Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of RB-Modified Universal Adhesive
2.2. Specimen Preparation and Bonding Procedures
2.3. Micro-Tensile Bond Strength
2.4. MMP-2 and Cathepsin-K Activity Determination
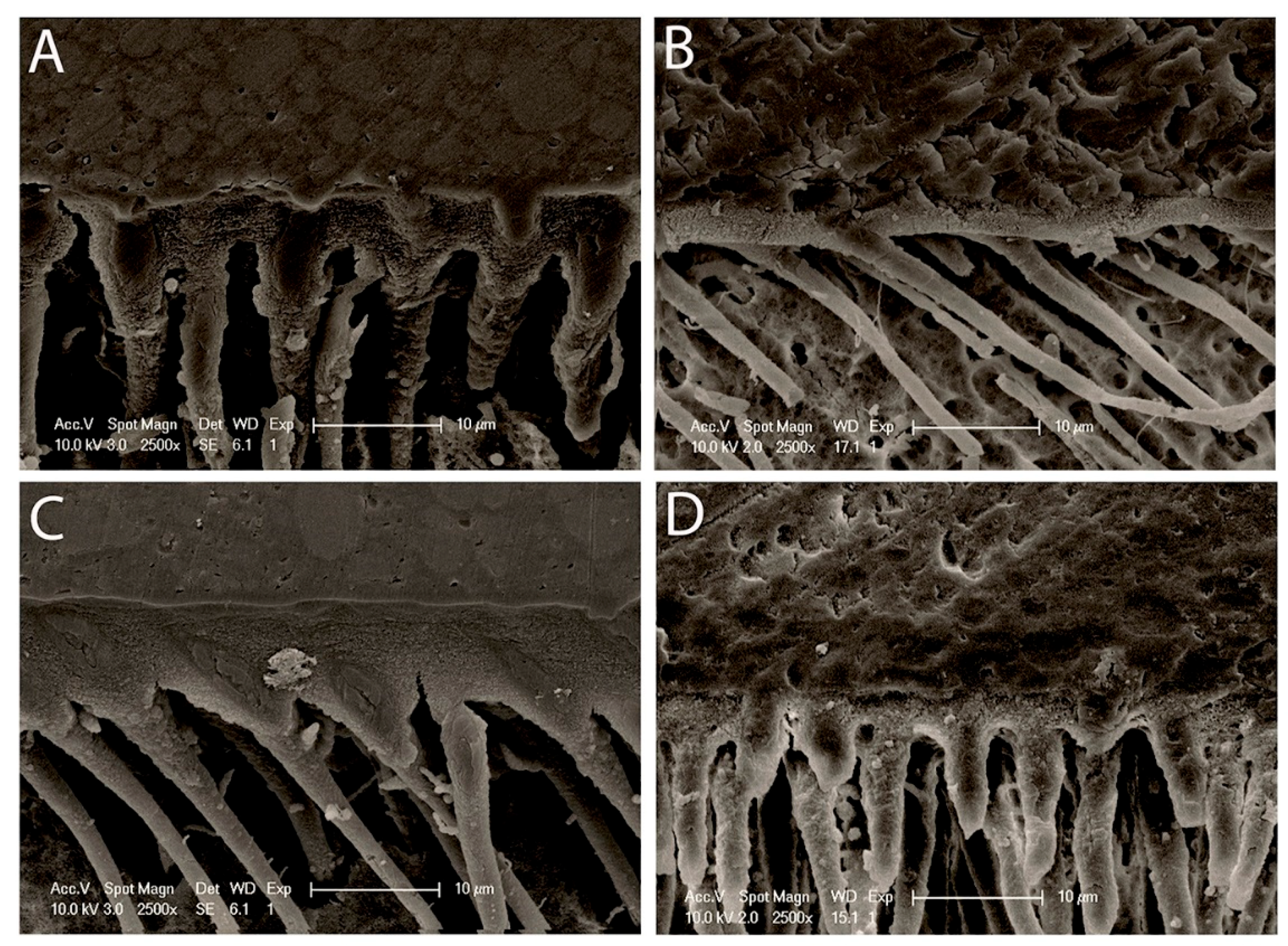
2.5. Morphology of Resin–Dentin Interface
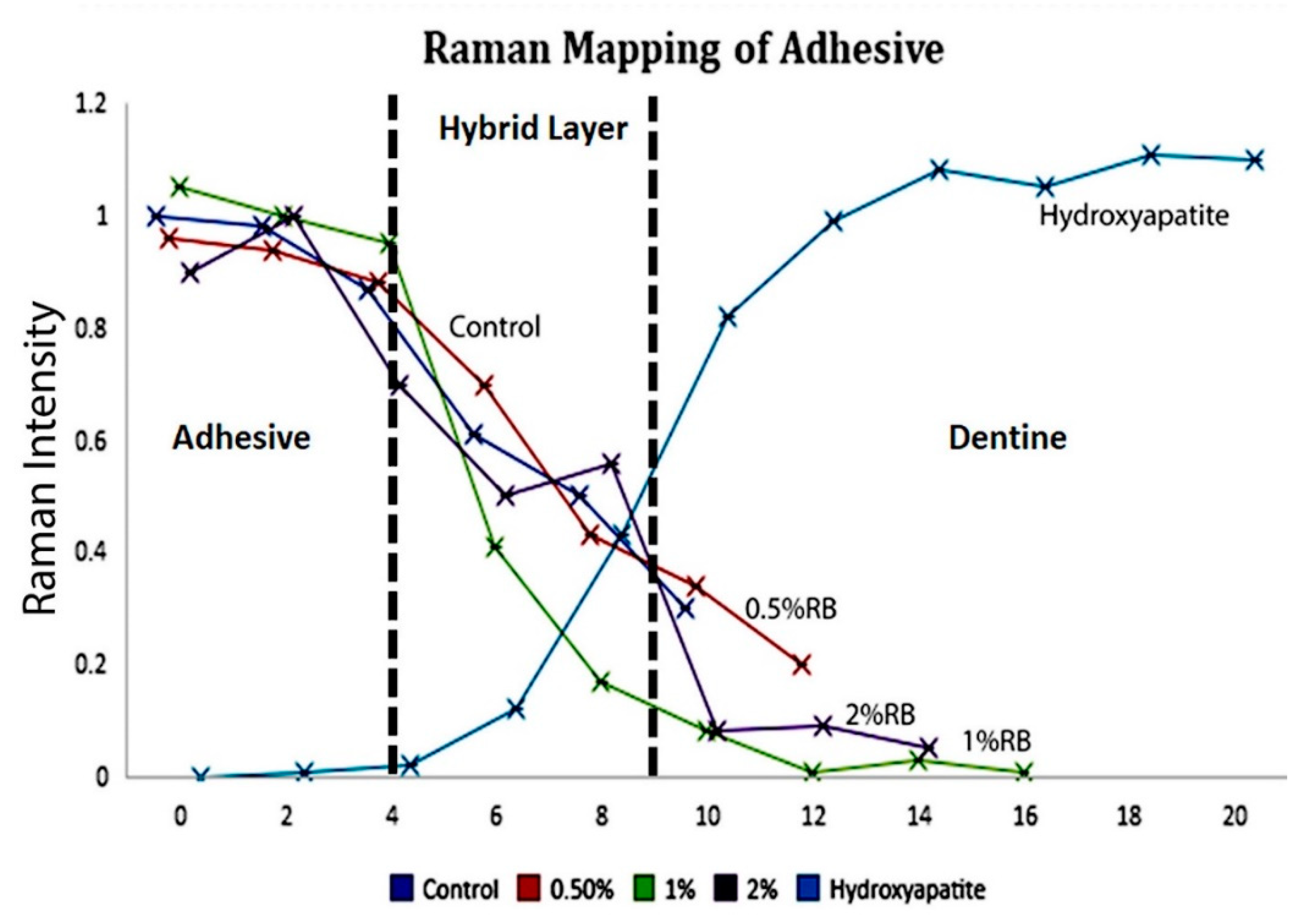
2.6. Micro-Raman Spectroscopy
2.7. Adhesive Contact Angle
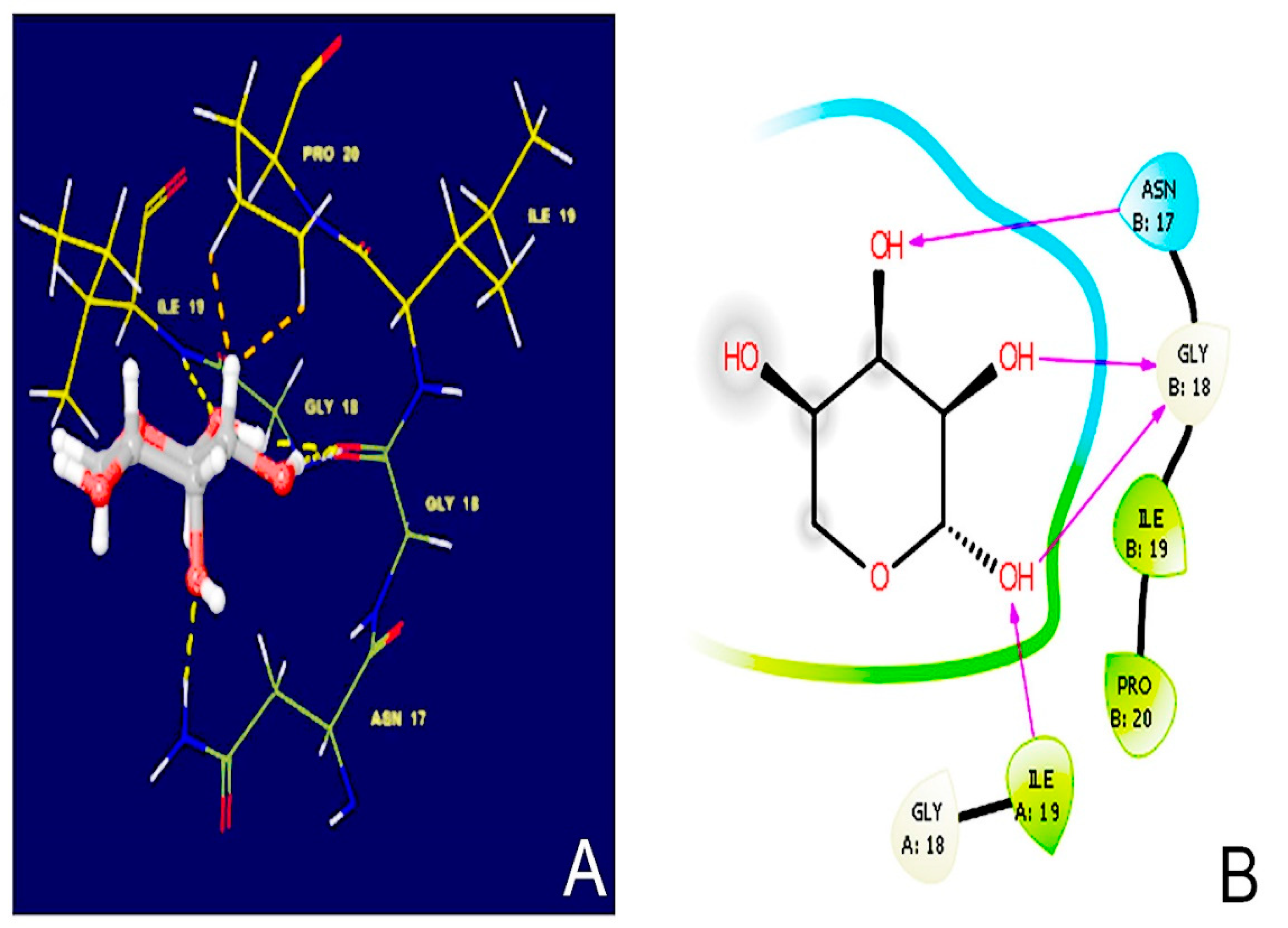
2.8. Molecular Docking Simulations
2.9. Nanoleakage Analysis
2.10. Statistical Analysis
3. Results
3.1. Micro-Tensile Bond Strength
3.2. MMP-2 and Cathepsin-K Activity Determination
3.3. Morphology of Resin–Dentin Interface
3.4. Micro-Raman Spectroscopy
3.5. Adhesive Contact Angle
3.6. Molecular Docking Simulations
3.7. Nanoleakage Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Current perspectives on dental adhesion:(1) Dentin adhesion–not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Zecin-Deren, A.; Lukomska-Szymanska, M.; Szczesio-Wlodarczyk, A.; Piwonski, I.; Sokolowski, J.; Lapinska, B. The Influence of Application Protocol of Simplified and Universal Adhesives on the Dentin Bonding Performance. Appl. Sci. 2019, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.V.; De Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current aspects on bonding effectiveness and stability in adhesive dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability-A literature review. Dent. Mater. 2016, 32, 41–53. [Google Scholar] [CrossRef]
- Hass, V.; Luque-Martinez, I.V.; Gutierrez, M.F. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. 2016, 32, 732–741. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.E.; Da Rosa, W.L.O.; Lund, R.G.; Da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [CrossRef]
- Wagner, A.; Wendler, M.; Petschelt, A.; Belli, R.; Lohbauer, U. Bonding performance of universal adhesives in different etching modes. J. Dent. 2014, 42, 800–807. [Google Scholar] [CrossRef]
- Lukomska-Szymanska, M.; Sokolowski, J.; Lapinska, B. Current views on adhesive bonding systems. J. Stomatol. 2017, 70, 384–393. [Google Scholar] [CrossRef]
- Cuevas-Suárez, C.E.; Ramos, T.S.; Rodrigues, S.B.; Collares, F.M.; Zanchi, C.H.; Lund, R.G. Impact of shelf-life simulation on bonding performance of universal adhesive systems. Dent. Mater. 2019, 35, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Münchow, E.A.; Bottino, M.C. Recent advances in adhesive bonding: The role of biomolecules, nanocompounds, and bonding strategies in enhancing resin bonding to dental substrates. Curr. Oral Health Rep. 2017, 4, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Yiu, C.K.; Niu, L.N.; Tay, F.R. Effect of a novel quaternary ammonium silane on dentin protease activities. J. Dent. 2017, 58, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bafail, A.; Carrilho, M.R.; Kishen, A.; Prakki, A. Effect of protease inhibitor specificity on dentin matrix properties. J. Mech. Behav. Biomed. Mater. 2020, 109, 103861. [Google Scholar] [CrossRef]
- De Moraes, I.Q.S.; Do Nascimento, T.G.; Da Silva, A.T.; De Lira, L.M.S.S.; Parolia, A.; Porto, I.C.C.M. Inhibition of matrix metalloproteinases: A troubleshooting for dentin adhesion. Restor. Dent. Endod. 2020, 45, 31. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.L.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef]
- Gutiérrez, M.F.; Alegría-Acevedo, L.F.; Méndez-Bauer, L.; Bermudez, J.; Dávila-Sánchez, A.; Buvinic, S. Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J. Dent. 2019, 82, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Deng, S.; Koneski, I.; Awad, M.M.; Akram, Z.; Matinlinna, J. Multiscale in-vitro analysis of photo-activated riboflavin incorporated in an experimental universal adhesive. J. Mech. Behav. Biomed. Mater. 2020, 112, 104082. [Google Scholar] [CrossRef]
- Sampaio, P.C.P.; Kruly, P.C.; Ribeiro, C.C.; Hilgert, L.A.; Pereira, P.; Scaffa, P. Comparative bonding ability to dentin of a universal adhesive system and monomer conversion as functions of extended light curing times and storage. J. Mech. Behav. Biomed. Mater. 2017, 75, 41–49. [Google Scholar] [CrossRef]
- Zecin-Deren, A.; Sokolowski, J.; Szczesio-Wlodarczyk, A.; Piwonski, I.; Lukomska-Szymanska, M.; Lapinska, B. Multi-Layer Application of Self-Etch and Universal Adhesives and the Effect on Dentin Bond Strength. Molecules 2019, 24, 345. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.Y.; DI Nicoló, R.; Bresciani, E. Influence of ethanol-wet dentin, adhesive mode of application, and aging on bond strength of universal adhesive. Braz. Oral Res. 2018, 32, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanatvarakorn, O.; Prasansuttiporn, T.; Takahashi, M.; Thittaweerat, S.; Foxton, R.M.; Ichinose, S. Effect of Scrubbing Technique with Mild Self-etching Adhesives on Dentin Bond Strengths and Nanoleakage Expression. J. Adhes. Dent. 2016, 18, 197–204. [Google Scholar] [CrossRef]
- Mena-Serrano, A.; Costa, T.R.; Patzlaff, R.T.; Loguercio, A.D.; Reis, A. Effect of sonic application mode on the resin-dentin bond strength and dentin permeability of self-etching systems. J. Adhes. Dent. 2014, 16, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; De Munck, J.; Van Landuyt, K.; Peumans, M.; Yoshihara, K.; Van Meerbeek, B. Do Universal Adhesives Benefit from an Extra Bonding Layer? J. Adhes. Dent. 2019, 21, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Stape, T.H.S.; Wik, P.; Mutluay, M.M.; Al-Ani, A.A.S.; Tezvergil-Mutluay, A. Selective dentin etching: A potential method to improve bonding effectiveness of universal adhesives. J. Mech. Behav. Biomed. Mater. 2018, 86, 14–22. [Google Scholar] [CrossRef]
- Daood, U.; Akram, Z.; Matinlinna, J.P.; Fawzy, A.S. Dentine collagen cross-linking using tiopronin-protected Au/EDC nanoparticles formulations. Dent. Mater. 2019, 35, 1017–1030. [Google Scholar] [CrossRef]
- Daood, U.; Omar, H.; Qasim, S.; Nogueira, L.P.; Pichika, M.R.; Mak, K.K. New antimicrobial and collagen crosslinking formulated dentin adhesive with improved bond durability. J. Mech. Behav. Biomed. Mater. 2020, 110, 103927. [Google Scholar] [CrossRef]
- Daood, U.; Tsoi, J.; Neelakantan, P.; Matinlinna, J.; Omar, H.; Al-Nabulsi, M. In vitro assessment of ribose modified two-step etch-and-rinse dentine adhesive. Dent. Mater. 2018, 34, 1175–1187. [Google Scholar] [CrossRef]
- Bailey, A.J.; Light, N.D.; Atkins, E.D. Chemical cross-linking restrictions on models for the molecular organization of the collagen fibre. Nature 1980, 288, 408–410. [Google Scholar] [CrossRef]
- Gu, L.; Shan, T.; Ma, Y.X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Lu, Y.; Wei, Y.; Liu, Y. D-Ribose Induces Cellular Protein Glycation and Impairs Mouse Spatial Cognition. PLoS ONE 2011, 6, 24623. [Google Scholar] [CrossRef] [Green Version]
- Broom, A.D.; Townsend, L.B.; Jones, J.W.; Robins, R.K. Purine nucleosides. vi. further methylation studies of naturally occurring purine nucleosides. Biochemistry 1964, 3, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Balansin Rigon, R.; Kaessmeyer, S.; Wolff, C.; Hausmann, C.; Zhang, N.; Sochorová, M. Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin. Int. J. Mol. Sci. 2018, 19, 3521. [Google Scholar] [CrossRef] [Green Version]
- Elder, B.D.; Mohan, A.; Athanasiou, K.A. Beneficial effects of exogenous crosslinking agents on self-assembled tissue engineered cartilage construct biomechanical properties. J. Mech. Med. Biol. 2011, 11, 433–443. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Campodoni, E.; Dapporto, M.; Montesi, M.; Panseri, S. Ribose mediated crosslinking of collagen-hydroxyapatite hybrid scaffolds for bone tissue regeneration using biomimetic strategies. Mater. Sci. Eng. C 2017, 77, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Cerami, A. Nonenzymatic browning in vivo: Possible process for aging of long-lived proteins. Science 1981, 211, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Sroga, G.E.; Siddula, A.; Vashishth, D. Glycation of human cortical and cancellous bone captures differences in the formation of Maillard reaction products between glucose and ribose. PLoS ONE 2015, 10, e0117240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.; Dönmez, N.; Belli, S. Effect of artificial saliva contamination on bond strength to pulp chamber dentine. Eur. J. Dent. 2008, 2, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, S.; Geraldeli, S.; Maia, R.; Raposo, L.H.; Soares, C.J.; Yamagawa, J. Adhesion to tooth structure: A critical review of “micro” bond strength test methods. Dent. Mater. 2010, 26, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Sameh, E.; Adam, Z.; Martin, S.; Beat, E.; Angelo, V. A molecular-modeling toolbox aimed at bridging the gap between medicinal chemistry and computational sciences. Int. J. Mol. Sci. 2013, 14, 684–700. [Google Scholar]
- Mai, C.W.; Kang, Y.B.; Hamzah, A.S.; Pichika, M.R. Comparative efficacy of Vanilloids in inhibiting Toll-like Receptor-4 (TLR-4)/Myeloid differentiation factor (MD-2) homodimerization. Food Funct. 2018, 9, 3344–3350. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Sauro, S.; Pichika, M.R.; Omar, H.; Liang Lin, S.; Fawzy, A.S. Novel riboflavin/VE-TPGS modified universal dentine adhesive with superior dentine bond strength and self-crosslinking potential. Dent. Mater. 2020, 36, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Saboia, V.P.A.; Nato, F.; Mazzoni, A.; Orsini, G.; Putignano, A.; Giannini, M. Adhesion of a two-step etch-and-rinse adhesive on collagen-depleted dentin. J. Adhes. Dent. 2008, 10, 419–422. [Google Scholar]
- Takahashi, M.; Kushida, K.; Ohishi, T.; Kouichi, K.; Hoshino, H.; Uchiyama, A. Quantitative analysis of crosslinks pyridinoline and pentosidine in articular cartilage of patients with bone and joint disorders. Arthritis Rheumatol. 1994, 37, 724–728. [Google Scholar] [CrossRef]
- Karim, L.; Tang, S.Y.; Sroga, G.E.; Vashishth, D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos. Int. 2013, 24, 2441–2447. [Google Scholar] [CrossRef] [Green Version]
- Guilbert, M.; Roig, B.; Terryn, C.; Garnotel, R.; Jeannesson, P.; Sockalingum, G.D.; Piot, O. Highlighting the impact of aging on type I collagen: Label-free investigation using confocal reflectance microscopy and diffuse reflectance spectroscopy in 3D matrix model. Oncotarget 2016, 7, 8546–8555. [Google Scholar] [CrossRef]
- Valcourt, U.; Merle, B.; Gineyts, E.; Viguet-Carrin, S.; Delmas, P.D.; Garnero, P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J. Biol. Chem. 2007, 8, 5691–5703. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, E.A.; Schaible, E.; Bale, H.; Barth, H.D.; Tang, S.Y.; Reichert, P. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc. Natl. Acad. Sci. USA 2011, 108, 14416–14421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gostynska, N.; Krishnakumar, G.S.; Campodoni, E.; Panseri, S.; Montesi, M.; Sprio, S.; Sandri, M. 3D porous collagen scaffolds reinforced glycation with ribose for tissue engineering application. Biomed. Mater. 2017, 12, 055002. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charulatha, V.; Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials 2003, 24, 759–767. [Google Scholar] [CrossRef]
- Kumagai, R.Y.; Hirata, R.; Pereira, P.N.R.; Reis, A.F. Moist vs over-dried etched dentin: FE-SEM/TEM and bond strength evaluation of resin-dentin interfaces produced by universal adhesives. J. Esthet. Restorage Dent. 2020, 32, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.F.M.A.; Islam, R.; Alam, A.; Matsumoto, M.; Yamauti, M.; Carvalho, R.M.; Sano, H. Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. Int. J. Mol. Sci. 2019, 20, 5381. [Google Scholar] [CrossRef] [Green Version]
- Bertinetti, L.; Drouet, C.; Combes, C.; Rey, C.; Tampieri, A.; Coluccia, A. Surface characteristics of nanocrystalline apatities: Effect of Mg surface enrichment on morphology, surface hydration species, and cationic environments. Langmuir 2009, 25, 5647–5654. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A. Controlling the porosity and microstructure of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterisation of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. A 2013, 101, 1080–1094. [Google Scholar] [CrossRef]
- Rehman, I.; Movasaghi, Z.; Rehman, S. Vibrational Spectroscopy for Tissue Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–15. [Google Scholar]
- Ruyter, I.E. The chemistry of adhesive agents. Oper. Dent. 1992, 5, 32–43. [Google Scholar]
- Migliori, M.; Gabriele, D.; Di Sanzo, R.; De Cindio, B.; Correra, S. Viscosity of multicomponent solutions of simple and complex sugars in water. J. Chem. Eng. Data 2007, 52, 1347–1353. [Google Scholar] [CrossRef]
- Daood, U.; Heng, C.; Lian, J.; Fawzy, A.S. In vitro analysis of riboflavin-modified, experimental, two-step etch-and-rinse dentin adhesive: Fourier transform infrared spectroscopy and micro-Raman studies. Int. J. Oral Sci. 2015, 7, 110–124. [Google Scholar] [CrossRef] [PubMed]


| Material and Manufacturer | Composition | pH | Instructions for Use |
|---|---|---|---|
| Prime&Bond activeTM; Dentsply DeTrey GmbH, Konstanz, Germany | bisacrylamide 1 (25–50%), 10-methacryloxydecyl dihydrogen phosphate (10-MDP) (10–25%), bisacrylamide 2 (2.5–10%), 4-(dimethylamino)benzonitrile (0.1–1%), dipentaerythritol pentacrylate phosphate (PENTA) propan-2-ol (10–25%) water (20%) | 2.5 | apply adhesive, slight agitation (20 s), mild air-blowing (5 s), light-curing (20 s) |
| Groups | μTBS | MMP-2 | Cathepsin-K | Contact Angle | ||
|---|---|---|---|---|---|---|
| Time | 24 h | 7 days | 14 days | 7 days | 14 days | 5 min |
| Control group | 36.21 ± 13.8 A | 7.80 ± 1.7 A | 11.10 ± 1.8 A | 4.11 ± 2 A | 5.7 ± 1.9 A | 28.17 ± 8.1 C |
| 0.5% Ribose | 34.44 ± 5 B | 4.10 ± 0.9 B | 7.80 ± 1.4 B | 2.90 ± 0.6 B | 1.11 ± 2 A | 8.650 ± 4.2 A |
| 1% Ribose | 39.44 ± 7.7 C | 1.80 ± 1.2 C | 4.40 ± 1.6 C | 1.20 ± 0.3 C | 0.51 ± 0.2 A | 13.950 ± 3.9 A,B |
| 2% Ribose | 31.31 ± 6.7 D | 0.90 ± 0.5 D | 2.20 ± 0.7 D | 0.40 ± 0.09 D | 0.2 ± 0.1 A | 22.025 ± 6.2 B,C |
| Groups | Nanoleakage Score | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | p-Value | |
| Control | 0 | 30 | 5 | 25 | 40 | p < 0.08 |
| 0.5% RB | 5 | 25 | 10 | 45 | 25 | p < 0.04 |
| 1% RB | 30 | 10 | 15 | 30 | 15 | p < 0.05 |
| 2% RB | 0 | 10 | 10 | 35 | 45 | p < 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgi, R.; Daood, U.; Bijle, M.N.; Fawzy, A.; Ghaleb, M.; Hardan, L. Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry. Polymers 2021, 13, 704. https://doi.org/10.3390/polym13050704
Bourgi R, Daood U, Bijle MN, Fawzy A, Ghaleb M, Hardan L. Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry. Polymers. 2021; 13(5):704. https://doi.org/10.3390/polym13050704
Chicago/Turabian StyleBourgi, Rim, Umer Daood, Mohammed Nadeem Bijle, Amr Fawzy, Maroun Ghaleb, and Louis Hardan. 2021. "Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry" Polymers 13, no. 5: 704. https://doi.org/10.3390/polym13050704








