Advances in Nano-Enabled Platforms for the Treatment of Depression
Abstract
:1. Introduction
2. Designing Sustained-Release Formulations for the Delivery of Antidepressant Drugs
2.1. The Oral Route of Antidepressant Drug Delivery
2.2. Intranasal Route of Administration of Antidepressants
2.3. Parenteral Route for the Delivery of Antidepressants
3. Nanocarriers Employed as Therapeutic Delivery Platforms of Antidepressants
3.1. Dendrimers
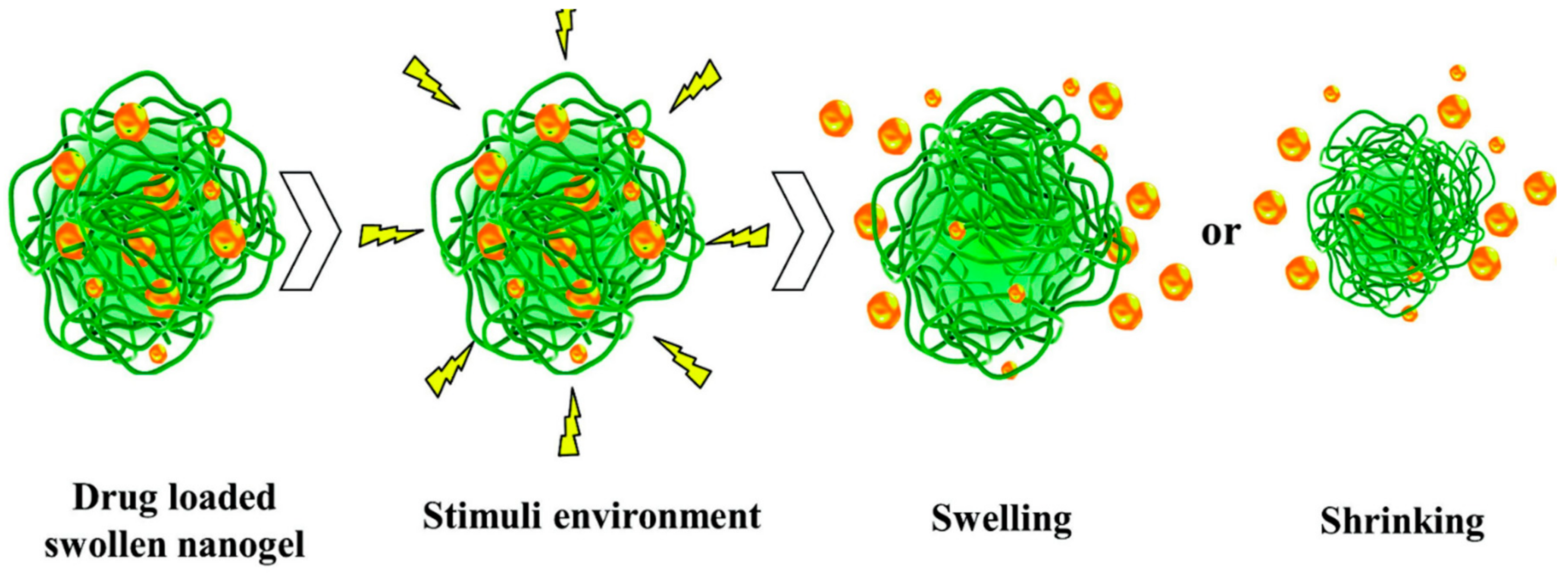
3.2. Nanogels
3.3. Polymeric Micelles
3.4. Nanoliposomes
3.5. Carbon Nanotubes (CNT)
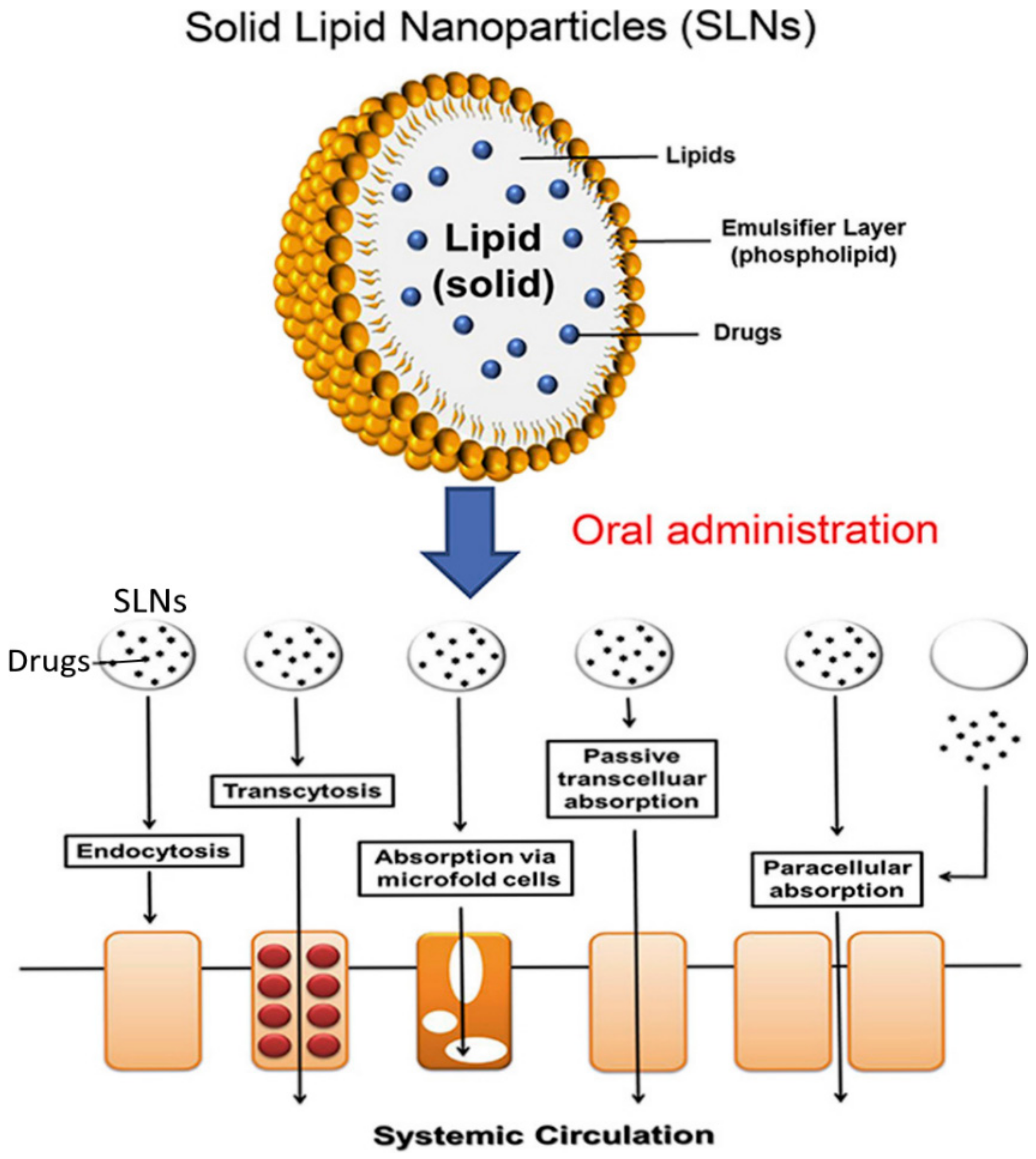
3.6. Solid-Lipid Nanoparticles (SLN)
3.7. Polymeric Nanoparticles
3.8. Magnetic Nanoparticles
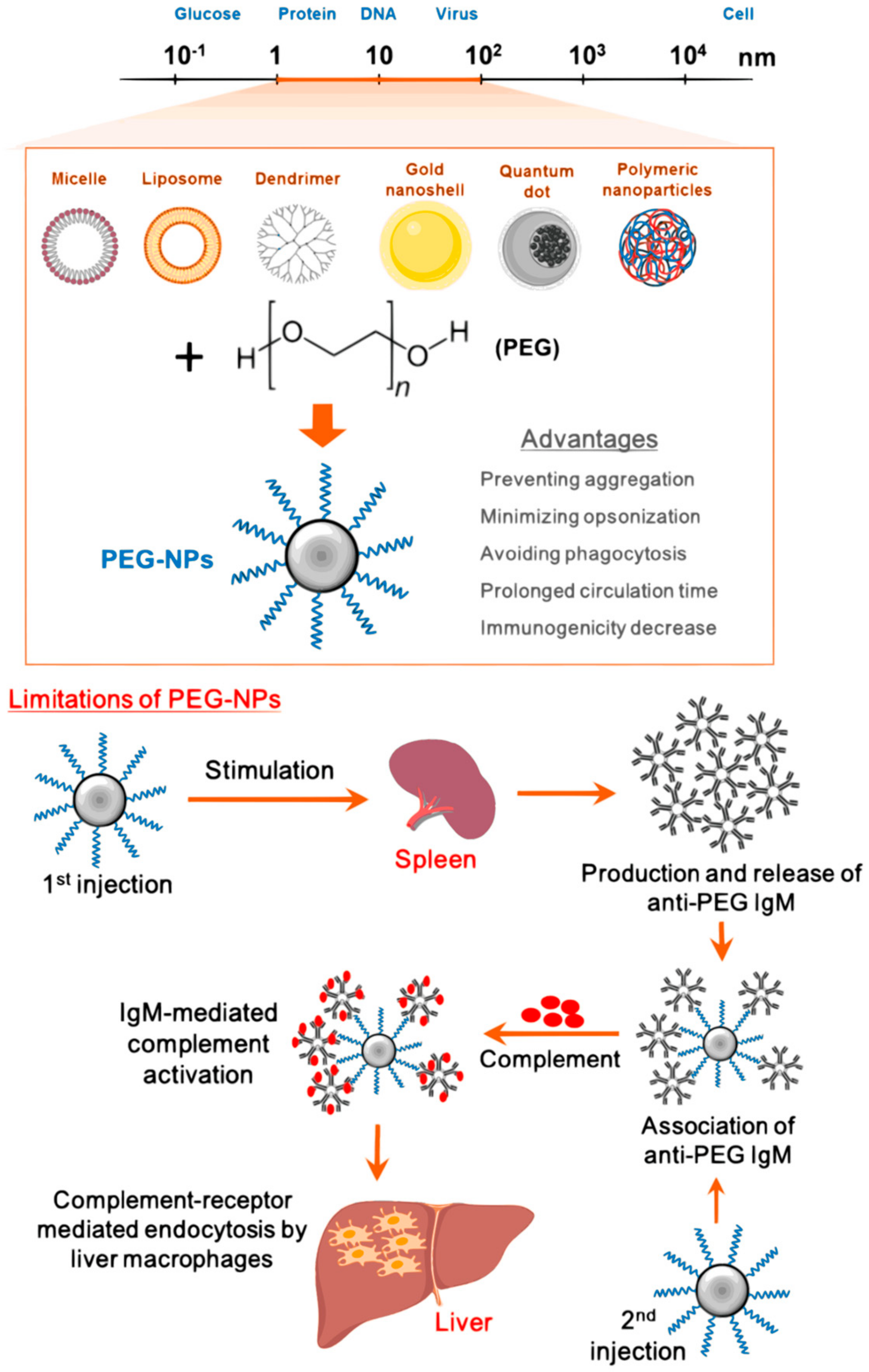
4. Surface Modification of Nanoparticles for Targeted Delivery of Antidepressants
Use Of Ligands to Improve the Specificity of Antidepressants and to Enhance Neuro Bioavailability
5. Toxicity of Nano-Based Antidepressants
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Atnafie, S.A.; Muluneh, N.Y.; Getahun, K.A.; Woredekal, A.T.; Kahaliw, W. Depression, anxiety, stress, and associated factors among khat chewers in Amhara region, Northwest Ethiopia. Depression Res. Treat. 2020, 2020, 7934892. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World Psychiatry 2010, 9, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Meltzer, Y.H. Psychopharmacology; The Fourth Generation of Progress. In The Serotonin Hypothesis of Major Depression; Raven Press: New York, NY, USA, 1994; pp. 933–944. [Google Scholar]
- Strawn, J.R.; Geracioti, L.; Rajdev, N.; Clemenza, K.; Levine, A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: An evidence-based treatment review. Expert Opin. Pharmacother. 2018, 19, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Ruhela, R.K.; Medhi, B. Nanomedicine in Central Nervous System (CNS) disorders: A present and future prospective. Adv. Pharm. Bull. 2016, 6, 319–335. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; Kondiah, P.J.; Du Toit, L.C.; Kumar, P.; Pillay, V. Nanotechnological paradigms for neurodegenerative disease interventions. In Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering; Elsevier BV: Cambridge, MA, USA, 2020; pp. 277–292. [Google Scholar]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. Ligand-appended BBB-targeted nanocarriers (LABTNs). Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 149–180. [Google Scholar] [CrossRef]
- Kondiah, P.P.; Choonara, Y.E.; Kondiah, P.J.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Nanocomposites for therapeutic application in multiple sclerosis. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 391–408. [Google Scholar]
- Parajapati, S.K.; Maurya, S.D.; Das, M.K.; Tilak, V.K.; Verma, K.K.; Dhakar, R.C. Potential application of dendrimers in drug delivery: A concise review and update. J. Drug Deliv. Ther. 2016, 6, 71–85. [Google Scholar] [CrossRef]
- Deore, S.; Shahi, S.; Dabir, P. Nanoparticle: As targeted drug delivery system for depression. Int. J. Curr. Pharm. 2016, 8, 7–11. [Google Scholar]
- Swain, S.; Behera, A.; Dinda, S.; Patra, C.; Jammula, S.; Beg, S.; Rao, M.E.B. Formulation design, optimization and pharmacodynamic evaluation of sustained release mucoadhesive microcapsules of venlafaxine HCl. Indian J. Pharm. Sci. 2019, 76, 354. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, C.-H.; Lin, Z.-C.; Fang, J.-Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017, 25, 219–234. [Google Scholar] [CrossRef]
- Dimitrijevic, I.; Pantic, I. Application of nanoparticles in psychophysiology and psychiatry research. Rev. Adv. Mater. Sci. 2014, 38, 1–6. [Google Scholar]
- Watanabe, S.; Suemaru, K.; Inoue, N.; Imai, K.; Aimoto, T.; Araki, H. Pharmacokinetic and pharmacodynamic studies of drug interaction following oral administration of imipramine and sodium alginate in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 85–91. [Google Scholar] [CrossRef]
- Rajput, R.; Kumar, S.; Nag, P.; Singh, M. Fabrication and characterization of chitosan based polymeric Escitalopram nanoparticles. J. Appl. Pharm. Sci. 2016, 6, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Chivere, V.T.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Nanotechnology-based biopolymeric oral delivery platforms for advanced cancer treatment. Cancers 2020, 12, 522. [Google Scholar] [CrossRef] [Green Version]
- Alia, E.; Afreen, S.; Saleem, U.; Rauf, A. Formulation and evaluation of microspheres of fluoxetine hydro-chloride using different biopolymers. ResearchGate 2016, 33, 759–770. [Google Scholar]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Thi, T.T.H.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Neha, B.; Ganesh, B.; Preeti, K.; Guru, S. Drug delivery to the brain using polymeric nanoparticles: A review. Int. J. Pharm. Life Sci. 2013, 2, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Lahkar, S.; Das, M.K. Surface modified polymeric nanoparticles for brain targeted drug delivery. ResearchGate 2013, 7, 914–926. [Google Scholar]
- Abourehab, M.A.; Ahmed, O.A.; Balata, G.F.; Almalki, W.H. Self-assembled biodegradable polymeric micelles to improve dapoxetine delivery across the blood–brain barrier. Int. J. Nanomed. 2018, ume 13, 3679–3687. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-Santos, B.; Chorilli, M. The uses of resveratrol for neurological diseases treatment and insights for nanotechnology based-drug delivery systems. Int. J. Pharm. 2020, 589, 119832. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2017, 25, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, Y.-R.; Kou, N.; Hu, M.-J.; Wang, Q.-S.; Cui, Y.-L. Intranasal delivery of icariin via a nanogel-thermoresponsive hydrogel compound system to improve its antidepressant-like activity. Int. J. Pharm. 2020, 586, 119550. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Md, S.; Fazil, M.; Kumar, M.; Sahni, J.K.; Ali, J.; Baboota, S. Venlafaxine loaded chitosan NPs for brain targeting: Pharmacokinetic and pharmacodynamic evaluation. Carbohydr. Polym. 2012, 89, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Md, S.; Sahni, J.K.; Ali, J.; Baboota, S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J. Psychiatr. Res. 2014, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dange, S.; Kamble, M.; Bhalerao, K.; Chaudhari, P.; Bhosale, A.; Nanjwade, B.; Shinde, S. Formulation and evaluation of venlafaxine nanostructured lipid carriers. J. Bionanosci. 2014, 8, 81–89. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, N.; Zhong, J. Nanostructures for Drug Delivery. In Nanosuspension Drug Delivery Dystem: Preparation, Characterization, Postproduction Processing, Dosage Form, and Application; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–443. [Google Scholar]
- Yuan, Z.T.; Qing, C.D.; Qing, G.J.; Lan, H.Y. Preparation of sertraline-loaded chitosan nanoparticles and the pharmacokinetics studies. Afr. J. Pharm. Pharmacol. 2016, 10, 26–33. [Google Scholar] [CrossRef] [Green Version]
- AlAbsi, A.; Khoudary, A.C.; Abdelwahed, W. The antidepressant effect of L-tyrosine-loaded nanoparticles: Behavioral aspects. Ann. Neurosci. 2016, 23, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 590, 119912. [Google Scholar] [CrossRef]
- Fuller, E.G.; Scheutz, G.M.; Jimenez, A.; Lewis, P.; Savliwala, S.; Liu, S.; Sumerlin, B.S.; Rinaldi, C. Theranostic nanocarriers combining high drug loading and magnetic particle imaging. Int. J. Pharm. 2019, 572, 118796. [Google Scholar] [CrossRef]
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122. [Google Scholar] [CrossRef] [Green Version]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Wu, Y. Dendrimer Advances for the Central Nervous System Delivery of Therapeutics. ACS Chem. Neurosci. 2013, 5, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Florendo, M.; Figacz, A.; Srinageshwar, B.; Sharma, A.; Swanson, D.; Dunbar, G.L.; Rossignol, J. Use of Polyamidoamine Dendrimers in Brain Diseases. Molecules 2018, 23, 2238. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Danish, A.; Sheikh, T.; Abrar, M.; Chaos, S.; Bagwan, R.; Kulkarni, A. Nanogel: A versatile nano-scopic platform for oral drug delivery. World J. Pharm. Sci. 2018, 7, 685–693. [Google Scholar]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef] [Green Version]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Casolaro, M.; Casolaro, I. Polyelectrolyte hydrogel platforms for the delivery of antidepressant drugs. Gels 2016, 2, 24. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Polymeric micelles for drug targeting and delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 167–202. [Google Scholar]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef] [Green Version]
- Subramani, K.; Ahmed, W. Emerging Nanotechnologies in Dentistry. In Nanoparticulate Drug Delivery Systems for Oral Cancer Treatment; William Andrew Publishing: Boston, MA, USA, 2012; pp. 333–345. [Google Scholar]
- Mohapatra, S.S.; Ranjan, S.; Dasgupta, N.; Mishra, R.K.; Thomas, S. Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. Dove Med. Press 2016, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomani, M.S.; Samy, J.G. Nanoliposome: An alternative approach for drug delivery system. Int. J. Adv. Pharm. Med. Bioallied. Sci. 2016, 2016, 1–10. [Google Scholar]
- Mura, P. Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: A review. Int. J. Pharm. 2020, 579, 119181. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Stealth and cationic nanoliposomes as drug delivery systems to increase andrographolide BBB permeability. Pharmaceutics 2018, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Daeihamed, M.; Dadashzadeh, S.; Haeri, A.; Akhlaghi, M.F. Potential of liposomes for enhancement of oral drug absorption. Curr. Drug Deliv. 2016, 13, 1. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; Pereira, M.D.C. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2019, 10, 380–402. [Google Scholar] [CrossRef]
- Mutlu, N.B.; DeĞim, Z.; Yılmaz, Ş.; Eşsiz, D.; Nacar, A. New perspective for the treatment of Alzheimer diseases: Liposomal rivastigmine formulations. Drug Dev. Ind. Pharm. 2011, 37, 775–789. [Google Scholar] [CrossRef]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; de Backer, M.E.; Rip, J.; Nabuurs, R.J.; Gaillard, P.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J. Control. Release 2015, 203, 40–50. [Google Scholar] [CrossRef]
- Medyantseva, E.P.; Brusnitsyn, D.V.; Varlamova, R.M.; Maksimov, A.A.; Konovalova, O.A.; Budnikov, H.C. Surface modification of electrodes by carbon nanotubes and gold and silver nanoparticles in monoaminoxidase biosensors for the determination of some antidepressants. J. Anal. Chem. 2017, 72, 362–370. [Google Scholar] [CrossRef]
- Komane, P.P.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Kondiah, P.P.D.; Modi, G.; Pillay, V.; Komane, P.P. Diagnosis and treatment of neurological and ischemic disorders employing carbon nanotube technology. J. Nanomater. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sengel, C.T.; Alpturk, O. Nanoconjugate Nanocarriers for Drug Delivery. In Carbon Nanotubes for Drug Delivery; Apple Academic Press: New York, NY, USA, 2018; pp. 348–376. [Google Scholar]
- Pitroda, J.; Jethwa, B.; Dave, S.K. A critical review on carbon nanotubes. Int. J. Constr. Res. Civ. Eng. 2016, 2, 36–42. [Google Scholar] [CrossRef]
- Guo, Q.; You, H.; Yang, X.; Lin, B.; Zhu, Z.; Lu, Z.; Li, X.; Zhao, Y.; Mao, L.; Shen, S.; et al. Functional single-walled carbon nanotubes ‘CAR’ for targeting dopamine delivery into the brain of parkinsonian mice. Nanoscale 2017, 9, 10832–10845. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-Santos, B.; Chorilli, M.; Gremião, M.P.D. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef] [Green Version]
- Noack, A.; Hause, G.; Mäder, K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 423, 440–451. [Google Scholar] [CrossRef]
- Vijayanand, P.; Jyothi, V.; Aditya, N.; Mounika, A. Development and characterization of solid lipid nanoparticles containing herbal extract: In vivo antidepressant activity. J. Drug Deliv. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Parry, O.; Downing, C.; Aleisaei, E.; Jones, C.; Coward, K. Nanomedicine applications in women’s health: State of the art. Int. J. Nanomed. 2018, 13, 1963–1983. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.-K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Beiranvand, S.; Sorori, M.M. Pain management using nanotechnology approaches. Artif. Cells Nanomed. Biotechnol. 2019, 47, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, N.; Jagani, H.V.; Alex, A.T.; Volety, S.M.; Rao, J.V. Strategies for drug delivery to the central nervous system by systemic route. Drug Deliv. 2015, 22, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Midha, K.; Kaur, S.; Marwah, S. An overview of the nanoparticles in CNS targeted drug delivery: An emerging trend. Curr. Trends Pharm. Clin. Trials 2019, 2, 2–5. [Google Scholar]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic nanoparticles in the central nervous system: Targeting principles, applications and safety issues. Molecules 2017, 23, 9. [Google Scholar] [CrossRef] [Green Version]
- Denkbaş, E.B.; Çelik, E.; Erdal, E.; Kavaz, D.; Akbal, Ö.; Kara, G.; Bayram, C. Magnetically based nanocarriers in drug delivery. Nanobiomater. Drug Deliv. 2016, 285–331. [Google Scholar] [CrossRef]
- Saeidienik, F.; Shahraki, M.R.; Fanaei, H.; Badini, F. The effects of iron oxide nanoparticles administration on depression symptoms induced by LPS in male wistar rats. Basic Clin. Neurosci. J. 2018, 9, 209–216. [Google Scholar] [CrossRef]
- Gutiérrez, L.; De La Cueva, L.; Moros, M.; Mazarío, E.; De Bernardo, S.; De La Fuente, J.M.; Morales, M.D.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A promising approach for delivery of neuroprotective drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Siafaka, P.I.; Okur, N.Ü.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. Front. 2020, 1–20. [Google Scholar] [CrossRef]
- Essa, D.; Choonara, Y.E.; Kondiah, P.P.D.; Pillay, V. Comparative nanofabrication of PLGA-chitosan-PEG systems employing microfluidics and emulsification solvent evaporation techniques. Polymers 2020, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, W.; Kim, J. Surface modification of functional nanoparticles for controlled drug delivery. J. Dispers. Sci. Technol. 2003, 24, 475–487. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Lee, S.-S.; Bhattacharya, M.; Nam, J.-S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, B.; Fan, Y.; Wang, M.; Kebebe, D.; Li, J.; Liu, Z. Traditional Chinese medicine combined with hepatic targeted drug delivery systems: A new strategy for the treatment of liver diseases. Biomed. Pharmacother. 2019, 117, 109128. [Google Scholar] [CrossRef]
- Muro, S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release 2012, 164, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Sikorski, A.F.; Toporkiewicz, M.; Meissner, J.; Matusewicz, L.; Czogalla, A. Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: Principles, hopes, and challenges. Int. J. Nanomed. 2015, 10, 1399–1414. [Google Scholar] [CrossRef] [Green Version]
- Prado-Audelo, P.-A.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; Gonzá-lez-Torres, M.; Florán, E.; Cortés, H.; Leyva-Gómez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Impact of nanoparticles on brain health: An up to date overview. J. Clin. Med. 2018, 7, 490. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Yin, Q.; Fei, Y.; Zhu, J.; Qiu, Y.; Fang, W.; Li, Y. Research progress of mechanisms for tight junction damage on blood–brain barrier inflammation. Arch. Physiol. Biochem. 2020, 1–12. [Google Scholar] [CrossRef]
- Tao, X.; Xie, Y.; Zhang, Q.; Qiu, X.; Yuan, L.; Wen, Y.; Li, M.; Yang, X.; Tao, T.; Xie, M.; et al. Cholesterol-modified amino-pullulan nanoparticles as a drug carrier: Comparative study of cholesterol-modified carboxyethyl pullulan and pullulan nanoparticles. Nanomaterials 2016, 6, 165. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]


| Biopolymers | Benefits | Disadvantages | Drug | References |
|---|---|---|---|---|
| Oral | ||||
| sodium alginate HMC | Sustained release from microcapsules due to swelling properties of sodium alginate/hydroxypropyl methylcellulose copolymers at pH 7.4 and improved bioavailability due to mucoadhesive properties of sodium alginate when they bind to the epithelial mucous membrane lining. | Drug release is dependent on the concentration of the biopolymers | Venlafaxine | [14] |
| Sodium alginate | Increased circulation period enabled due to the mucoadhesive properties of sodium alginate which allows a delay in gastric emptying. | Solubility of alginate is dependent on pH of the solvent | Imipramine | [17] |
| Chitosan | Improved sustained release and circulation. Enhanced permeation due to mucoadhesive properties of chitosan which allows the nanoparticles to bind with the mucosa via the ionic interaction | Solubility of chitosan is affected by pH. | escitalopram | [18] |
| Chitosan-Arabinoxylan | Improved entrapping rate due to the swelling properties of the biopolymers. Sustained release from the microspheres due to swelling properties of chitosan/ arabinoxylan copolymer under acidic conditions of pH 1.2, due to protonation of the free amine groups on the copolymers | Encapsulation rate is directly proportional to the concentration of chitosan | Fluoxetine HCL | [20] |
| PEG-PLGA | Improved circulation, half-life and bioavailability due to amphiphilic copolymers of PEG-PLGA. | PLGA is not stable on its own | Dapoxetine | [25] |
| Intranasal | ||||
| Chitosan-PLGA | Sustained release profile due to hydration and swelling properties of CN/PLGA copolymers. Enhanced drug uptake rate and bioavailability as a result mucoadhesive and cationic properties of chitosan which increases the retention time of the nanoparticles in the nasal passage | PLGA cannot be stabilized by chitosan on its own | Desvenlafaxine | [27] |
| Chitosan sodium TPP | Amplified drug intranasal uptake and bioavailability as a result of mucoadhesive properties of chitosan and the interaction of cationic charges on the chitosan and anionic charges on the tight junction of the mucosal epithelium cells | Solubility of chitosan is affected by pH | Venlafaxine | [29] |
| Alginate | Higher mucoadhesive properties and permeation and sustained release. Enhanced therapeutic efficacy. | Covalent cross linking can result in cell toxicity | Venlafaxine | [30] |
| Nanostructured lipids | Increased drug release and drug efficacy due to improved residential time of the nanoparticles in the nasal cavity due to HPMC biopolymer. | Requires a stabilizer | Venlafaxine | [31] |
| Parenteral | ||||
| Chitosan | Improved half-life, entrapping rate and bioavailability owing it to mucoadhesive, encapsulation efficacy and delayed clearance properties of chitosan | Solubility of chitosan is affected by pH. | Sertraline | [21,33] |
| Polycaprolactone | Enhanced entrapping efficiency and sustained release. | More efficient with hydrophobic drugs Requires a stabilizer | L–tyrosine | [34] |
| Type of Nanocarrier | Drug Delivery Characteristics | Structure | Drawbacks | References |
|---|---|---|---|---|
| Dendrimers | Rapid cellular entry, high drug loading capacity, improved half-life, biocompatibility | Highly branched, Monodisperse structure, | Non-degradable in physiological environment, Large particle size | [12,40] |
| Nanogels | Large surface area, high entrapping rate, biocompatible, high loading capacity, | Hydrogels, cross-linked hydrophilic polymer networks, | Physically cross-linked nanogels are less stable | [42,43] |
| Polymeric micelles | Increased half-life, solubility and stability, biodegradable, biocompatible | Amphiphilic Block copolymers, | Low drug loading capacity, Premature leaking, | [48,49,50,72] |
| Nanoliposomes | Enhanced encapsulating rate, biocompatible, biodegradable, improved intracellular uptake | Lipid vesicles, amphiphilic phospholipids | poor stability in aqueous | [53,55] |
| Carbon nanotubes | Improved cell-penetrating ability, biocompatibility, high drug entrapping rate, | Tubular morphology, two or more layers, allotropes of carbon | Mechanism is not known, too small, low solubility, permeability can be affected with temperature | [6,62] |
| Solid Lipid Nanoparticles | Excellent drug release profile, stable, biodegradable, large surface area | Spherical structure, | Poor incorporation rate, prone to gelation, loading capacity depends on length of the hydrocarbon chain, | [15,66] |
| Polymeric nanoparticles | High cell-penetrating rate, prolong duration, biodegradable, enhanced stability, | Spherical shape, | Easily eliminated in the bloodstream | [23,73] |
| Magnetic nanoparticles | High stability, biocompatible, improve drug targeting | Spherical structure, crystals. | Easily eliminated from the body, prone to aggregation | [75,77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutingwende, F.P.; Kondiah, P.P.D.; Ubanako, P.; Marimuthu, T.; Choonara, Y.E. Advances in Nano-Enabled Platforms for the Treatment of Depression. Polymers 2021, 13, 1431. https://doi.org/10.3390/polym13091431
Mutingwende FP, Kondiah PPD, Ubanako P, Marimuthu T, Choonara YE. Advances in Nano-Enabled Platforms for the Treatment of Depression. Polymers. 2021; 13(9):1431. https://doi.org/10.3390/polym13091431
Chicago/Turabian StyleMutingwende, Fadzai P., Pierre P. D. Kondiah, Philemon Ubanako, Thashree Marimuthu, and Yahya E. Choonara. 2021. "Advances in Nano-Enabled Platforms for the Treatment of Depression" Polymers 13, no. 9: 1431. https://doi.org/10.3390/polym13091431
APA StyleMutingwende, F. P., Kondiah, P. P. D., Ubanako, P., Marimuthu, T., & Choonara, Y. E. (2021). Advances in Nano-Enabled Platforms for the Treatment of Depression. Polymers, 13(9), 1431. https://doi.org/10.3390/polym13091431









