Enhancement of Adhesion Characteristics of Low-Density Polyethylene Using Atmospheric Plasma Initiated-Grafting of Polyethylene Glycol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LDPE Thin Sheets and LDPE-Al Laminate
2.3. Surface Modification of LDPE Surface Using Corona Discharge
2.4. Grafting PEG/PEO onto LDPE
2.5. Grafting Efficiency (GE) Evaluation
2.6. Determination of Surface Wettability
2.7. Determination of the Adhesion Strength of LDPE/Al Laminate
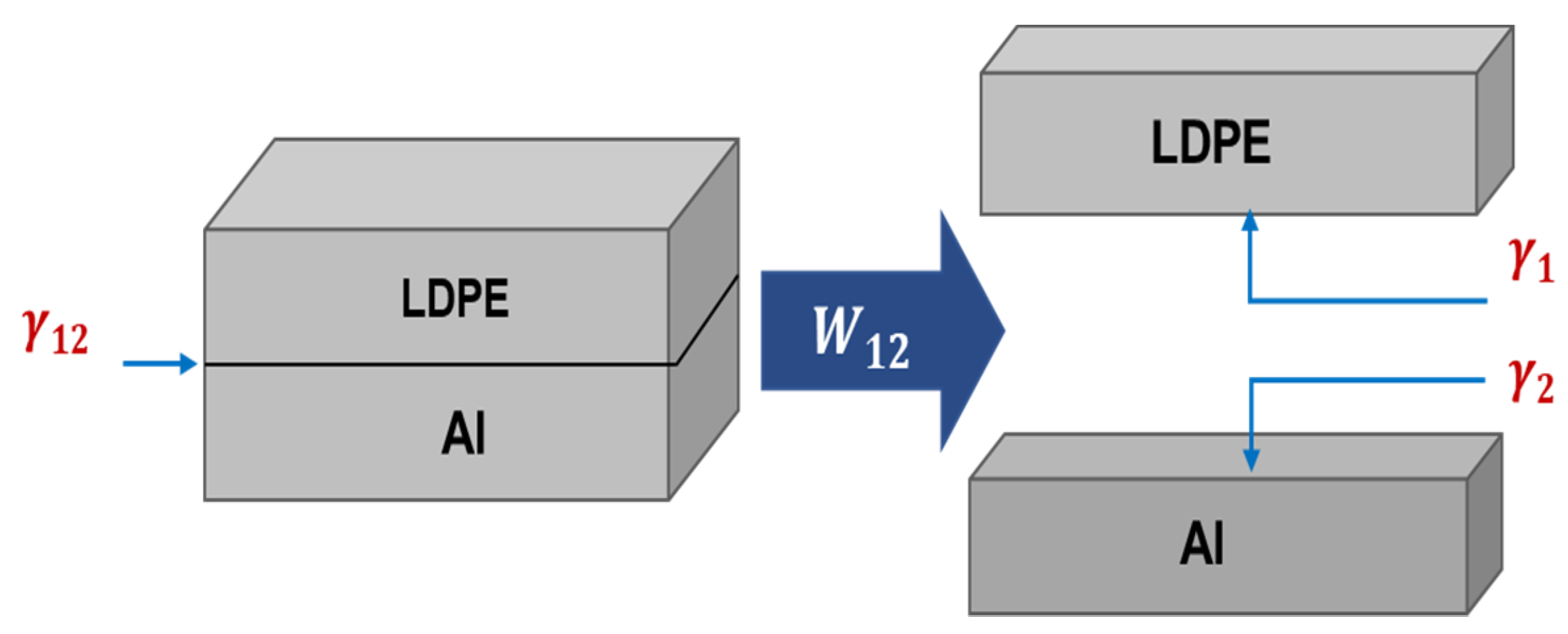
2.8. Calculation of the Work of Adhesion
2.9. Surface Morphology Analysis
2.10. Surface Composition Evaluation
3. Results
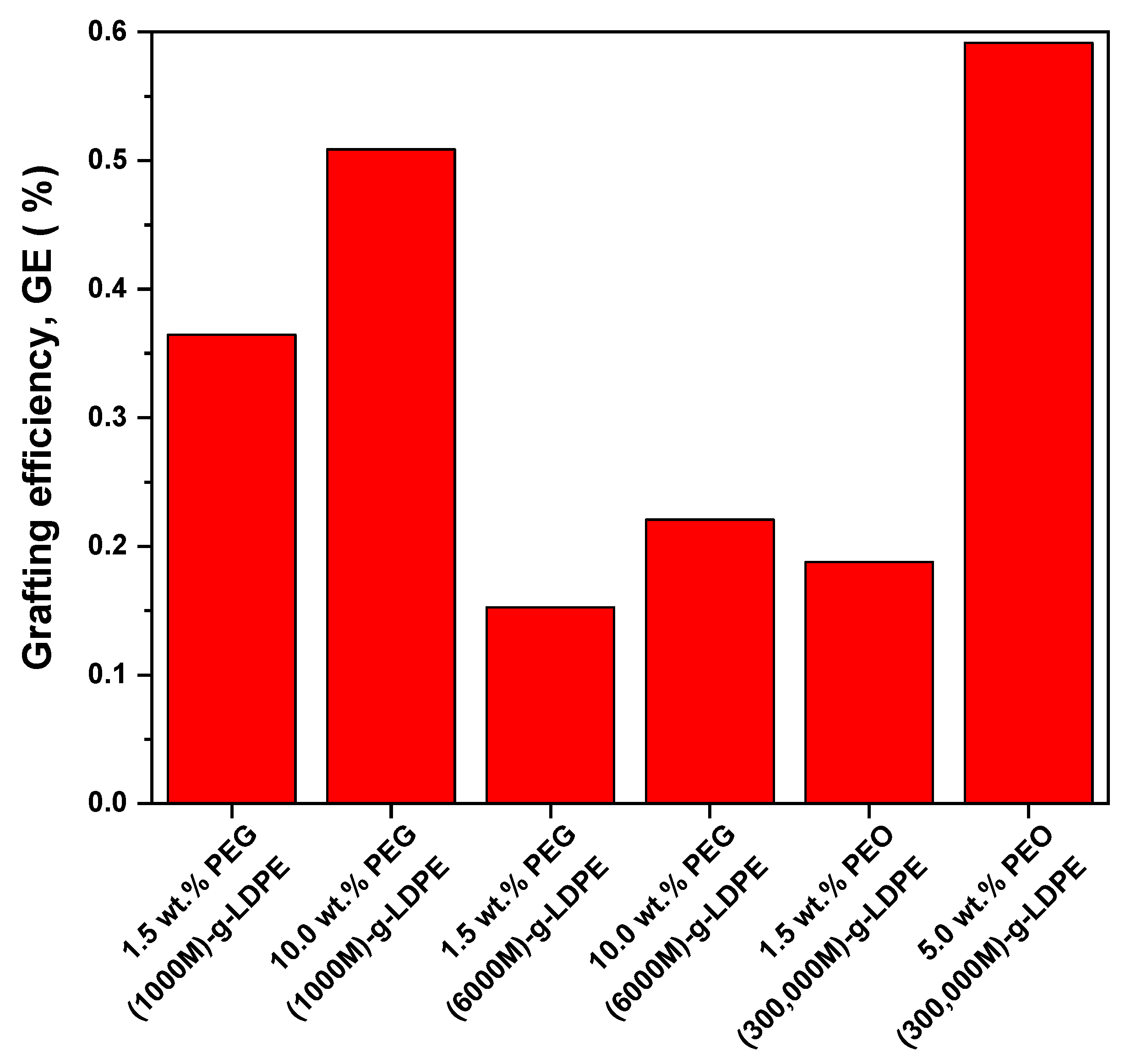
3.1. Grafting Efficiency (GE)
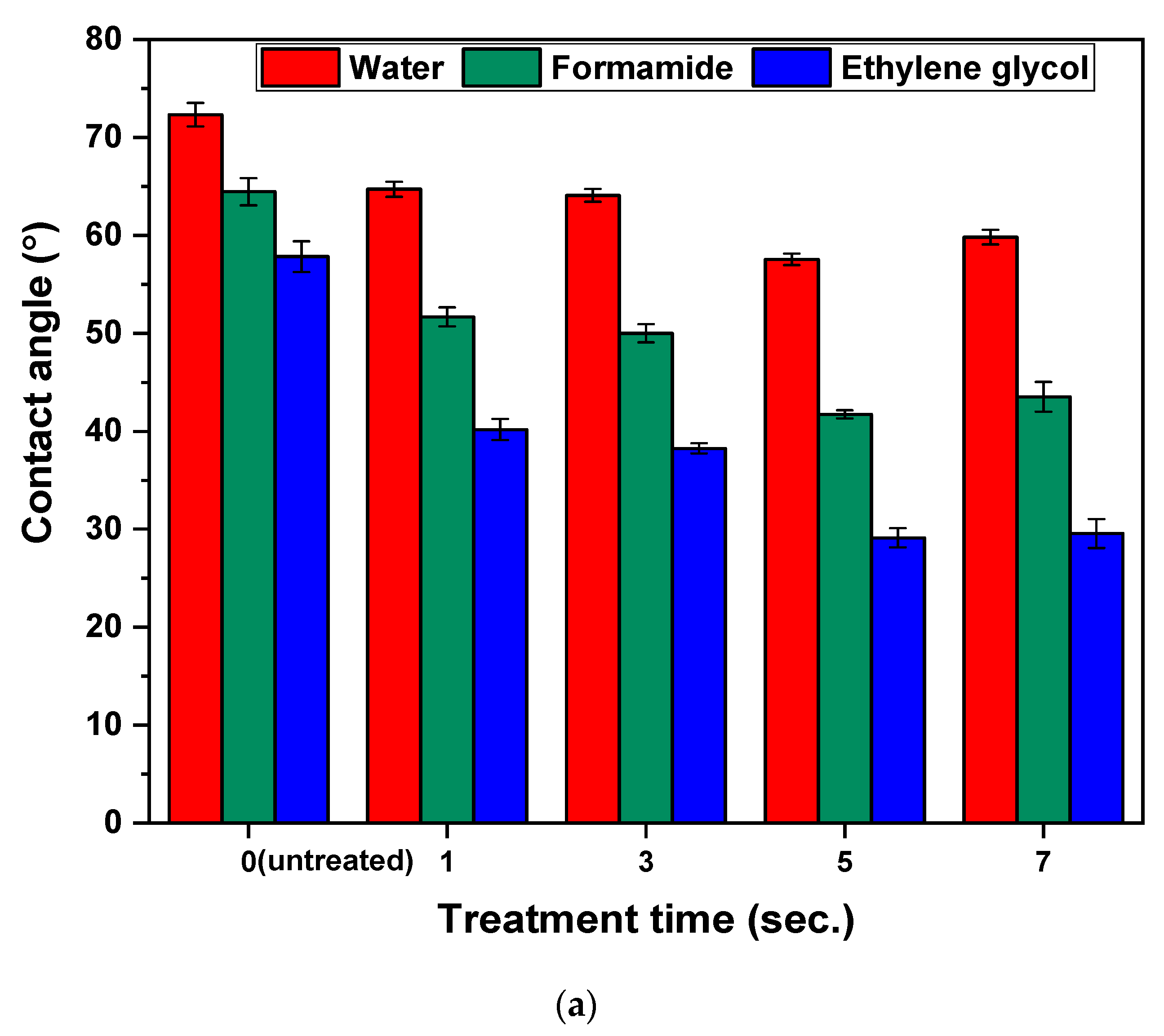
3.2. Surface Wettability Analysis
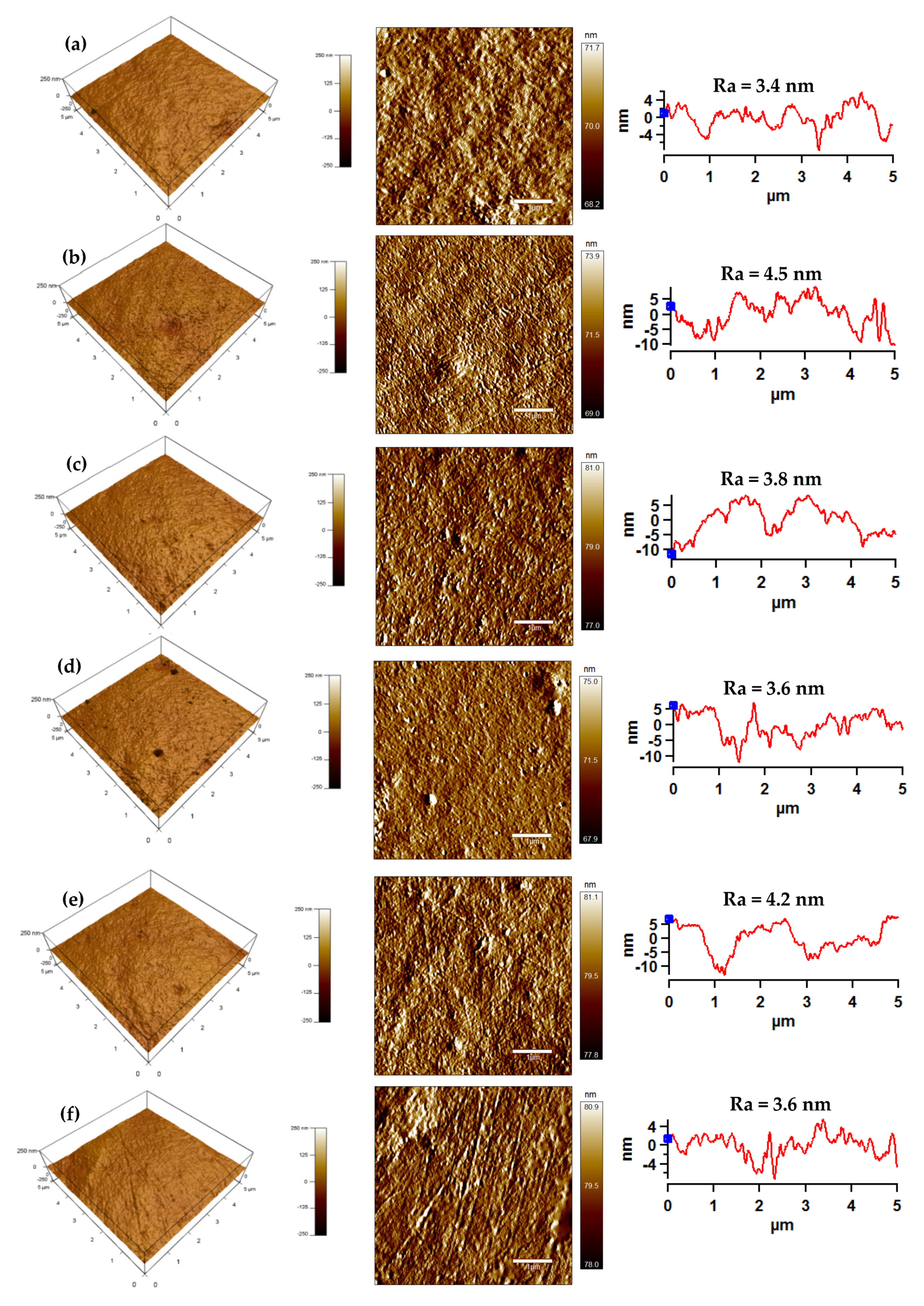
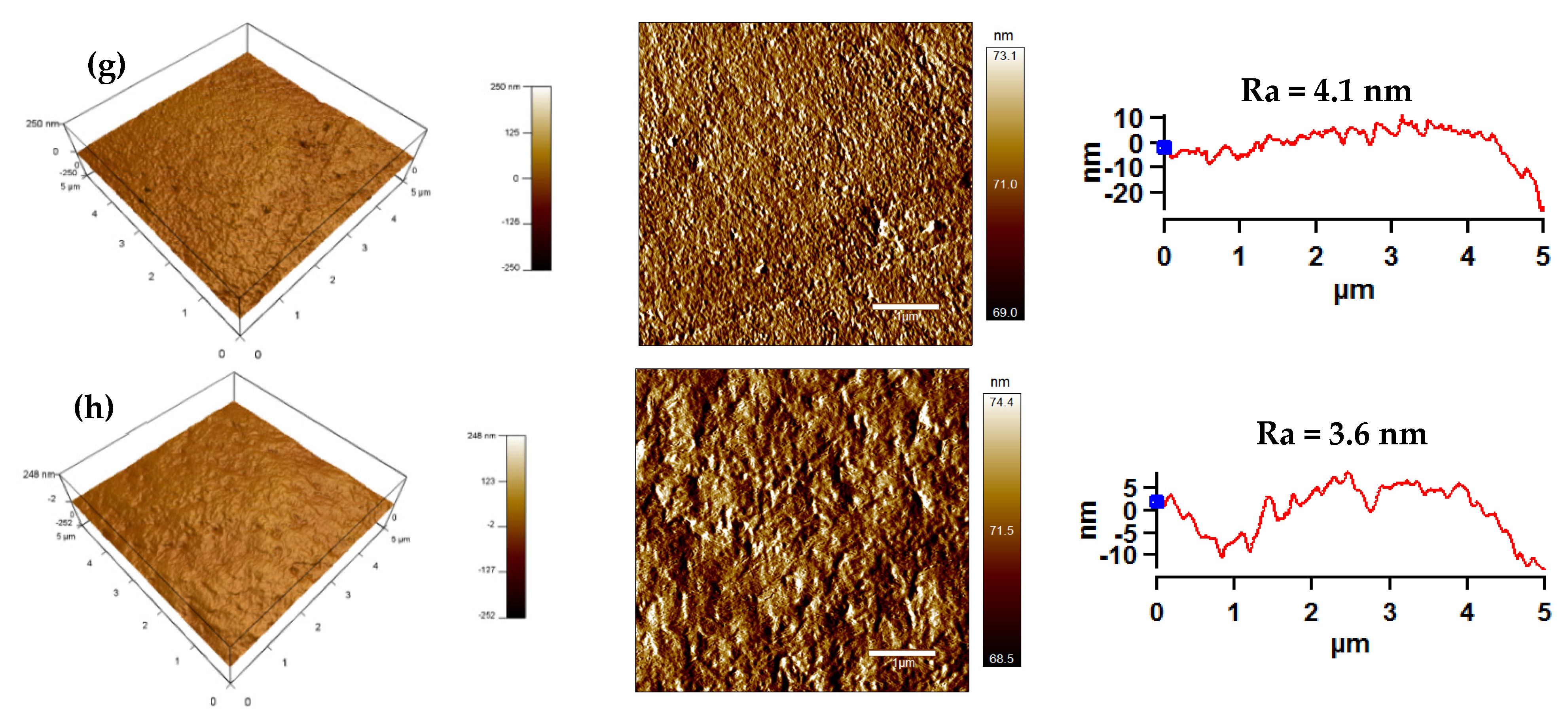
3.3. Surface Morphology Analysis
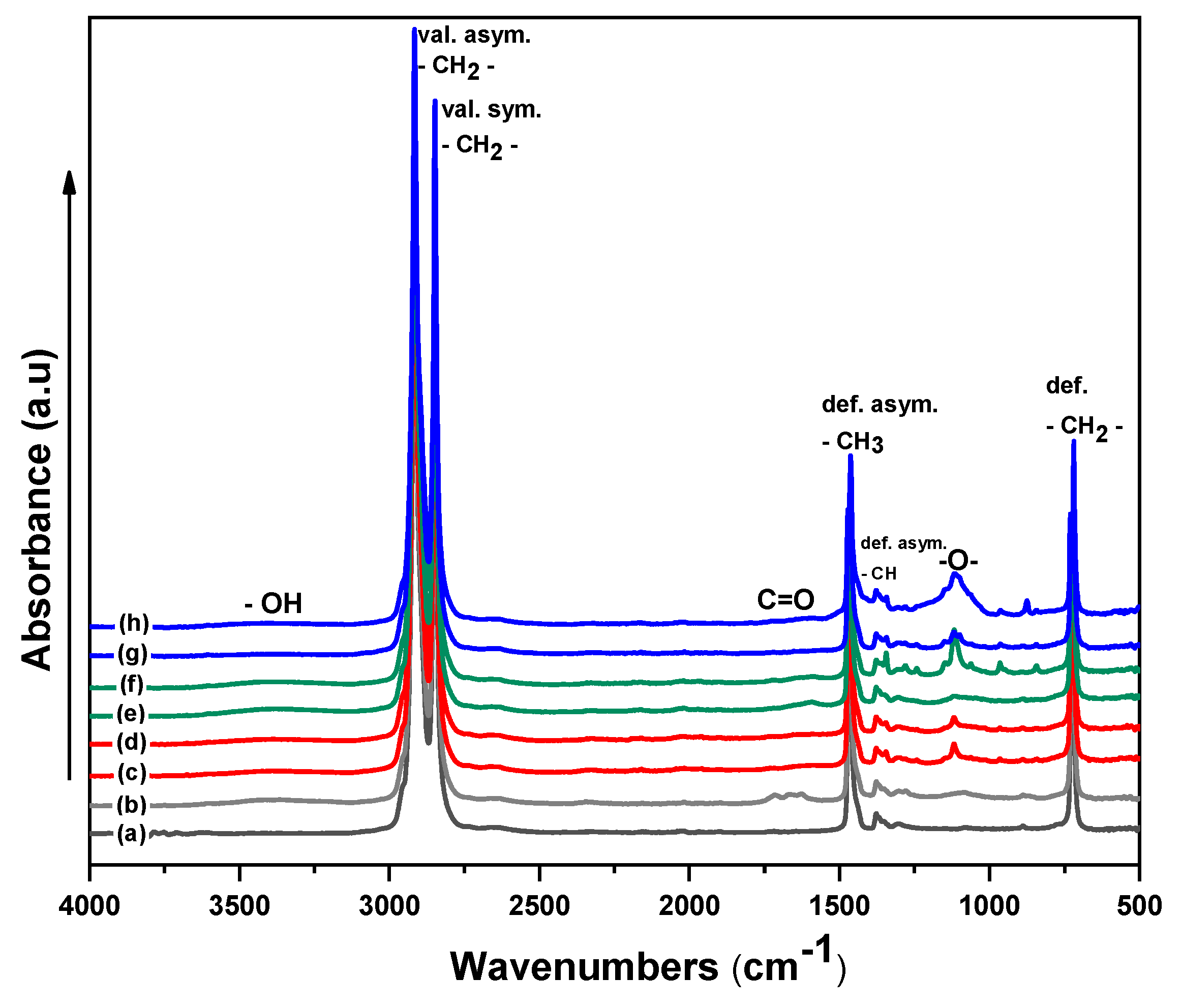
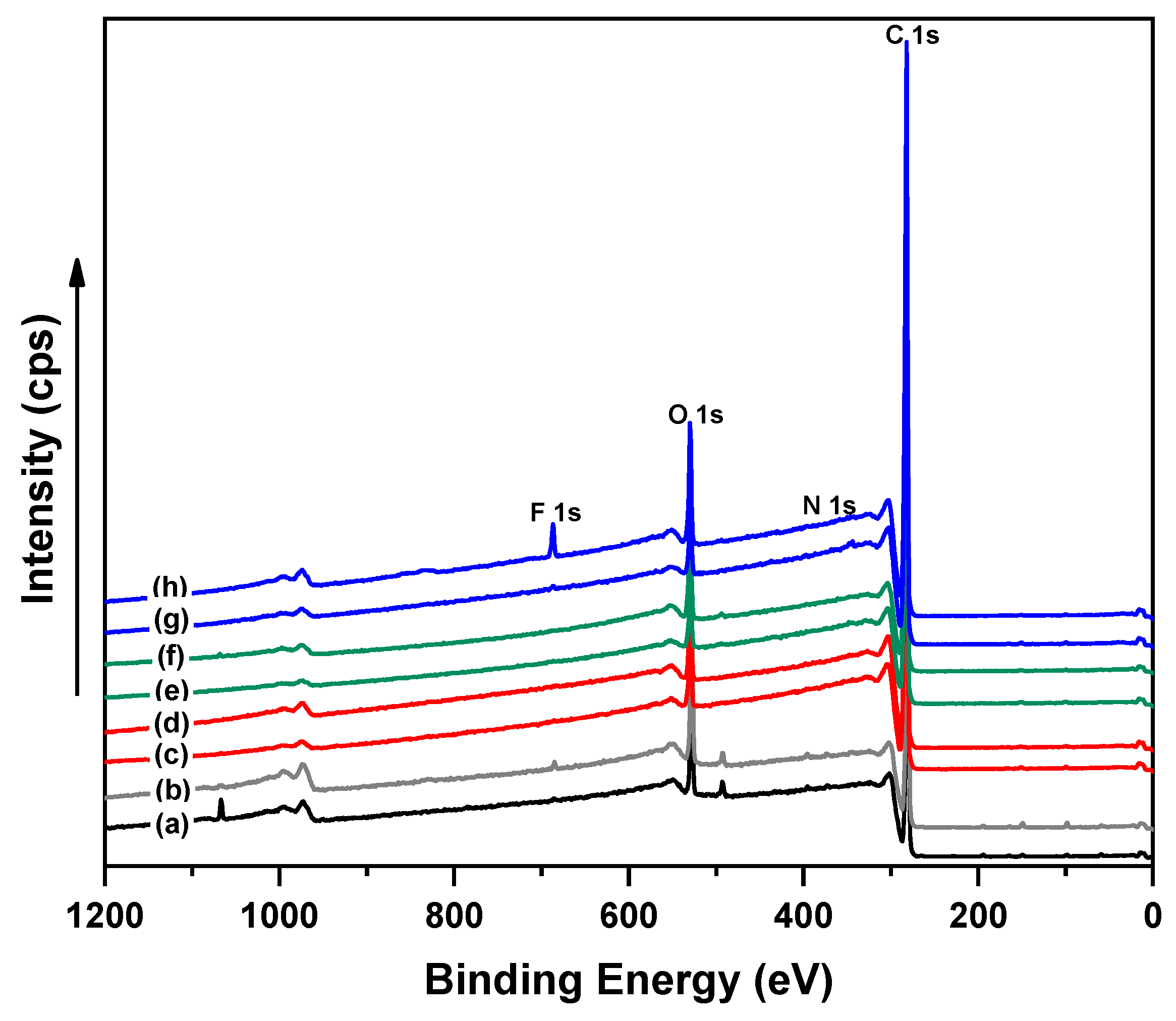
3.4. Chemical Composition Investigation
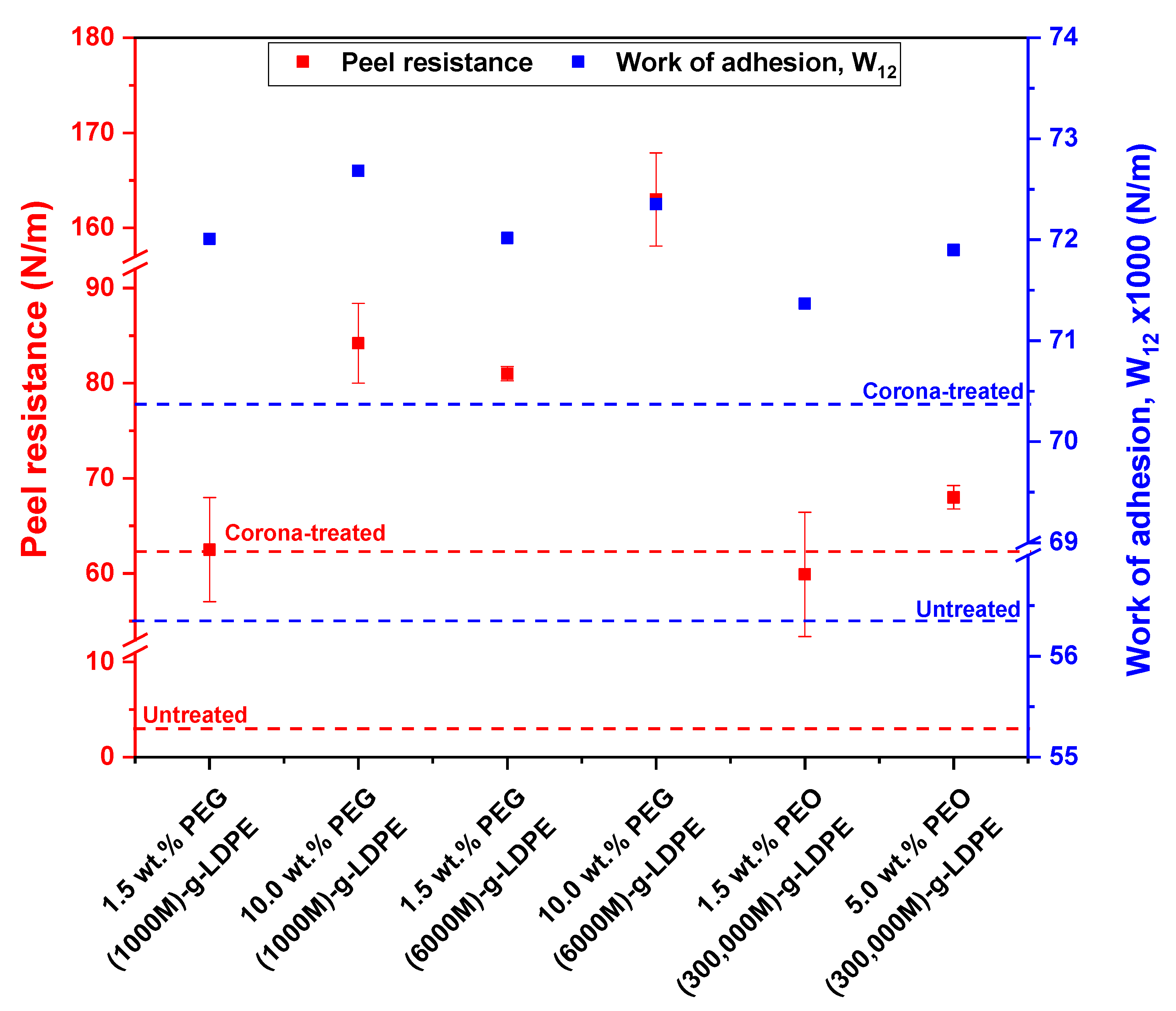
3.5. Adhesive Strength Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manjula, B.; Reddy, A.B.; Sadiku, E.R.; Sivanjineyulu, V.; Molelekwa, G.F.; Jayaramudu, J.; Kumar, K.R. Use of polyolefins in hygienic applications. In Polyolefin Fibres; Elsevier: Amsterdam, The Netherlands, 2017; pp. 539–560. [Google Scholar]
- Olabisi, O. Polyolefins. In Handbook of Thermoplastics; Olabisi, O., Adewale, K., Eds.; CRC Pres: Boca Raton, FL, USA, 2015; pp. 1–2. [Google Scholar]
- Petrie, E.M. Adhesive Bonding of Polyolefin. Polyurethane 2013, 38, 85. [Google Scholar]
- Lohse, D.J. POLYOLEFINS. In Applied Polymer Science: 21st Century; Craver, C.D., Carraher, C.E., Eds.; Pergamon: Oxford, UK, 2000; pp. 73–91. [Google Scholar]
- AlMa’adeed, M.A.-A.; Krupa, I. Polyolefin compounds and materials. In Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2016; pp. 271–284. [Google Scholar]
- Posch, W. 3-Polyolefins. In Applied Plastics Engineering Handbook; Kutz, M., Ed.; William Andrew Publishing: Oxford, UK, 2011; pp. 23–48. [Google Scholar]
- Yang, F.; Wang, X.; Ma, Z.; Wang, B.; Pan, L.; Li, Y. Copolymerization of propylene with higher α-olefins by a Pyridylamidohafnium catalyst: An effective approach to polypropylene-based elastomer. Polymers 2020, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.M.S.; Rico, B.P.M.; Vieira, M.C. 9-Food packaging and migration. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 281–301. [Google Scholar]
- Kasalkova, N.S.; Slepicka, P.; Kolska, Z.; Svorcik, V. Wettability and other surface properties of modified polymers. Wetting Wettability 2015, 12, 323–356. [Google Scholar]
- Drnovska, H.; Lapčík, L.; Buršíková, V.; Zemek, J.; Barros-Timmons, A.M. Surface properties of polyethylene after low-temperature plasma treatment. Colloid Polym. Sci. 2003, 281, 1025–1033. [Google Scholar] [CrossRef]
- Lindner, M.; Rodler, N.; Jesdinszki, M.; Schmid, M.; Sängerlaub, S. Surface energy of corona treated PP, PE and PET films, its alteration as function of storage time and the effect of various corona dosages on their bond strength after lamination. J. Appl. Polym. Sci. 2018, 135, 45842. [Google Scholar] [CrossRef]
- Gilliam, M. Polymer Surface Treatment and Coating Technologies. In Handbook of Manufacturing Engineering and Technology; Nee, A., Ed.; Springer: London, UK, 2013; pp. 1–23. [Google Scholar]
- Sharpe, P. Making Plastics: From Monomer to Polymer. Chem. Eng. Prog. 2015, 111, 24–29. [Google Scholar]
- Ozdemir, M.; Yurteri, C.U.; Sadikoglu, H. Physical polymer surface modification methods and applications in food packaging polymers. Crit. Rev. Food Sci. Nutr. 1999, 39, 457–477. [Google Scholar] [CrossRef]
- Pinto, A.G.; Magalhaes, A.; da Silva, F.G.; Baptista, A.M. Shear strength of adhesively bonded polyolefins with minimal surface preparation. Int. J. Adhes. Adhes. 2008, 28, 452–456. [Google Scholar] [CrossRef] [Green Version]
- Pascual, M.; Sanchis, R.; Sánchez, L.; García, D.; Balart, R. Surface modification of low density polyethylene (LDPE) film using corona discharge plasma for technological applications. J. Adhes. Sci. Technol. 2008, 22, 1425–1442. [Google Scholar] [CrossRef]
- Santos, L.P.; Bernardes, J.S.; Galembeck, F. Corona-treated polyethylene films are macroscopic charge bilayers. Langmuir 2013, 29, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Amirou, S.; Zerizer, A.; Haddadou, I.; Merlin, A. Effects of corona discharge treatment on the mechanical properties of biocomposites from polylactic acid and Algerian date palm fibres. Sci. Res. Essays 2013, 8, 946–952. [Google Scholar]
- Chapter 17-Adhesive Bonding. In Handbook of Plastics Joining, 2nd ed.; Troughton, M.J. (Ed.) William Andrew Publishing: Boston, MA, USA, 2009; pp. 145–173. [Google Scholar]
- Chapter 3-Material Surface Preparation Techniques. In Adhesives Technology Handbook, 2nd ed.; Ebnesajjad, S. (Ed.) William Andrew Publishing: Norwich, NY, USA, 2009; pp. 37–46. [Google Scholar]
- Nikolic, V.; Velickovic, S.; Popovic, A. Biodegradation of polystyrene-graft-starch copolymers in three different types of soil. Environ. Sci. Pollut. Res. 2014, 21, 9877–9886. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.D.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L. Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.-H.; Kim, S.W. Polymer-based delivery of glucagon-like peptide-1 for the treatment of diabetes. Int. Sch. Res. Not. 2012, 2012, 340632. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi Sejoubsari, R.; Martinez, A.P.; Kutes, Y.; Wang, Z.; Dobrynin, A.V.; Adamson, D.H. “Grafting-through”: Growing polymer brushes by supplying monomers through the surface. Macromolecules 2016, 49, 2477–2483. [Google Scholar] [CrossRef]
- Pergal, M.V.; Antic, V.V.; Tovilovic, G.; Nestorov, J.; Vasiljevic-Radovic, D.; Djonlagic, J. In vitro biocompatibility evaluation of novel urethane–siloxane co-polymers based on poly (ϵ-caprolactone)-block-poly (dimethylsiloxane)-block-poly (ϵ-caprolactone). Biomater. Sci. Polym. Ed. 2012, 23, 1629–1657. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Chapter 18-Application of compatibilized polymer blends in biomedical fields. In Compatibilization of Polymer Blends; Ajitha, A.R., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 511–537. [Google Scholar]
- McPherson, T.B.; Shim, H.S.; Park, K. Grafting of PEO to glass, nitinol, and pyrolytic carbon surfaces by gamma irradiation. J. Biomed. Mater. Res. 1997, 38, 289–302. [Google Scholar] [CrossRef]
- Lakshmi, S.; Jayakrishnan, A. Migration resistant, blood-compatible plasticized polyvinyl chloride for medical and related applications. Artif. Organs 1998, 22, 222–229. [Google Scholar] [CrossRef]
- Cheng, G.; Cai, Z.; Wang, L. Biocompatibility and biodegradation of poly(hydroxybutyrate)/poly(ethylene glycol) blend films. J. Mater. Sci. Mater. Med. 2003, 14, 1073–1078. [Google Scholar] [CrossRef]
- Zacchigna, M.; Cateni, F.; Drioli, S.; Bonora, G.M. Multimeric, multifunctional derivatives of poly (ethylene glycol). Polymers 2011, 3, 1076–1090. [Google Scholar] [CrossRef]
- Sun, D.; Iqbal, K.; Siddiqui, M.O.R. 20-Thermal analysis of temperature responsive fibrous materials. In Thermal Analysis of Textiles and Fibers; Jaffe, M., Menczel, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 335–353. [Google Scholar]
- Hutanu, D.; Frishberg, M.D.; Guo, L.; Darie, C.C. Recent applications of polyethylene glycols (PEGs) and PEG derivatives. Mod. Chem. Appl. 2014, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Bandara, S.; Carson, L.E.; Kommalapati, R.R. Association of Polyethylene Glycol Solubility with Emerging Membrane Technologies, Wastewater Treatment, and Desalination. In Water Quality-Science, Assessments and Policy; IntechOpen: London, UK, 2019. [Google Scholar]
- Zia, F.; Anjum, M.N.; Saif, M.J.; Jamil, T.; Malik, K.; Anjum, S.; BiBi, I.; Zia, M.A. Chapter 16-Alginate-Poly(Ethylene) Glycol and Poly(Ethylene) Oxide Blend Materials. In Algae Based Polymers, Blends, and Composites; Zia, K.M., Zuber, M., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 581–601. [Google Scholar]
- Gelardi, G.; Mantellato, S.; Marchon, D.; Palacios, M.; Eberhardt, A.B.; Flatt, R.J. 9-Chemistry of chemical admixtures. In Science and Technology of Concrete Admixtures; Aïtcin, P.-C., Flatt, R.J., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 149–218. [Google Scholar]
- Liu, L.; Gao, Y.; Zhao, J.; Yuan, L.; Li, C.; Liu, Z.; Hou, Z. A mild method for surface-grafting PEG onto segmented poly (ester-urethane) film with high grafting density for biomedical purpose. Polymers 2018, 10, 1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adib, H.; Raisi, A. Surface modification of a PES membrane by corona air plasma-assisted grafting of HB-PEG for separation of oil-in-water emulsions. RSC Adv. 2020, 10, 17143–17153. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Landrock, A.H. Chapter 2-Surface Tension and Its Measurement. In Adhesives Technology Handbook, 3rd ed.; Ebnesajjad, S., Landrock, A.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2015; pp. 19–34. [Google Scholar]
- Wu, S. Adhesion: Basic Concept and Locus of Failure. In Polymer Interface and Adhesion, 1; CRC Press: Boca Raton, FL, USA; Taylor & Francis: London, UK, 1982; pp. 337–354. [Google Scholar]
- Leslie, S.A.; Mitchell, J.C. Removing gold coating from SEM samples. Palaeontology 2007, 50, 1459–1461. [Google Scholar] [CrossRef]
- Takke, V.; Behary, N.; Perwuelz, A.; Campagne, C. Surface and adhesion properties of poly (ethylene glycol) on polyester (polyethylene terephthalate) fabric surface: Effect of air-atmospheric plasma treatment. Appl. Polym. Sci. 2011, 122, 2621–2629. [Google Scholar] [CrossRef]
- Kumar, B.R.; Rao, T.S. AFM studies on surface morphology, topography and texture of nanostructured zinc aluminum oxide thin films. Digest J. Nanomater. Biostructures 2012, 7, 1881–1889. [Google Scholar]
- Gaff, M.; Gašparík, M.; Kvietková, M. Evaluation of wood surface quality depending on the embossing parameters. Wood Res. 2017, 62, 751–762. [Google Scholar]
- Chastain, J.; King Jr, R.C. Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Chen, G.; Zhang, Y.; Zhu, Y.; Xu, J. Grafting of PEG400 onto the surface of LLDPE/SMA film. Front. Chem. Eng. China 2007, 1, 128–131. [Google Scholar] [CrossRef]
- Zanini, S.; Müller, M.; Riccardi, C.; Orlandi, M. Polyethylene glycol grafting on polypropylene membranes for anti-fouling properties. Plasma Chem. Plasma Process. 2007, 27, 446–457. [Google Scholar] [CrossRef]
- Popelka, A.; Krupa, I.; Novák, I.; Al-Maadeed, M.A.S.; Ouederni, M. Improvement of aluminum/polyethylene adhesion through corona discharge. Phys. D Appl. Phys. 2016, 50, 035204. [Google Scholar] [CrossRef]
- Barish, J.; Goddard, J. Polyethylene Glycol Grafted Polyethylene: A Versatile Platform for Nonmigratory Active Packaging Applications. Food Sci. 2011, 76, E586–E591. [Google Scholar] [CrossRef] [PubMed]
- Ganji, M.; Docter, M.; Le Grice, S.F.; Abbondanzieri, E.A. DNA binding proteins explore multiple local configurations during docking via rapid rebinding. Nucleic Acids Res. 2016, 44, 8376–8384. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Fujioka, M.; Asano, K.; Shoji, A.; Athipettah, J.; Yoshida, Y. Surface modification of poly(ethylene terephthalate) by plasma polymerization of poly(ethylene glycol). Mater. Sci. Mater. Med. 2007, 18, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Horgen, D.; Orski, S.; Rodriguez, C.V.; Beers, K.; Balazs, G.; Jones, T.; Work, T.; Brignac, K.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2017, 127. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M. Rheological and mechanical investigation into the effect of different molecular weight poly (ethylene glycol) s on polycaprolactone-ciprofloxacin filaments. ACS Omega 2019, 4, 5412–5423. [Google Scholar] [CrossRef]





| LDPE Properties | Description |
|---|---|
| Density at temperature 23 °C | 0.918 g/cm3 (ASTM D-1505) |
| Melt flow index | 8.0 g/10 min, 190 °C/2.16 kg (ASTM D-1238) |
| Crystalline melting point | 105 °C |
| Recommended uses | Extrusion coating at high speed |
| Testing Liquid | |||
|---|---|---|---|
| Water | 72.1 | 19.9 | 52.2 |
| Formamide | 56.9 | 23.5 | 33.4 |
| Ethylene glycol | 48.0 | 29.0 | 19.0 |
| Samples | Element, Atomic Conc. (at. %) | ||
|---|---|---|---|
| C 1s | O 1s | N 1s | |
| (a) Untreated-LDPE | 91.2 | 8.6 | 0.2 |
| (b) Corona-treated LDPE | 88.5 | 11.3 | 0.2 |
| (c) 1.5 wt.% PEG (1000M)-g-LDPE | 95.2 | 4.6 | 0.2 |
| (d) 10.0 wt.% PEG (1000M)-g-LDPE | 93.6 | 6.1 | 0.3 |
| (e) 1.5 wt.% PEG (6000M)-g-LDPE | 95.3 | 4.5 | 0.00 |
| (f) 10.0 wt.% PEG (6000M)-g-LDPE | 92.3 | 7.5 | 0.04 |
| (g) 1.5 wt.% PEO (300,000M)-g-LDPE | 95.1 | 5.2 | 0.03 |
| (h) 5.0 wt.% PEO (300,000M)-g-LDPE | 90.6 | 9.2 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Gunaid, T.A.; Krupa, I.; Ouederni, M.; Krishnamoorthy, S.K.; Popelka, A. Enhancement of Adhesion Characteristics of Low-Density Polyethylene Using Atmospheric Plasma Initiated-Grafting of Polyethylene Glycol. Polymers 2021, 13, 1309. https://doi.org/10.3390/polym13081309
Al-Gunaid TA, Krupa I, Ouederni M, Krishnamoorthy SK, Popelka A. Enhancement of Adhesion Characteristics of Low-Density Polyethylene Using Atmospheric Plasma Initiated-Grafting of Polyethylene Glycol. Polymers. 2021; 13(8):1309. https://doi.org/10.3390/polym13081309
Chicago/Turabian StyleAl-Gunaid, Taghreed Abdulhameed, Igor Krupa, Mabrouk Ouederni, Senthil Kumar Krishnamoorthy, and Anton Popelka. 2021. "Enhancement of Adhesion Characteristics of Low-Density Polyethylene Using Atmospheric Plasma Initiated-Grafting of Polyethylene Glycol" Polymers 13, no. 8: 1309. https://doi.org/10.3390/polym13081309






