Antibacterial Activity of Chitosan–Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemicals
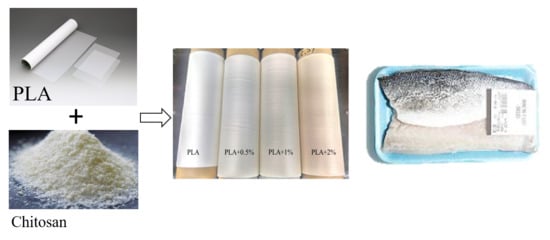
2.2. Antimicrobial Films’ Preparation
2.3. Culture Conditions
2.4. Antimicrobial Activity of Films
2.5. Application of the Active Films on the Preservation of Fish Fillet
2.6. Films’ Physical and Mechanical Properties Determination
2.6.1. Water Vapor Permeability (WVP)
2.6.2. Moisture Content
2.6.3. Solubility
2.6.4. Mechanical Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Mechanical and Physical Properties of Films
3.2. Antimicrobial Activity of Films
3.3. Chitosan–PLA Film’s Application for Preservation Fish Fillet
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amos, B.; Sector, F.; Einarsson, H.; Eythorsdottir, A. Analysis of Quality Deterioration at Critical Steps/Points in Fish Handling in Uganda and Iceland and Suggestions for Improvement; United Nations University: Kalangala, Uganda, 2007. [Google Scholar]
- Labuza, T.P.; Breene, W. Application of ‘active packaging’technologies for the improvement of shelf-life and nutritional quality of fresh and extended shelf-life foods. In Nutritional Impact of Food Processing; Karger Publishers: Basel, Switzerland, 1989; Volume 43, pp. 252–259. [Google Scholar]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for Schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Liu, Y. Preservation technologies for fresh meat–A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Hong, S.-I.; Ha, C.-S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of poly (lactic acid)(PLA) fibre. Fibre Chem. 2010, 42, 68–78. [Google Scholar] [CrossRef]
- Gahleitner, M.; Grein, C.; Kheirandish, S.; Wolfschwenger, J. Nucleation of polypropylene homo-and copolymers. Int. Polym. Process. 2011, 26, 2–20. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Chen, J.-B.; Jiang, X.; Hsiao, B.S.; Zhong, G.-J.; Fu, Q.; Li, Z.-M. Strong and tough micro/nanostructured poly (lactic acid) by mimicking the multifunctional hierarchy of shell. Mater. Horiz. 2014, 1, 546–552. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chen, C.-H.; Tsai, G.-J. Effects of chitosan on Clostridium perfringens and application in the preservation of pork sausage. Mar. Drugs 2020, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.-J.; Tsai, M.-T.; Lee, J.-M.; Zhong, M.-Z. Effects of chitosan and a low-molecular-weight chitosan on Bacillus cereus and application in the preservation of cooked rice. J. Food Prot. 2006, 69, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.-J.; Su, W.-H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Tsai, G.; Su, W.-H.; Chen, H.-C.; Pan, C.-L. Antimicrobial activity of shrimp chitin and chitosan from different treatments. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.-J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Wu, J.; Ge, S.; Liu, H.; Wang, S.; Chen, S.; Wang, J.; Li, J.; Zhang, Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Packag. Shelf Life 2014, 2, 7–16. [Google Scholar] [CrossRef]
- Cao, R.; Xue, C.-H.; Liu, Q. Changes in microbial flora of Pacific oysters (Crassostrea gigas) during refrigerated storage and its shelf-life extension by chitosan. Int. J. Food Microbiol. 2009, 131, 272–276. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruits and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar]
- Remya, S.; Mohan, C.; Bindu, J.; Sivaraman, G.; Venkateshwarlu, G.; Ravishankar, C. Effect of chitosan based active packaging film on the keeping quality of chilled stored barracuda fish. J. Food Sci. Technol. 2016, 53, 685–693. [Google Scholar] [CrossRef]
- Grande, R.; Carvalho, A.J. Compatible ternary blends of chitosan/poly (vinyl alcohol)/poly (lactic acid) produced by oil-in-water emulsion processing. Biomacromolecules 2011, 12, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Sébastien, F.; Stéphane, G.; Copinet, A.; Coma, V. Novel biodegradable films made from chitosan and poly (lactic acid) with antifungal properties against mycotoxinogen strains. Carbohydr. Polym. 2006, 65, 185–193. [Google Scholar] [CrossRef]
- Soares, F.C.; Yamashita, F.; Müller, C.M.; Pires, A.T. Thermoplastic starch/poly (lactic acid) sheets coated with cross-linked chitosan. Polym. Test. 2013, 32, 94–98. [Google Scholar] [CrossRef]
- Bonilla, J.; Fortunati, E.; Vargas, M.; Chiralt, A.; Kenny, J.M. Effects of chitosan on the physicochemical and antimicrobial properties of PLA films. J. Food Eng. 2013, 119, 236–243. [Google Scholar] [CrossRef]
- Tôei, K.; Kohara, T. A conductometric method for colloid titrations. Anal. Chim. Acta 1976, 83, 59–65. [Google Scholar] [CrossRef]
- Conway, E.J. Microdiffusion analysis and volumetric error. In Microdiffusion Analysis and Volumetric Error; Crosby Lockwood and Son, Ltd.: London, UK, 1947. [Google Scholar]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- ASTM. ASTM. ASTM D882-02. Standard Method for Tensile Properties of Thin Plastic Sheeting. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Roberts, G.A. Chitin Chemistry; Macmillan International Higher Education: Heidelberg, Germany, 1992. [Google Scholar]
- Suyatma, N.E.; Copinet, A.; Tighzert, L.; Coma, V. Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J. Polym. Environ. 2004, 12, 1–6. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Krajewska, B.; Wydro, P.; Jańczyk, A. Probing the modes of antibacterial activity of chitosan. Effects of pH and molecular weight on chitosan interactions with membrane lipids in Langmuir films. Biomacromolecules 2011, 12, 4144–4152. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N. A novel active food packaging film for shelf-life extension of minced beef meat. J. Food Saf. 2018, 38, e12569. [Google Scholar] [CrossRef]
- Fathima, P.; Panda, S.K.; Ashraf, P.M.; Varghese, T.; Bindu, J. Polylactic acid/chitosan films for packaging of Indian white prawn (Fenneropenaeus indicus). Int. J. Biol. Macromol. 2018, 117, 1002–1010. [Google Scholar] [CrossRef]






| Composition | Tensile Strength (kgf/cm2) | Elongation at Break (%) | Tearing Strength (gf) | |||
|---|---|---|---|---|---|---|
| MD | TD | MD | TD | MD | TD | |
| PLA | 448 ± 19 a | 271 ± 18 a | 318 ± 16 b | 498 ± 41 a | 54 ± 7 c | 220 ± 14 c |
| 0.5% CH–PLA | 261 ± 23 b | 139 ± 8 b | 376 ± 37 a | 416 ± 39 a | 81 ± 7 b | 282 ± 14 a |
| 1% CH–PLA | 216 ± 3 c | 133 ± 12 b | 320 ± 7 b | 414 ± 67 a | 97 ± 12 a | 252 ± 13 b |
| 2% CH–PLA | 155 ± 8 d | 79 ± 6 c | 255 ± 11 c | 271 ± 32 b | 84 ±8 b | 226 ± 24c |
| Composition | Water Vapor Transmission Rate (g mm/m2 day kPa) | Moisture Content (%) | Film Solubility (%) |
|---|---|---|---|
| PLA | 0.53 ± 0.00 d | 0.26 ± 0.37 b | 0.82 ± 0.68 a |
| 0.5% CH–PLA | 0.71 ± 0.03 c | 1.68 ± 0.32 a | 1.73 ± 1.01 a |
| 1% CH–PLA | 0.96 ± 0.02 b | 2.09 ± 1.81 a | 0.49 ± 1.33 a |
| 2% CH–PLA | 1.25 ± 0.36 a | 2.62 ± 0.35 a | 0.23 ± 0.97 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-H.; Chen, Y.-J.; Tseng, H.-J.; Hsiao, H.-I.; Chai, H.-J.; Shang, K.-C.; Pan, C.-L.; Tsai, G.-J. Antibacterial Activity of Chitosan–Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet. Polymers 2021, 13, 696. https://doi.org/10.3390/polym13050696
Chang S-H, Chen Y-J, Tseng H-J, Hsiao H-I, Chai H-J, Shang K-C, Pan C-L, Tsai G-J. Antibacterial Activity of Chitosan–Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet. Polymers. 2021; 13(5):696. https://doi.org/10.3390/polym13050696
Chicago/Turabian StyleChang, Shun-Hsien, Ying-Ju Chen, Hsiang-Jung Tseng, Hsin-I Hsiao, Huey-Jine Chai, Kuo-Chung Shang, Chorng-Liang Pan, and Guo-Jane Tsai. 2021. "Antibacterial Activity of Chitosan–Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet" Polymers 13, no. 5: 696. https://doi.org/10.3390/polym13050696
APA StyleChang, S.-H., Chen, Y.-J., Tseng, H.-J., Hsiao, H.-I., Chai, H.-J., Shang, K.-C., Pan, C.-L., & Tsai, G.-J. (2021). Antibacterial Activity of Chitosan–Polylactate Fabricated Plastic Film and Its Application on the Preservation of Fish Fillet. Polymers, 13(5), 696. https://doi.org/10.3390/polym13050696