.; Chen, B.; Zhao, Y.; Xiao, Z.; Yang, B.; Shi, Y.; Fang, Y.; Ma, X.; et al. Collagen particles with collagen-binding bone morphogenetic protein-2 promote vertebral laminar regeneration in infant rabbits. Biomed. Mater. 2020, 15, 055008. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-C.; Wang, H.; Li, J.-H.; Zhang, D.; Yin, L.-Q.; Yan, Y.-F.; Ma, X.; Xia, H.-F. Repair of alveolar cleft bone defects by bone collagen particles combined with human umbilical cord mesenchymal stem cells in rabbit. Biomed. Eng. Online 2020, 19, 1–19. [Google Scholar] [CrossRef]
- Colin, W.; Donoff, R. Nerve Regeneration Through Collagen Tubes. J. Dent. Res. 1984, 63, 987–993. [Google Scholar] [CrossRef]
- Itoh, S.; Takakuda, K.; Kawabata, S.; Aso, Y.; Kasai, K.; Itoh, H.; Shinomiya, K. Evaluation of cross-linking procedures of collagen tubes used in peripheral nerve repair. Biomaterials 2002, 23, 4475–4481. [Google Scholar] [CrossRef]
- Lopes, F.R.P.; Campos, L.C.D.M.; Corrêa, J.D.; Balduino, A.; Lora, S.; Langone, F.; Borojevic, R.; Martinez, A.M.B. Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp. Neurol. 2006, 198, 457–468. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.-K. Micro/Nano Multilayered Scaffolds of PLGA and Collagen by Alternately Electrospinning for Bone Tissue Engineering. Nanoscale Res. Lett. 2016, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dhand, C.; Ong, S.T.; Dwivedi, N.; Diaz, S.M.; Venugopal, J.R.; Navaneethan, B.; Fazil, M.H.; Liu, S.; Seitz, V.; Wintermantel, E.; et al. Bio-inspired in situ crosslinking and mineralization of electrospun collagen scaffolds for bone tissue engineering. Biomaterials 2016, 104, 323–338. [Google Scholar] [CrossRef]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 31, 155–168. [Google Scholar] [CrossRef]
- Minamide, A.; Yoshida, M.; Kawakami, M.; Yamasaki, S.; Kojima, H.; Hashizume, H.; Boden, S.D. The Use of Cultured Bone Marrow Cells in Type I Collagen Gel and Porous Hydroxyapatite for Posterolateral Lumbar Spine Fusion. Spine 2005, 30, 1134–1138. [Google Scholar] [CrossRef]
- Hao, W.; Hu, Y.-Y.; Wei, Y.-Y.; Pang, L.; Lv, R.; Bai, J.-P.; Xiong, Z.; Jiang, M. Collagen I Gel Can Facilitate Homogenous Bone Formation of Adipose-Derived Stem Cells in PLGA-β-TCP Scaffold. Cells Tissues Organs 2008, 187, 89–102. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, J.S.; Jang, J.W.; Shim, Y.B.; Lee, K.-I. Tendon-bone interface healing using an injectable rhBMP-2-containing collagen gel in a rabbit extra-articular bone tunnel model. J. Tissue Eng. Regen. Med. 2015, 11, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, I.L.; Gudovan, D.; Ficai, D.; Ficai, A.; Andronescu, E.; Albu-Kaya, M.G.; Neacsu, P.; Ion, R.N.; Cimpean, A.; Mitran, V. Collagen/hydroxyapatite bone grafts manufactured by homogeneous/heterogeneous 3D printing. Mater. Lett. 2018, 231, 179–182. [Google Scholar] [CrossRef]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Lee, H.; Yang, G.H.; Kim, M.; Lee, J.; Huh, J.; Kim, G. Fabrication of micro/nanoporous collagen/dECM/silk-fibroin biocomposite scaffolds using a low temperature 3D printing process for bone tissue regeneration. Mater. Sci. Eng. C 2018, 84, 140–147. [Google Scholar] [CrossRef]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; Hosseinkhani, H.; Heirani-Tabasi, A.; Havakhah, S.; Nekooei, S.; Jafarian, A.H.; Rezaie, F.; Peivandi, M.T.; et al. Bone defect healing is induced by collagen sponge/polyglycolic acid. J. Mater. Sci. Mater. Med. 2019, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmady, H.H.; Elazeem, A.F.A.; Ahmed, N.E.-M.B.; Shawkat, W.M.; Elmasry, M.; Abdelrahman, M.A.; Abderazik, M.A. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J. Cranio Maxillofac. Surg. 2018, 46, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Luo, Q.; Deng, B.; Morita, Y.; Ju, Y.; Song, G. Construction of tendon replacement tissue based on collagen sponge and mesenchymal stem cells by coupled mechano-chemical induction and evaluation of its tendon repair abilities. Acta Biomater. 2018, 74, 247–259. [Google Scholar] [CrossRef]
- Lim, S.; Lyu, H.-Z.; Lee, J.-R.; Han, S.H.; Lee, J.H.; Kim, B.-S. Umbilical Cord Mesenchymal Stem Cell-Derived Nanovesicles Potentiate the Bone-Formation Efficacy of Bone Morphogenetic Protein 2. Int. J. Mol. Sci. 2020, 21, 6425. [Google Scholar] [CrossRef]
- Groeneveld, E.H.J.; Van Den Bergh, J.P.A.; Holzmann, P.; Ten Bruggenkate, C.M.; Tuinzing, D.B.; Burger, E.H. Mineralization processes in demineralized bone matrix grafts in human maxillary sinus floor elevations. J. Biomed. Mater. Res. 1999, 48, 393–402. [Google Scholar] [CrossRef]
- Won, Y.-H.; Kim, S.-G.; Oh, J.-S.; Lim, S.-C. Clinical Evaluation of Demineralized Bone Allograft for Sinus Lifts in Humans: A Clinical and Histologic Study. Implant. Dent. 2011, 20, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [Green Version]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [Green Version]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Chen, G.; Kawazoe, N. Collagen-Based Porous Scaffolds for Tissue Engineering. In Biomaterials from Nature for Advanced Devices and Therapies; Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 1–15. [Google Scholar]
- Suesca, E.; Dias, A.; Braga, M.; De Sousa, H.; Fontanilla, M. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater. Sci. Eng. C 2017, 77, 333–341. [Google Scholar] [CrossRef]
- Jo, W.; Kim, J.; Yoon, J.; Jeong, D.; Cho, S.; Jeong, H.; Yoon, Y.J.; Kim, S.C.; Gho, Y.S.; Park, J. Large-scale generation of cell-derived nanovesicles. Nanoscale 2014, 6, 12056–12064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, A.; Princ, A.; Korus, G.; Ellinghaus, A.; Leemhuis, H.; Herrera, A.; Klaumünzer, A.; Schreivogel, S.; Woloszyk, A.; Schmidt-Bleek, K.; et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat. Commun. 2018, 9, 4430. [Google Scholar] [CrossRef] [Green Version]
- Moncayo, D.; Rico-Llanos, G.A.; Garzón-Alvarado, D.; Becerra, J.; Visser, R.; Fontanilla, M.R. A collagen scaffold with unidirectional pores increases bone formation in vivo. Tissue Eng Part A 2021. [Google Scholar]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Yao, C.; Markowicz, M.; Pallua, N.; Noah, E.M.; Steffens, G. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials 2008, 29, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Twardowski, T.; Fertala, A.; Orgel, J.P.R.O.; Antonio, J.D.S. Type I Collagen and Collagen Mimetics as Angiogenesis Promoting Superpolymers. Curr. Pharm. Des. 2007, 13, 3608–3621. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Yao, C.; Grieb, G.; Prével, P.; Noah, E.M.; Steffens, G.C.M. Enhancing angiogenesis in collagen matrices by covalent incorporation of VEGF. J. Mater. Sci. Mater. Electron. 2006, 17, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Patel, R.; Yadav, U.C.S.; Vasita, R. Dual drug-delivering polycaprolactone-collagen scaffold to induce early osteogenic differentiation and coupled angiogenesis. Biomed. Mater. 2020, 15, 045008. [Google Scholar] [CrossRef]
- Borrego-González, S.; Romero-Sánchez, L.B.; Blázquez, J.; Díaz-Cuenca, A. Nanostructured hybrid device mimicking bone extracellular matrix as local and sustained antibiotic delivery system. Microporous Mesoporous Mater. 2018, 256, 165–176. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-H.; Tsai, W.-B. In situ UV-crosslinking gelatin electrospun fibers for tissue engineering applications. Biofabrication 2013, 5, 035008. [Google Scholar] [CrossRef]
- Qu, T.; Jing, J.; Jiang, Y.; Taylor, R.J.; Feng, J.Q.; Geiger, B.; Liu, X. Magnesium-Containing Nanostructured Hybrid Scaffolds for Enhanced Dentin Regeneration. Tissue Eng. Part A 2014, 20, 2422–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Ma, P.X. Phase separation, pore structure, and properties of nanofibrous gelatin scaffolds. Biomaterials 2009, 30, 4094–4103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, L.M.; Bayon, Y.; Pandit, A.; Zeugolis, D.I. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng. Part B Rev. 2015, 21, 298–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and Mechanical Properties Significantly Influence Cell Attachment, Proliferation, and Migration Within Collagen Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef]
- Borrego-González, S.; Rico-Llanos, G.; Becerra, J.; Díaz-Cuenca, A.; Visser, R. Sponge-like processed D-periodic self-assembled atelocollagen supports bone formation in vivo. Mater. Sci. Eng. C 2021, 120, 111679. [Google Scholar] [CrossRef]
- Lynn, A.; Yannas, I.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2009, 339, 131–153. [Google Scholar] [CrossRef]
- Maia, F.R.; Bidarra, S.J.; Granja, P.L.; Barrias, C.C. Functionalization of biomaterials with small osteoinductive moieties. Acta Biomater. 2013, 9, 8773–8789. [Google Scholar] [CrossRef]
- Takaoka, K.; Koezuka, M.; Nakahara, H. Telopeptide-depleted bovine skin collagen as a carrier for bone morphogenetic protein. J. Orthop. Res. 1991, 9, 902–907. [Google Scholar] [CrossRef]
- Wang, E.A.; Rosen, V.; D’Alessandro, J.S.; Bauduy, M.; Cordes, P.; Harada, T.; Israel, D.I.; Hewick, R.M.; Kerns, K.M.; Lapan, P. Recombinant human bone morphogenetic protein induces bone formation. Proc. Natl. Acad. Sci. USA 1990, 87, 2220–2224. [Google Scholar] [CrossRef] [Green Version]
- Sampath, T.; Maliakal, J.; Hauschka, P.; Jones, W.; Sasak, H.; Tucker, R.; White, K.; Coughlin, J.; Tucker, M.; Pang, R. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J. Biol. Chem. 1992, 267, 20352–20362. [Google Scholar] [CrossRef]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Perry, C.R.; Cole, J.D.; Cook, S.D.; Cierny, G.; Muschler, G.F.; Zych, G.A.; Calhoun, J.H.; Laforte, A.J.; Yin, S. Osteogenic Protein-1 (Bone Morphogenetic Protein-7) in the Treatment of Tibial Nonunions. J. Bone Jt. Surg. Am. 2001, 83, S1–S151. [Google Scholar] [CrossRef]
- Charles, L.F.; Woodman, J.L.; Ueno, D.; Gronowicz, G.; Hurley, M.M.; Kuhn, L.T. Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp. Gerontol. 2015, 64, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, R.; Arrabal, P.M.; Santos-Ruiz, L.; Becerra, J.; Cifuentes, M.; Rueda, M.C. Basic fibroblast growth factor enhances the osteogenic differentiation induced by bone morphogenetic protein-6 in vitro and in vivo. Cytokine 2012, 58, 27–33. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, S.; Baek, H.; Lee, K.M.; Chang, B.; Lee, C. Synergistic induction of early stage of bone formation by combination of recombinant human bone morphogenetic protein-2 and epidermal growth factor. J. Tissue Eng. Regen. Med. 2014, 9, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoma, D.S.; Sapata, V.M.; Jung, R.E.; Hüsler, J.; Cha, J.-K.; Jung, U.-W. Localized bone regeneration around dental implants using recombinant bone morphogenetic protein-2 and platelet-derived growth factor-BB in the canine. Clin. Oral Implant. Res. 2016, 28, 1334–1341. [Google Scholar] [CrossRef] [Green Version]
- Rico-Llanos, G.A.; Becerra, J.; Visser, R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J. Biomed. Mater. Res. Part A 2017, 105, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Zhou, G.; Liu, H.; Zhang, J.; Liu, M.; Xiao, X.; Fei, J.; Guan, X.; Fan, Y. Sequential releasing of VEGF and BMP-2 in hydroxyapatite collagen scaffolds for bone tissue engineering: Design and characterization. Int. J. Biol. Macromol. 2019, 123, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.P.; Raftery, R.M.; Chen, G.; Heise, A.; O’Brien, F.J.; Cryan, S. Rapid healing of a critical-sized bone defect using a collagen-hydroxyapatite scaffold to facilitate low dose, combinatorial growth factor delivery. J. Tissue Eng. Regen. Med. 2019, 13, 1843–1853. [Google Scholar] [CrossRef]
- Oliveira, É.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
- King, W.J.; Krebsbach, P.H. Growth factor delivery: How surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012, 64, 1239–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Arrighi, I.; Mark, S.; Alvisi, M.; Von Rechenberg, B.; Hubbell, J.A.; Schense, J.C. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials 2009, 30, 1763–1771. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [Green Version]
- Addi, C.; Murschel, F.; De Crescenzo, G. Design and Use of Chimeric Proteins Containing a Collagen-Binding Domain for Wound Healing and Bone Regeneration. Tissue Eng. Part B Rev. 2017, 23, 163–182. [Google Scholar] [CrossRef]
- Tuan, T.-L.; Cheung, D.T.; Wu, L.-T.; Yee, A.; Gabriel, S.; Han, B.; Morton, L.; Nimni, M.E.; Hall, F.L. Engineering, Expression and Renaturation of Targeted TGF-Beta Fusion Proteins. Connect. Tissue Res. 1996, 34, 1–9. [Google Scholar] [CrossRef]
- Andrades, J.A.; Han, B.; Becerra, J.; Sorgente, N.; Hall, F.L.; Nimni, M.E. A Recombinant Human TGF-β1 Fusion Protein with Collagen-Binding Domain Promotes Migration, Growth, and Differentiation of Bone Marrow Mesenchymal Cells. Exp. Cell Res. 1999, 250, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Andrades, J.A.; Santamaría, J.A.; Wu, L.T.; Hall, F.L.; Nimni, M.E.; Becerra, J. Production of a recombinant human basic fibroblast growth factor with a collagen binding domain. Protoplasma 2001, 218, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, B.; Sun, W.; Zhao, W.; Zhao, Y.; Dai, J. The effect of collagen-targeting platelet-derived growth factor on cellularization and vascularization of collagen scaffolds. Biomaterials 2006, 27, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, L.; Zhao, Y.; Sun, W.; Chen, B.; Lin, H.; Wang, X.; Zhang, L.; Xu, B.; Dai, J. Collagen-Targeting Vascular Endothelial Growth Factor Improves Cardiac Performance After Myocardial Infarction. Circulation 2009, 119, 1776–1784. [Google Scholar] [CrossRef]
- Visser, R.; Arrabal, P.M.; Becerra, J.; Rinas, U.; Cifuentes, M. The effect of an rhBMP-2 absorbable collagen sponge-targeted system on bone formation in vivo. Biomaterials 2009, 30, 2032–2037. [Google Scholar] [CrossRef]
- Visser, R.; Bodnarova, K.; Arrabal, P.M.; Cifuentes, M.; Becerra, J. Combining bone morphogenetic proteins-2 and -6 has additive effects on osteoblastic differentiationin vitroand accelerates bone formationin vivo. J. Biomed. Mater. Res. Part A 2015, 104, 178–185. [Google Scholar] [CrossRef]
- Saremba, S.; Nickel, J.; Seher, A.; Kotzsch, A.; Sebald, W.; Mueller, T.D. Type I receptor binding of bone morphogenetic protein 6 is dependent on N-glycosylation of the ligand. FEBS J. 2007, 275, 172–183. [Google Scholar] [CrossRef]
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. J. Control. Release 2016, 244, 122–135. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nat. Cell Biol. 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Reyes, C.D.; García, A.J. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J. Biomed. Mater. Res. Part A 2003, 65, 511–523. [Google Scholar] [CrossRef]
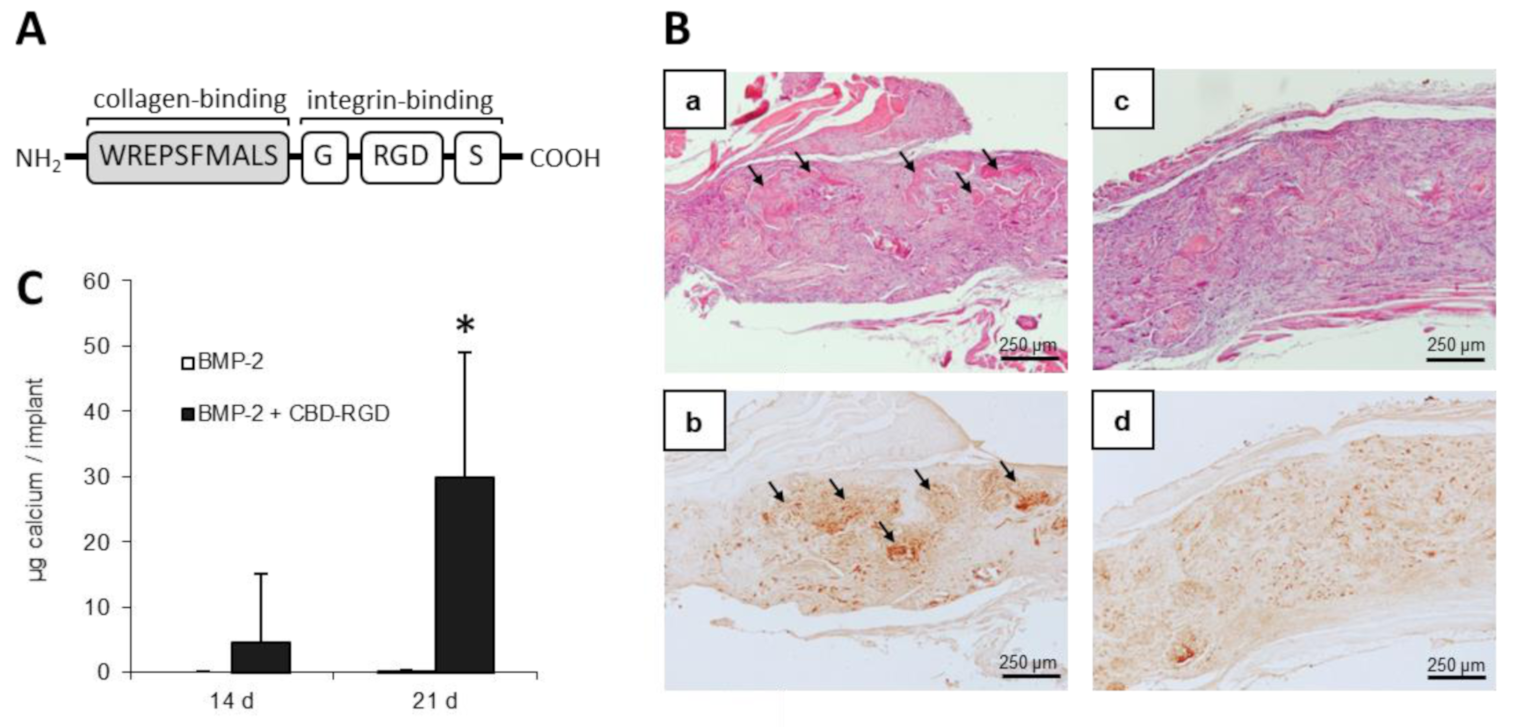
- Visser, R.; Arrabal, P.M.; Santos-Ruiz, L.; Fernandez-Barranco, R.; Becerra, J.; Cifuentes, M. A Collagen-Targeted Biomimetic RGD Peptide to Promote Osteogenesis. Tissue Eng. Part A 2014, 20, 34–44. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Furno, D.L.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-D collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically Inspired Collagen/Apatite Composite Biomaterials for Potential Use in Bone Tissue Regeneration—A Review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Hu, M.; Xiao, Y.; Cui, Y.; Yan, J.; Yang, G.; Zhang, F.; Lin, G.; Yi, H.; Han, L.; et al. Preparation recombination human-like collagen/fibroin scaffold and promoting the cell compatibility with osteoblasts. J. Biomed. Mater. Res. Part A 2021, 109, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk Fibroin/Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials toward Bone Tissue Regeneration. Materials 2020, 13, 3433. [Google Scholar] [CrossRef]
- Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. [Google Scholar] [CrossRef]

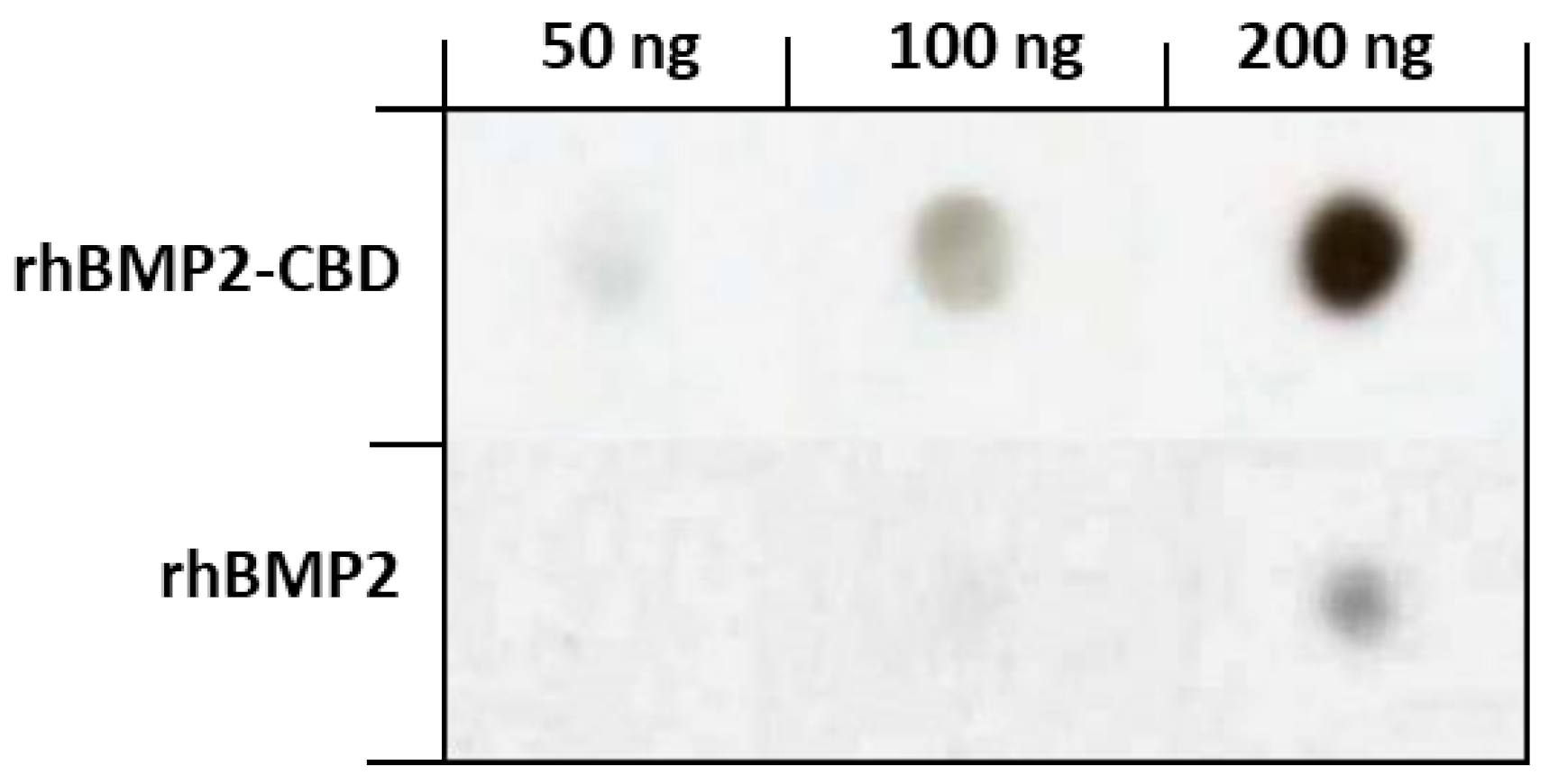
| Collagen Biomaterial Form | Combination/Modification | Biological Model | Main Results | References |
|---|---|---|---|---|
| Powders/particles | CBD-hFGF-2 | Mouse femur fracture | Increase of callus volume and bone mineral content | [33] |
| CBD-rhBMP-2 | Vertebral laminar defects in rabbits | Greater bone regeneration. Signs of bone formation even without growth factor | [34] | |
| ucMSC | Rabbit alveolar cleft model | Formation of a significant amount of new bone, higher percentage of bone trabeculae but no more mineral density | [35] | |
| Fibers and tubes | - | Rat tibial nerve resection | Evidences of some degree of histological regeneration at surgical site | [36] |
| UV irradiation crosslinking | Rat sciatic nerve section | Space of the tube is preserved and nerve repair was comparable to isograft treatment | [37] | |
| BMSC | Mouse sciatic nerve section | Scaffolds loaded with cells induced better regeneration of peripheral nerve fibers | [38] | |
| Electrospun in combination with HA | In vitro cell viability and osteogenic differentiation assay | Good physicochemical properties and feasible manufacturing process. U2-OS cells remain viable and differentiate to osteoblast | [39] | |
| Electrospun with PLGA and HA nanorods | In vitro cell viability and osteogenic differentiation assay | MC3T3-E1 cells proliferate on the scaffold. Osteogenic differentiation is evidenced by different markers | [40] | |
| Electrospun collagen containing catecholamines and Ca2+ | In vitro human foetal osteoblasts viability and osteogenic differentiation | Good mechanical properties. Bio-inspired in situ chemical crosslinking and mineralization strategy. Osteogenic differentiation is evidenced by different markers | [41] | |
| Electrospun in combination with chitosan | Rat full-thickness cranial defects | Composite had improved physicochemical properties and induced almost a total regeneration 8 weeks after implantation | [42] | |
| Gels | HA particles and bone marrow cells | Rabbit posterolateral lumbar spine fusion model | Homogeneous new trabecular bone formation similar to autograft and BMP-HA group. | [43] |
| Adipose-Derived stem cells + PLGA-β-TCP scaffold | Rabbit intramuscular ectopic bone formation assay | Composites showed new bone formation evidenced by radiography, histology and histomorphometric bone occupation analysis | [44] | |
| rhBMP-2 | Rabbit tendon-bone interface injury model | Collagen-BMP-2 gel increased fusion rate between the bone tunnel and tendon | [45] | |
| 3D printed in combination with HA | In vitro viability and proliferation of Vero cells | Superior control over scaffold morphology and porosity. Supernatants of these gels incubated in medium were not cytotoxic | [46] | |
| 3D printed collagen containing rod-like nano-HA | - | Highly controlled 3D printed mesh-like structures with a homogeneous HA distribution. | [47] | |
| 3D printed in combination with decellularized extracellular matrix and silk fibroin | In vitro MC3T3 viability and osteogenic differentiation | Feasible hybrid 3D printing method. Cell proliferation and osteogenic differentiation was higher in comparison with only-collagen controls. | [48] | |
| Sponges | PGA + AD-MSC | Rabbit calvarial bone defect | Significant improvement of bone formation by CT scan imaging analysis induced by scaffolds with or without cells. | [49] |
| Bone marrow mononuclear cells + Nano- Hydroxyapatite + platelet-rich fibrin | Human patients with unilateral alveolar cleft defects | Patients exhibited less donor site complications, faster and better soft tissue healing, less postoperative pain and a higher rate of complete alveolar bone union. | [50] | |
| Precultured system of mesenchymal stem cells + mechano-chemical induction | Rat Achilles tendon repair model | Improved tenogenic differentiation in vitro. In vivo improvements in Achilles functional index, Young’s modulus and histology score | [51] | |
| Umbilical cord mesenchymal stem cell-derived nanovesicles + rhBMP-2 | Nude mouse calvarial defect model | Micro-CT imaging analysis evidenced increased bone volume and number of trabeculae. Histology revealed increased number of vessel structures. | [52] |
| Type of Crosslinking | Main Characteristics | |
|---|---|---|
| Chemical | Glutaraldehyde (GA) | Low cost. High reactivity. High water solubility. Cytotoxic. |
| Genipin | Less toxic than other chemical crosslinkers. Might promote osteoblastic differentiation. Not suitable for gelatin crosslinking. | |
| 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). | Zero-length crosslinker. Acts mainly as an intra-fibrillar crosslinker. Low cytotoxicity. | |
| Dialdehyde starch | Can be used for intra- and intermolecular crosslinking. Low cytotoxicity. Biodegradable. Has antiviral activity. | |
| Chitosan | Biodegradable. Non-toxic. Has antibacterial and antifungal activity. Poor water solubility. Tends to form polydisperse solutions. | |
| Physical | Dehydrothermal (DHT) treatment | Non-toxic. Provides sterilization. May cause collagen denaturation. |
| UV light | Faster than DHT. Non-toxic. Provides sterilization. May cause collagen denaturation. | |
| Enzymatic | Microbial transglutaminase | Similar to natural crosslinking. More expensive. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. https://doi.org/10.3390/polym13040599
Rico-Llanos GA, Borrego-González S, Moncayo-Donoso M, Becerra J, Visser R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers. 2021; 13(4):599. https://doi.org/10.3390/polym13040599
Chicago/Turabian StyleRico-Llanos, Gustavo A., Sara Borrego-González, Miguelangel Moncayo-Donoso, José Becerra, and Rick Visser. 2021. "Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering" Polymers 13, no. 4: 599. https://doi.org/10.3390/polym13040599
APA StyleRico-Llanos, G. A., Borrego-González, S., Moncayo-Donoso, M., Becerra, J., & Visser, R. (2021). Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers, 13(4), 599. https://doi.org/10.3390/polym13040599






