The Influence of the Structure of Selected Polymers on Their Properties and Food-Related Applications
Abstract
:1. Introduction
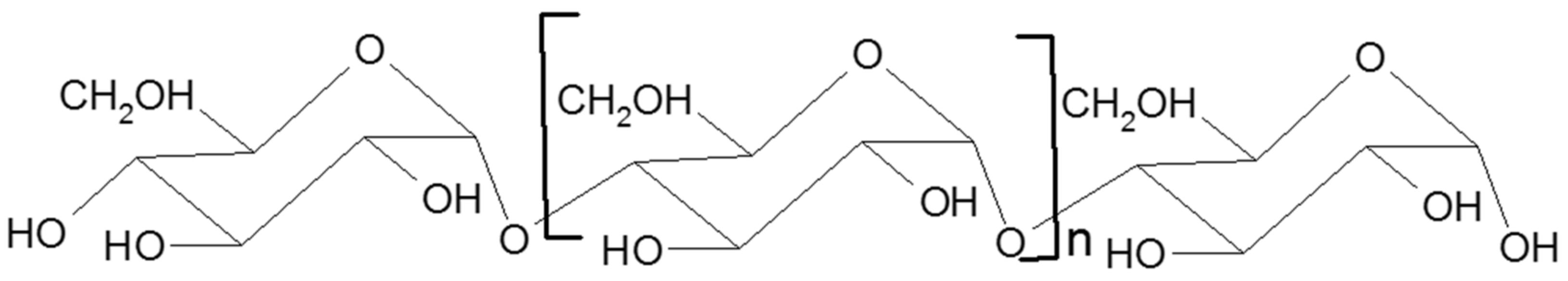
2. Starch
2.1. Structure
2.2. Properties and Applications
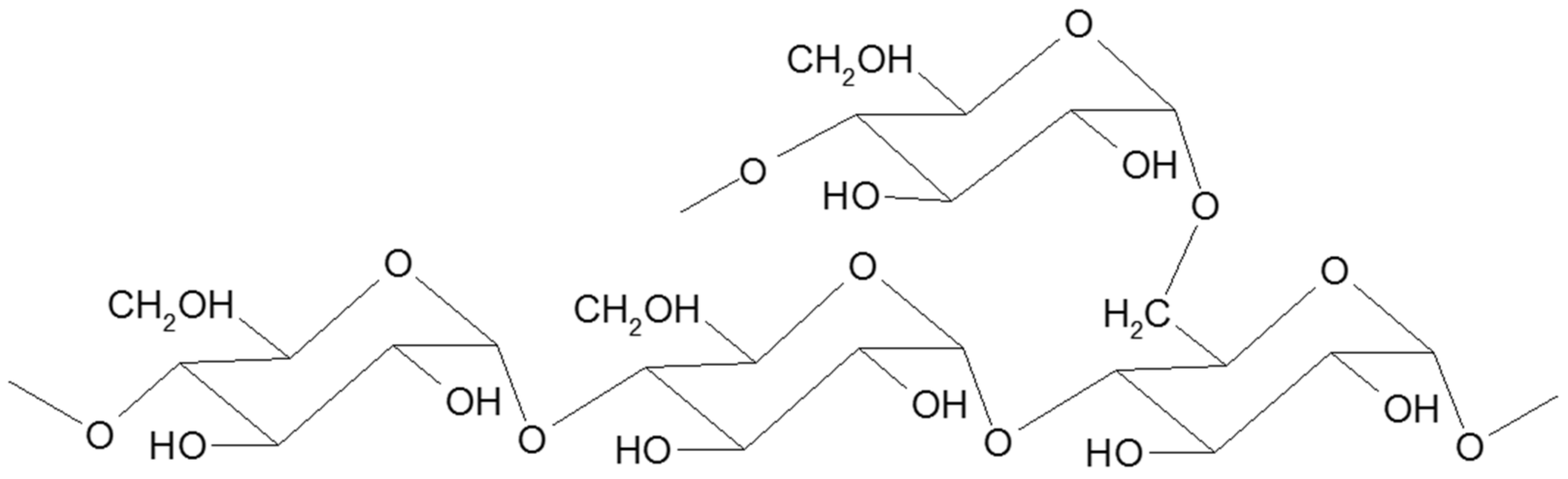
3. Pullulan
3.1. Structure
3.2. Properties
3.3. Applications
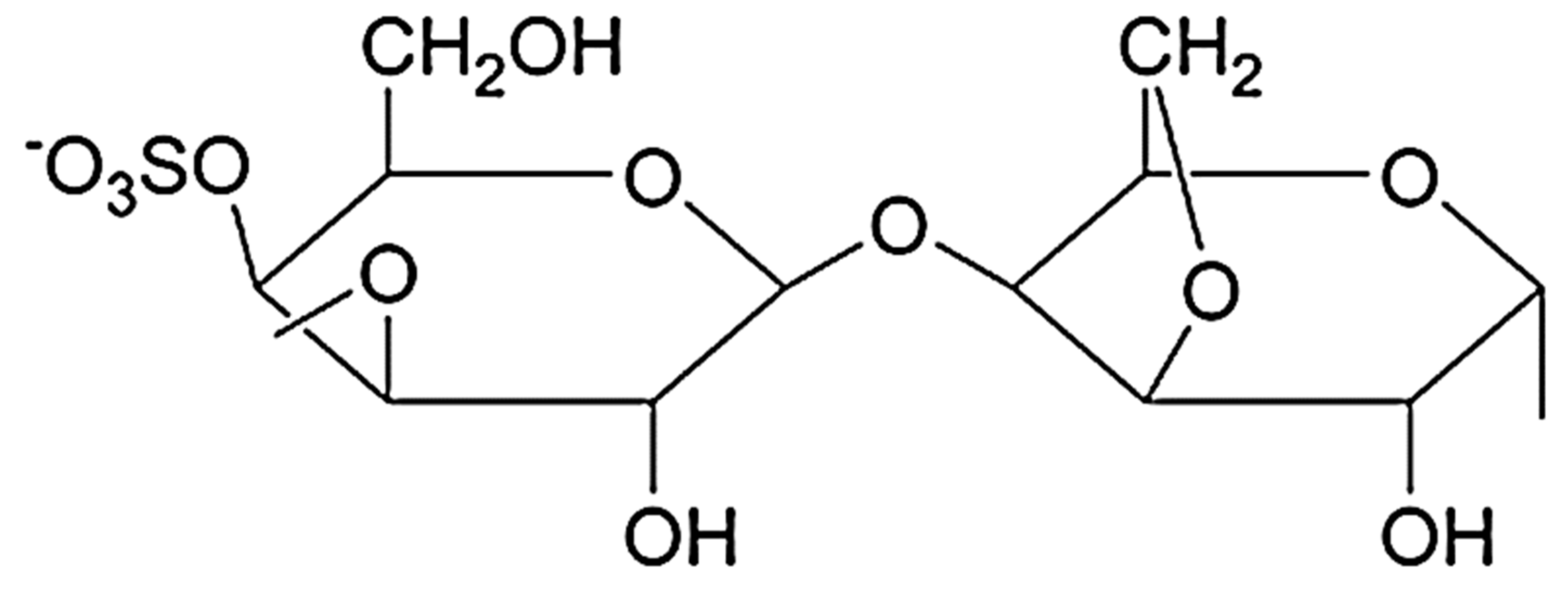
4. Carrageenan
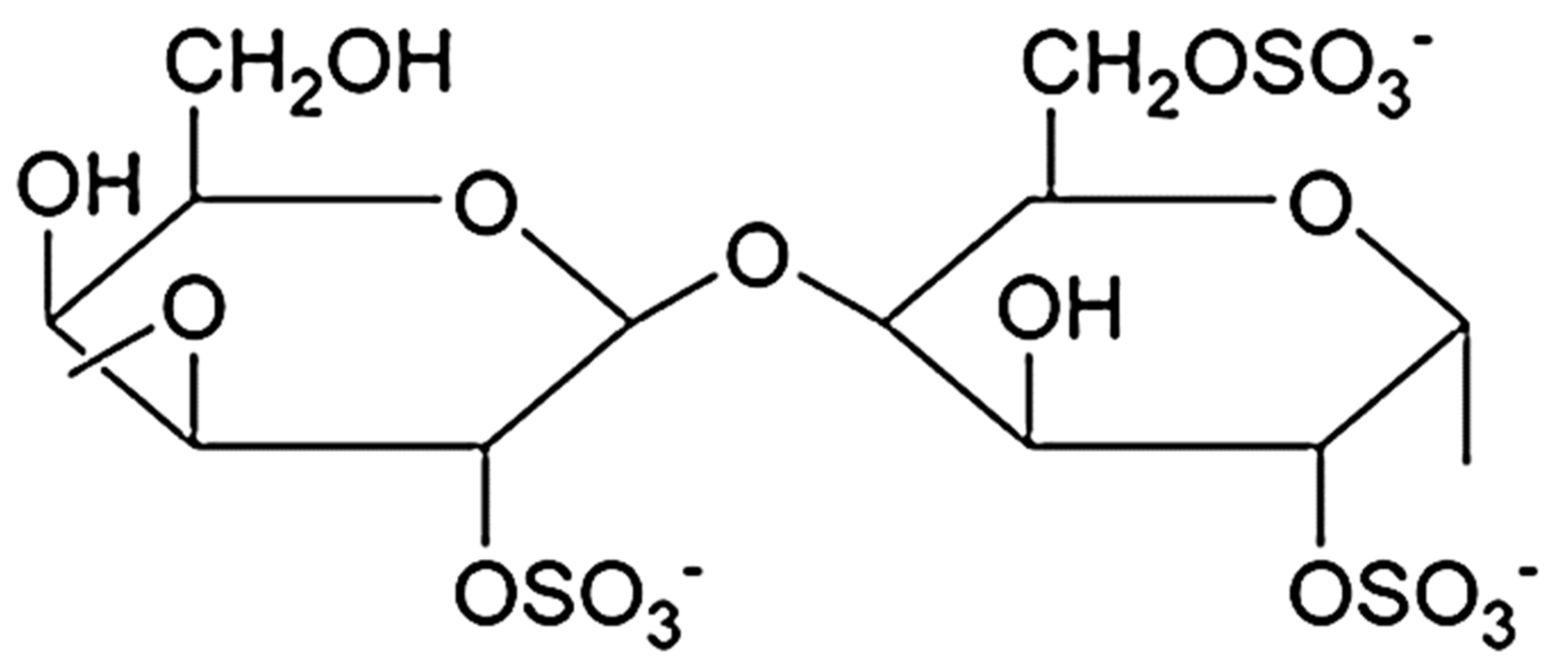
4.1. Structure
4.2. Properties and Applications
5. Pectins
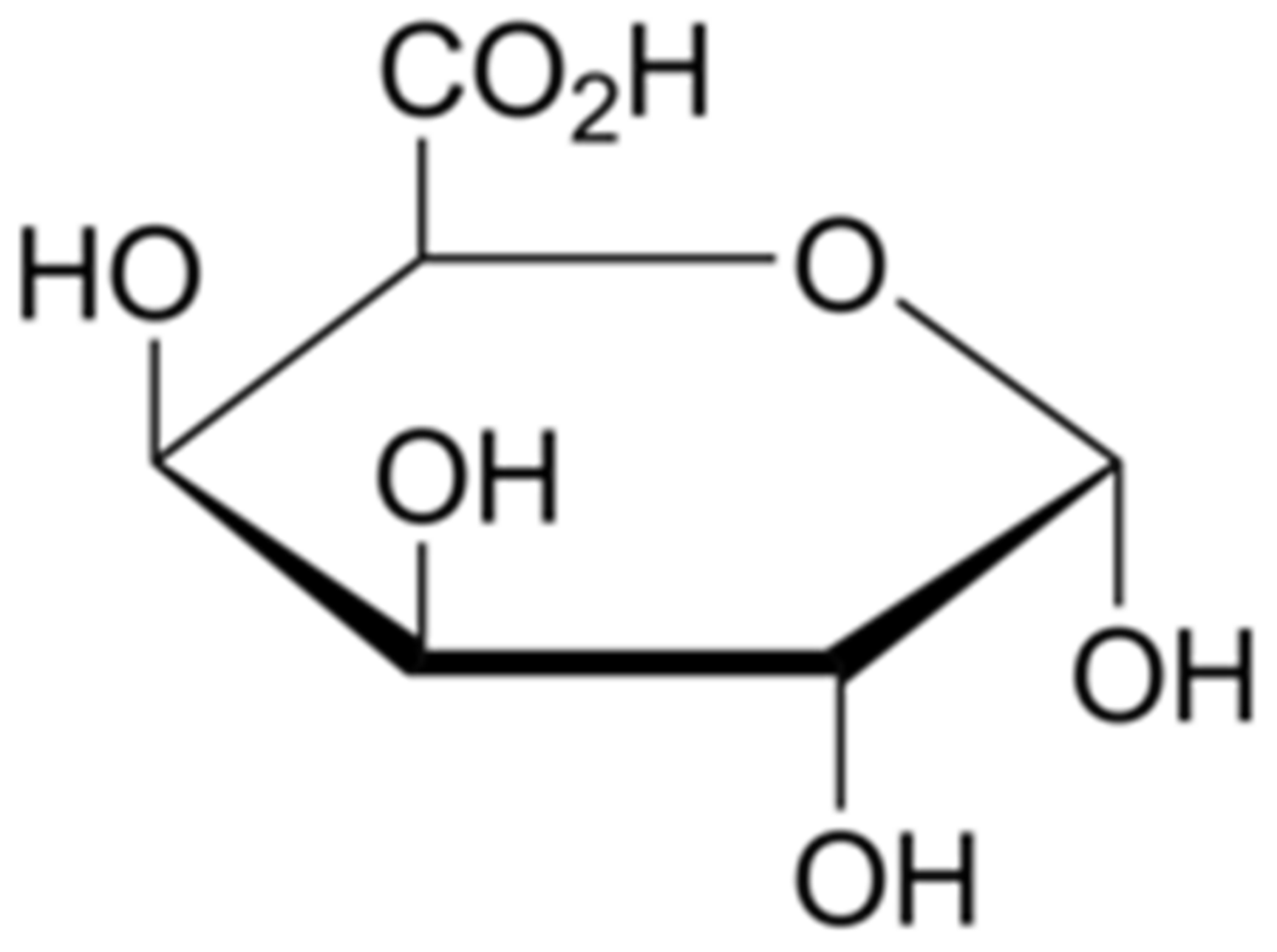
5.1. Structure
5.2. Properties and Applications
6. Agar
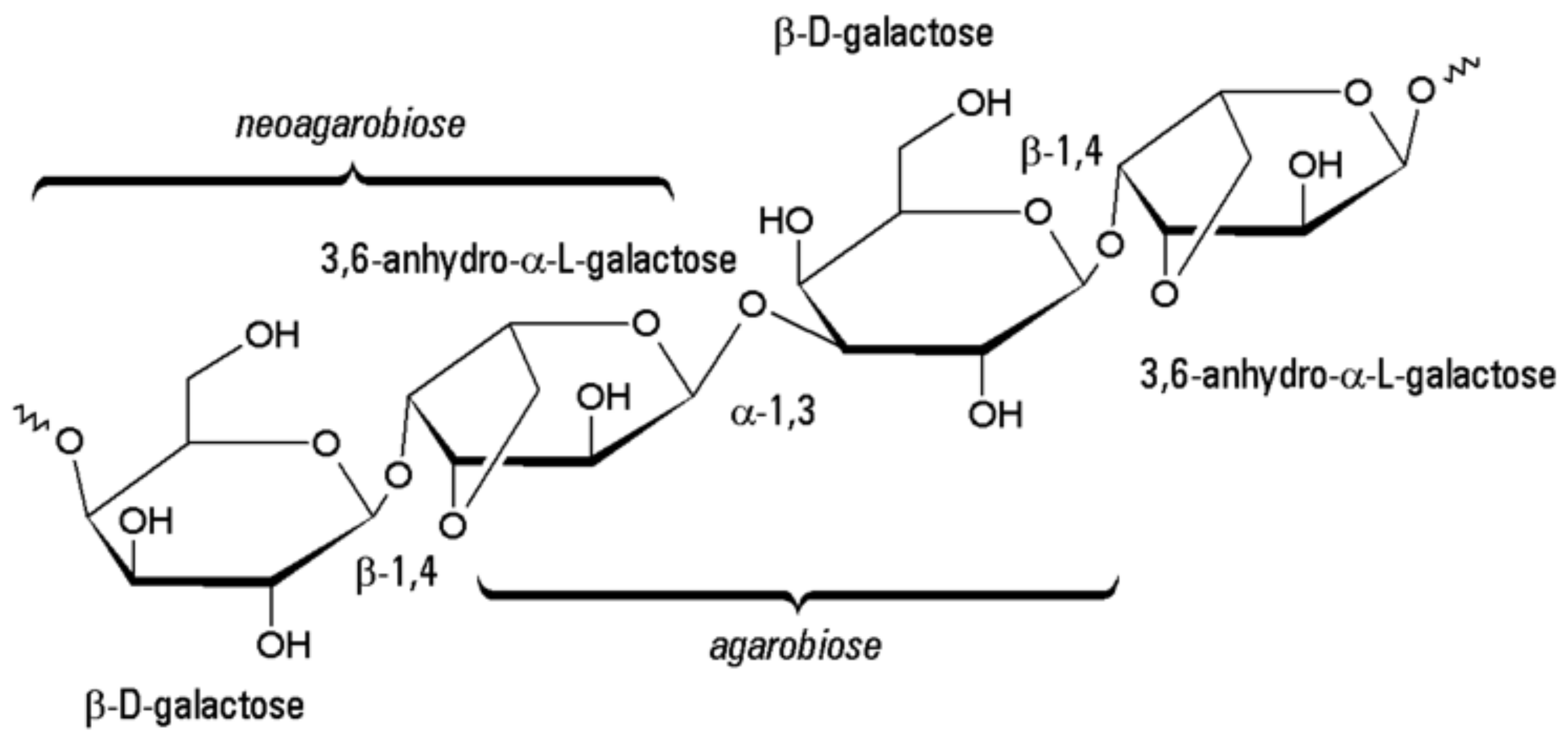
6.1. Structure
6.2. Properties and Applications
7. Nucleic Acids
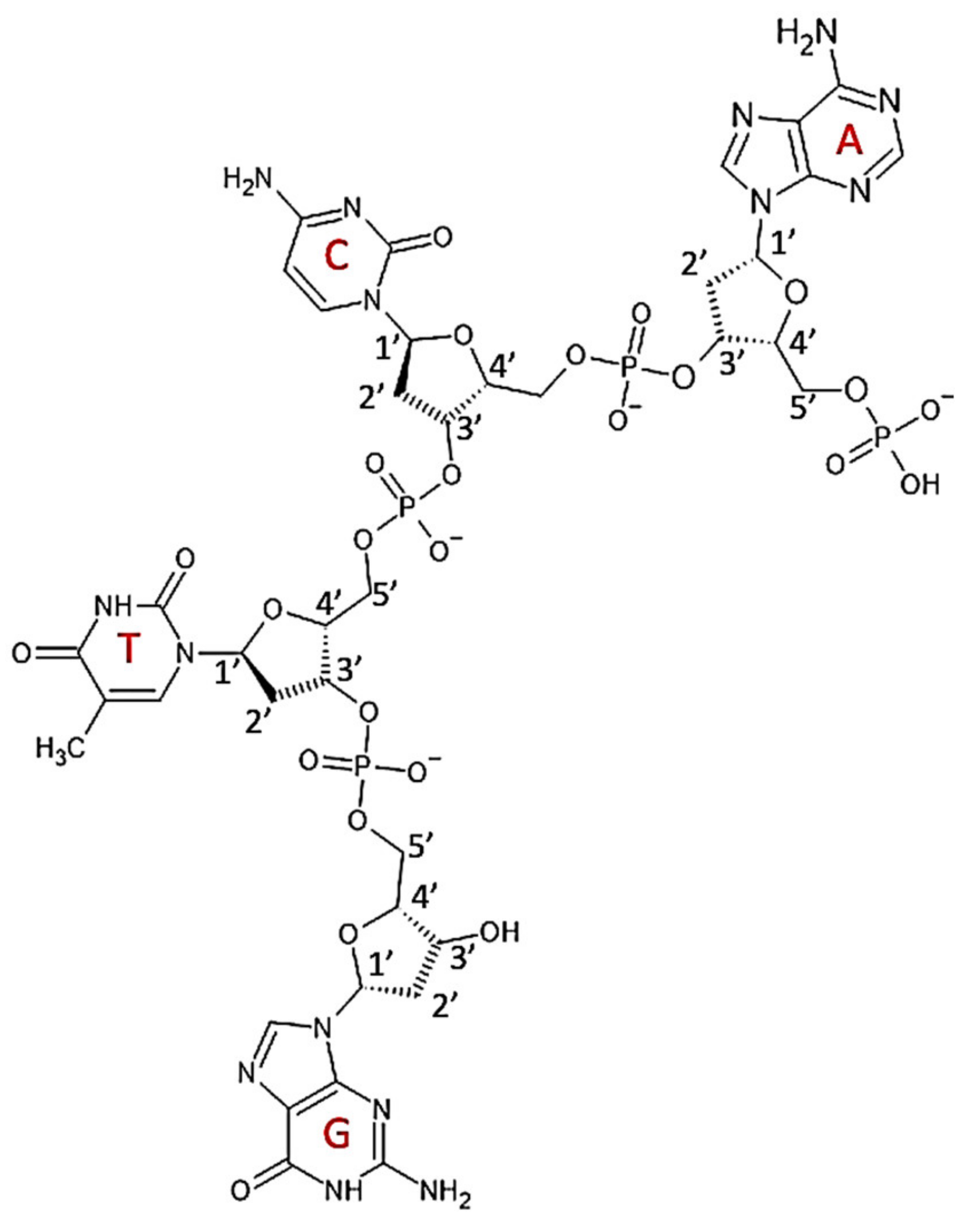
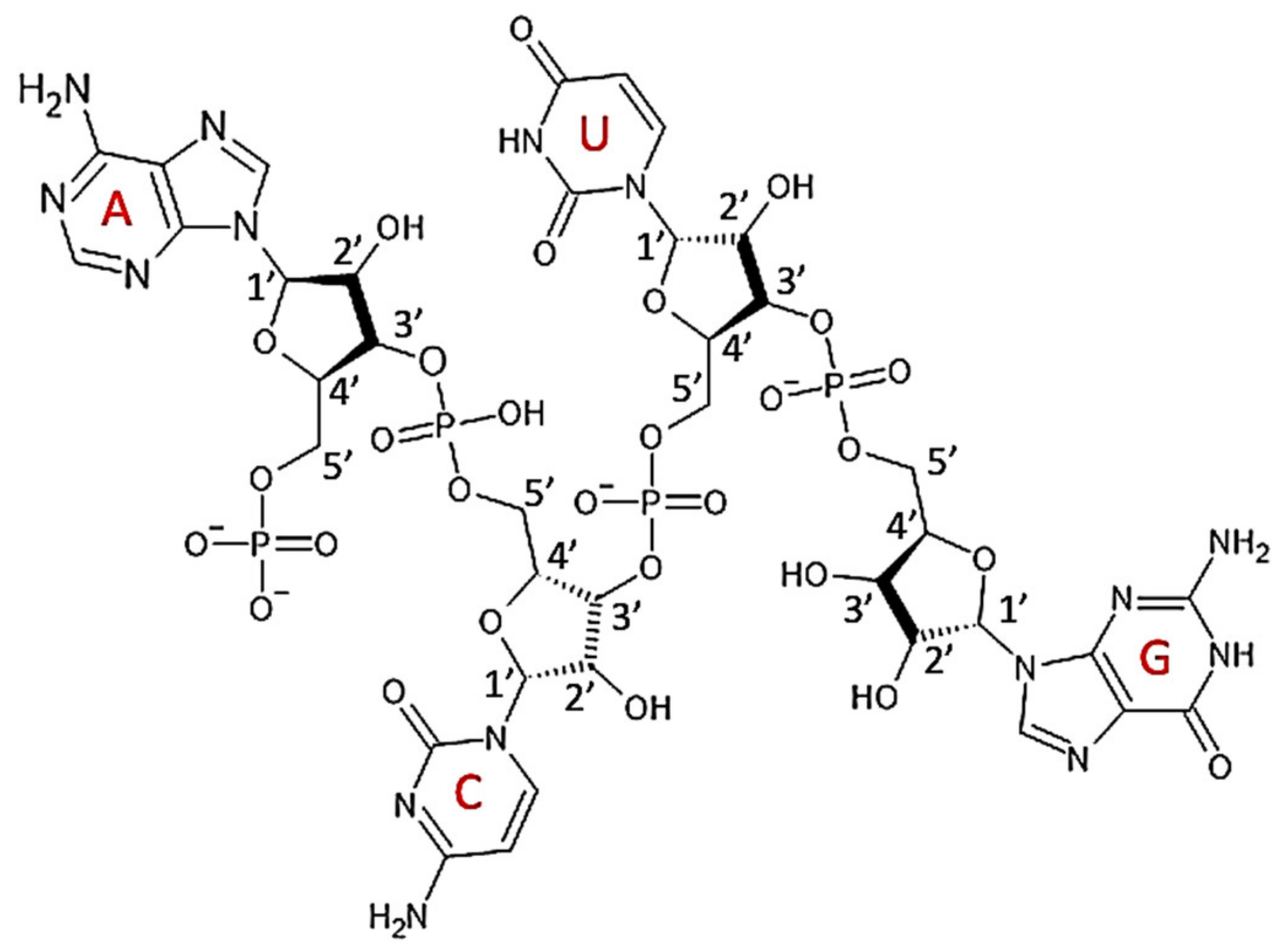
7.1. Structure
7.2. Properties and Applications
8. Proteins
8.1. Structure
8.2. Applications
8.3. Enzymes
8.4. Collagen
8.4.1. Structure
8.4.2. Properties and Applications
8.5. Gelatine
8.5.1. Structure
- Independent, randomly coiled α-chains of to ;
- α-chain dimers linked by one or more covalent bonds referred to as β-chains, of molecular weight ranging from to ;
8.5.2. Properties and Applications
9. Natural Rubber
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O. Dual modification of various starches: Synthesis, properties and applications. Food Chem. 2021, 342, 128325. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R. Functional and engineered colloids from edible materials for emerging applications in designing the food of the future. Adv. Funct. Mater. 2018, 30, 1806809. [Google Scholar] [CrossRef]
- Basiak, E.; Galus, S.; Lenart, A. Characterisation of composite edible films based on wheat starch and whey-protein isolate. Int. J. Food Sci. Technol. 2015, 50, 372–380. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications-A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Piecyk, M.; Wołosiak, R.; Drużynska, B.; Worobiej, E. Chemical composition and starch digestibility in flours from Polish processed legume seeds. Food Chem. 2012, 135, 1057–1064. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch digestibility: Past, present, and future. J. Sci. Food Agric. 2018, 100, 5009–5016. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of copper sulfide nanoparticles and limonene incorporated pullulan/carrageenan-based film with improved mechanical and antibacterial properties. Polymers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Dulong, V.; Kouassi, M.-C.; Labat, B.; Le Cerf, D.; Picton, L. Antioxidant properties and bioactivity of Carboxymethylpullulan grafted with ferulic acid and of their hydrogels obtained by enzymatic reaction. Food Chem. 2018, 262, 21–29. [Google Scholar] [CrossRef]
- Hirohara, H.; Nabeshima, S.; Fujimoto, M.; Nagase, T. Enzyme Immobilisation with Pullulan Gel. U.S. Patent 4,247,642, 27 January 1981. [Google Scholar]
- Jiang, J.-L.; Zhang, W.-Z.; Ni, W.-X.; Shao, J.-W. Insight on structure-property relationships of carrageenan from marine red algal: A review. Carbohydr. Polym. 2021, 257, 117642. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Abella, V.; Barral, L.; Bouza, R.; Farrag, Y.; Lago, F. Entrapment of chitosan, pectin or κ-carrageenan within methacrylate based hydrogels: Effect on swelling and mechanical properties. Mater. Sci. Eng. C 2019, 96, 583–590. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.-I.; Kim, C.W.; Lee, H.-R.; Kim, M. Antiviral activity of lambda-carrageenan against influenza viruses and severe acute respiratory syndrome coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Kuhnle, G.G.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16, e05238. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible films from carrageenan/orange essential oil/trehalose—Structure, optical properties, and antimicrobial activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Wusigale; Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, W.-Y.; Chen, T.-T.; Yan, J.-K. Effects of structural and conformational characteristics of citrus pectin on its functional properties. Food Chem. 2021, 339, 128064. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Huang, S.; Xiong, Y.; Zou, Y.; Dong, Q.; Ding, F.; Liu, X.; Li, H. A novel colorimetric indicator based on agar incorporated with Arnebia euchroma root extracts for monitoring fish freshness. Food Hydrocoll. 2019, 90, 198–205. [Google Scholar] [CrossRef]
- Kryndushkin, D.S.; Alexandrov, I.M.; Ter-Avanesyan, M.D.; Kushnirov, V.V. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J. Biol. Chem. 2003, 278, 49636–49643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedkar, G.D.; Prakash, B.; Khedkar, C.D.; Chopade, B.A. Nucleic acids. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 84–92. [Google Scholar]
- Nawaz, M.A.; Mesnage, R.; Tsatsakis, A.M.; Golokhvast, K.S.; Yang, S.H.; Antoniou, M.N.; Chung, G. Addressing concerns over the fate of DNA derived from genetically modified food in the human body: A review. Food Chem. Toxicol. 2019, 124, 423–430. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Dong, P.; An, R.; Xue, C.; Ge, Y.; Wei, L.; Liang, X. Digestion of nucleic acids starts in the stomach. Sci. Rep. 2015, 5, 11936. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Anglada, M.; Urtasun, N.; Pérez-Torras, S. Intestinal nucleoside transporters: Function, expression, and regulation. Compre. Physiol. 2018, 8, 1003–1017. [Google Scholar]
- Van der Goot, F.G.; Johannessen, L.E.; Spilsberg, B.; Wiik-Nielsen, C.R.; Kristoffersen, A.B.; Holst-Jensen, A.; Berdal, K.G. DNA-fragments are transcytosed across CaCo-2 cells by adsorptive endocytosis and vesicular mediated transport. PLoS ONE 2013, 8, e56671. [Google Scholar] [CrossRef]
- Matos, Â.P. Microalgae as a potential source of proteins. In Proteins: Sustainable Source, Processing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 63–96. [Google Scholar]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The association between purine-rich food intake and hyperuricemia: A cross-sectional study in Chinese adult residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef]
- Choi, H.K.; Liu, S.; Curhan, G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: The third national health and nutrition examination survey. Arthritis Rheum. 2005, 52, 283–289. [Google Scholar] [CrossRef]
- Hess, J.R.; Greenberg, N.A. The role of nucleotides in the immune and gastrointestinal systems. Nutr. Clin. Pract. 2012, 27, 281–294. [Google Scholar] [CrossRef]
- Dancey, C.P.; Attree, E.A.; Brown, K.F. Nucleotide supplementation: A randomised double-blind placebo controlled trial of IntestAidIB in people with irritable bowel syndrome [ISRCTN67764449]. Nutr. J. 2006, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Dewan, A.; Spisák, S.; Solymosi, N.; Ittzés, P.; Bodor, A.; Kondor, D.; Vattay, G.; Barták, B.K.; Sipos, F.; Galamb, O.; et al. Complete genes may pass from food to human blood. PLoS ONE 2013, 8, e69805. [Google Scholar] [CrossRef] [Green Version]
- Forsman, A.; Ushameckis, D.; Bindra, A.; Yun, Z.; Blomberg, J. Uptake of amplifiable fragments of retrotransposon DNA from the human alimentary tract. Mol. Genet. Genom. 2003, 270, 362–368. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Chen, W.L.; Kung, W.-H.; Huang, H.-D. Plant miRNAs found in human circulating system provide evidences of cross kingdom RNAi. BMC Genom. 2017, 18, 112. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Cong, L.; Lukiw, W.J. Plant and animal microRNAs (miRNAs) and their potential for inter-kingdom communication. Cell. Mol. Neurobiol. 2017, 38, 133–140. [Google Scholar] [CrossRef]
- Vries, J.; Meier, P.; Wackernagel, W. The natural transformation of the soil bacteriaPseudomonas stutzeriandAcinetobactersp. by transgenic plant DNA strictly depends on homologous sequences in the recipient cells. FEMS Microbiol. Lett. 2001, 195, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Colgrave, M.L.; Dominik, S.; Tobin, A.B.; Stockmann, R.; Simon, C.; Howitt, C.A.; Belobrajdic, D.P.; Paull, C.; Vanhercke, T. Perspectives on future protein production. J. Agric. Food Chem. 2021, 69, 15076–15083. [Google Scholar] [CrossRef]
- Littlechild, J.A. Protein structure and function. In Introduction to Biological and Small Molecule Drug Research and Development; Elsevier: Amsterdam, The Netherlands, 2013; pp. 57–79. [Google Scholar]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Hermans, W.J.H.; Senden, J.M.; Churchward-Venne, T.A.; Paulussen, K.J.M.; Fuchs, C.J.; Smeets, J.S.J.; van Loon, J.J.A.; Verdijk, L.B.; van Loon, L.J.C. Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double-blind randomized trial. Am. J. Clin. Nutr. 2021, 114, 934–944. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Wang, B.; Tian, R.; Li, J.; Liu, L.; Du, G.; Chen, J. Food synthetic biology-driven protein supply transition: From animal-derived production to microbial fermentation. Chin. J. Chem. Eng. 2021, 30, 29–36. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of food proteins. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2011; pp. 1–12. [Google Scholar]
- Shen, C.-H. Gene expression: Translation of the genetic code. In Diagnostic Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–116. [Google Scholar]
- Senthilkumaran, A.; Babaei-Ghazvini, A.; Nickerson, M.T.; Acharya, B. Comparison of protein content, availability, and different properties of plant protein sources with their application in packaging. Polymers 2022, 14, 1065. [Google Scholar] [CrossRef]
- Mihalca, V.; Kerezsi, A.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.; Dulf, F.; Socaci, S.; Fărcaș, A.; Mureșan, C.; et al. Protein-based films and coatings for food industry applications. Polymers 2021, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Fematt-Flores, G.E.; Aguiló-Aguayo, I.; Marcos, B.; Camargo-Olivas, B.A.; Sánchez-Vega, R.; Soto-Caballero, M.C.; Salas-Salazar, N.A.; Flores-Córdova, M.A.; Rodríguez-Roque, M.J. Milk protein-based edible films: Influence on mechanical, hydrodynamic, optical and antioxidant properties. Coatings 2022, 12, 196. [Google Scholar] [CrossRef]
- De Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Advanced technologies applied to enhance properties and structure of films and coatings: A review. Food Bioprocess Technol. 2022, 1, 1–24. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent trends in nanotechnology applications of bio-based packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Herrera-Vázquez, S.E.; Dublán-García, O.; Arizmendi-Cotero, D.; Gómez-Oliván, L.M.; Islas-Flores, H.; Hernández-Navarro, M.D.; Ramírez-Durán, N. Optimization of the physical, optical and mechanical properties of composite edible films of gelatin, whey protein and chitosan. Molecules 2022, 27, 869. [Google Scholar] [CrossRef]
- Salević, A.; Stojanović, D.; Lević, S.; Pantić, M.; Đorđević, V.; Pešić, R.; Bugarski, B.; Pavlović, V.; Uskoković, P.; Nedović, V. The structuring of sage (Salvia officinalis L.) extract-incorporating edible zein-based materials with antioxidant and antibacterial functionality by solvent casting versus electrospinning. Foods 2022, 11, 390. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of whey protein-based edible films and coatings in food industries: An updated overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Rantamäki, P.; Loimaranta, V.; Vasara, E.; Latva-Koivisto, J.; Korhonen, H.; Tenovuo, J.; Marnila, P. Edible films based on milk proteins release effectively active immunoglobulins. Food Qual. Saf. 2019, 3, 23–34. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kiat Kovis, T.J.; Yi, N.J. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [Green Version]
- Nurdiani, R.; Prihanto, A.A.; Firdaus, M. Seafood as source of protein-based functional foods. In Encyclopedia of Marine Biotechnology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 2987–2997. [Google Scholar]
- Kamdem, J.P.; Tsopmo, A. Reactivity of peptides within the food matrix. J. Food Biochem. 2019, 43, e12489. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Singhania, R.R.; Pandey, A. Production, purification, and application of microbial enzymes. In Biotechnology of Microbial Enzymes; Academic Press: Cambridge, MA, USA, 2017; pp. 13–41. [Google Scholar]
- Cheng, D.; Liu, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Bui, X.T. Sustainable enzymatic technologies in waste animal fat and protein management. J. Environ. Manag. 2021, 284, 112040. [Google Scholar] [CrossRef]
- Backes, E.; Kato-Schwartz, C.G.; Corrêa, R.C.G.; Moreira, R.D.F.P.M.; Peralta, R.A.; Barros, L.; Ferreira, I.C.; Zanin, G.M.; Bracht, A.; Peralta, R.M. Laccases in food processing: Current status, bottlenecks and perspectives. Trends Food Sci. Technol. 2021, 115, 445–460. [Google Scholar] [CrossRef]
- Purewal, S.S.; Sandhu, K.S. Debittering of citrus juice by different processing methods: A novel approach for food industry and agro-industrial sector. Sci. Hortic. 2021, 276, 109750. [Google Scholar] [CrossRef]
- Mahto, R.B.; Yadav, M.; Muthuraj, M.; Sharma, A.K.; Bhunia, B. Biochemical properties and application of a novel pectinase from a mutant strain of Bacillus subtilis. Biomass Convers. Biorefinery 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Zerva, A.; Limnaios, A.; Kritikou, A.S.; Thomaidis, N.S.; Taoukis, P.; Topakas, E. A novel thermophile β-galactosidase from Thermothielavioides terrestris producing galactooligosaccharides from acid whey. New Biotechnol. 2021, 63, 45–53. [Google Scholar] [CrossRef]
- Garcia, J.M.; Herrera-Rocha, F.; Cavajalino, A.S.; Baron, R.D.; Barrios, A.F.G.; Udenigwe, C.C. Effects of processing conditions on hydrolysates of proteins from whole whey and formation of Maillard reaction products. J. Food Process. Preserv. 2021, 45, e15469. [Google Scholar] [CrossRef]
- De Luca, V.; Perotti, M.C.; Wolf, I.V.; Meinardi, C.A.; Mandrich, L. The addition of the thermophilic esterase EST2 influences the fatty acids and volatile compound profiles of semi hard cheeses. Food Sci. Technol. 2019, 39, 711–720. [Google Scholar] [CrossRef] [Green Version]
- Dumina, M.; Zhgun, A.; Pokrovskaya, M.; Aleksandrova, S.; Zhdanov, D.; Sokolov, N.; El’Darov, M. Highly active thermophilic L-asparaginase from melioribacter roseus represents a novel large group of type II bacterial L-asparaginases from chlorobi-ignavibacteriae-bacteroidetes clade. Int. J. Mol. Sci. 2021, 22, 13632. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.T.; Kozak, S.M.; Lawton, M.R.; Alcaine, S.D. Lactose oxidase: An enzymatic approach to inhibit Listeria monocytogenes in milk. J. Dairy Sci. 2021, 104, 10594–10608. [Google Scholar] [CrossRef]
- Alvarez, R.G.; Karki, P.; Langleite, I.E.; Bakksjø, R.J.; Eichacker, L.A.; Furnes, C. Characterisation of a novel cold-adapted calcium-activated transglutaminase: Implications for medicine and food processing. FEBS Open Bio 2020, 10, 495–506. [Google Scholar] [CrossRef]
- Owczarzy, A.; Kurasiński, R.; Kulig, K.; Rogóż, W.; Szkudlarek, A.; Maciążek-Jurczyk, M. Collagen—Structure, properties and application. Eng. Biomater. 2020, 156, 17–23. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Domene, C.; Jorgensen, C.; Abbasi, S.W. A perspective on structural and computational work on collagen. Phys. Chem. Chem. Phys. 2016, 18, 24802–24811. [Google Scholar] [CrossRef] [Green Version]
- Traub, W.; Piez, K.A. The chemistry and structure of collagen. In Advances in Protein Chemistry and Structural Biology; Anfinsen, C.B., Jr., Edsall, J.T., Richards, F.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1971; Volume 25, pp. 243–352. [Google Scholar]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar] [CrossRef]
- Bella, J.; Berman, H.M. Crystallographic evidence for Cα–H···O=C hydrogen bonds in a collagen triple helix. J. Mol. Biol. 1996, 264, 734–742. [Google Scholar] [CrossRef]
- Brodsky, B.; Ramshaw, J.A.M. The collagen triple-helix structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2010, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, B.; Thiagarajan, G.; Madhan, B.; Kar, K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers 2008, 89, 345–353. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen based materials in cosmetic applications: A review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Ge, C.; Wu, Y. Animal by-products collagen and derived peptide, as important components of innovative sustainable food systems—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2021, 1–25. [Google Scholar] [CrossRef]
- García-Coronado, J.M.; Martínez-Olvera, L.; Elizondo-Omaña, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2018, 43, 531–538. [Google Scholar] [CrossRef]
- Miranda, R.B.; Weimer, P.; Rossi, R.C. Effects of hydrolyzed collagen supplementation on skin aging: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef]
- Schilling, M.W.; Mink, L.E.; Gochenour, P.S.; Marriott, N.G.; Alvarado, C.Z. Utilization of pork collagen for functionality improvement of boneless cured ham manufactured from pale, soft, and exudative pork. Meat Sci. 2003, 65, 547–553. [Google Scholar] [CrossRef]
- Pereira, A.G.T.; Ramos, E.M.; Teixeira, J.T.; Cardoso, G.P.; Ramos, A.d.L.S.; Fontes, P.R. Effects of the addition of mechanically deboned poultry meat and collagen fibers on quality characteristics of frankfurter-type sausages. Meat Sci. 2011, 89, 519–525. [Google Scholar] [CrossRef]
- Araújo, Í.B.S.; Lima, D.A.S.; Pereira, S.F.; Paseto, R.P.; Madruga, M.S. Effect of storage time on the quality of chicken sausages produced with fat replacement by collagen gel extracted from chicken feet. Poult. Sci. 2021, 100, 1262–1272. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Dolata, W. The application of collagen preparations as carriers of rosemary extract in the production of processed meat. Meat Sci. 2007, 75, 178–183. [Google Scholar] [CrossRef]
- Rama, G.R.; Dullius, D.; Agnol, W.D.; Esquerdo, V.M.; Lehn, D.N.; de Souza, C.F.V. Ricotta whey supplemented with gelatin and collagen for the encapsulation of probiotic lactic acid bacteria. Food Sci. Technol. 2021, 41, 576–586. [Google Scholar] [CrossRef]
- Da Mata Rigoto, J.; Ribeiro, T.H.S.; Stevanato, N.; Sampaio, A.R.; Ruiz, S.P.; Bolanho, B.C. Effect of açaí pulp, cheese whey, and hydrolysate collagen on the characteristics of dairy beverages containing probiotic bacteria. J. Food Proc. Eng. 2019, 42, e12953. [Google Scholar] [CrossRef] [Green Version]
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Probiotic fermented milk with collagen. Dairy 2020, 1, 126–134. [Google Scholar] [CrossRef]
- Fu, R.; Yao, K.; Zhang, Q.; Jia, D.; Zhao, J.; Chi, Y. Collagen hydrolysates of skin shavings prepared by enzymatic hydrolysis as a natural flocculant and their flocculating property. Appl. Biochem. Biotechnol. 2016, 182, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Fu, R.J.; Yao, K.; Jia, D.Y.; He, Q.; Chi, Y.L. Clarification effect of collagen hydrolysate clarifier on chrysanthemum beverage. LWT 2018, 91, 70–76. [Google Scholar] [CrossRef]
- Cao, H.; Zheng, X.; Liu, H.; Yuan, M.; Ye, T.; Wu, X.; Yin, F.; Li, Y.; Yu, J.; Xu, F. Cryo-protective effect of ice-binding peptides derived from collagen hydrolysates on the frozen dough and its ice-binding mechanisms. LWT 2020, 131, 109678. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Lima, Á.M.; Cerqueira, M.A.; Souza, B.W.S.; Santos, E.C.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. New edible coatings composed of galactomannans and collagen blends to improve the postharvest quality of fruits—Influence on fruits gas transfer rate. J. Food Eng. 2010, 97, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shibata, M.; Ma, Q.; Liu, F.; Lu, Q.; Shan, Q.; Hagiwara, T.; Bao, J. Characterization of fish collagen from blue shark skin and its application for chitosan-collagen composite coating to preserve red porgy (Pagrus major) meat. J. Food Biochem. 2020, 44, e13265. [Google Scholar] [CrossRef]
- Zając, M.; Pająk, P.; Skowyra, G. Characterization of edible collagen casings in comparison with the ovine casing and their effect on sausage quality. J. Sci. Food Agric. 2021, 101, 6001–6009. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron’ko, N.G. Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef] [Green Version]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Rbii, K.; Surel, O.; Brambati, N.; Buchert, A.M.; Violleau, F. Study of gelatin renaturation in aqueous solution by AFlFFF-MALS: Influence of a thermal pre-treatment applied on gelatin. Food Hydrocoll. 2011, 25, 511–514. [Google Scholar] [CrossRef]
- Johnston-Banks, F.A. Gelatine. In Food Gels; Springer: Dordrecht, The Netherlands, 1990; pp. 233–289. [Google Scholar]
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef]
- Heckbert, P.S. Ray tracing JELL-OTM brand gelatin. In Proceedings of the 14th Annual Conference on Computer Graphics and Interactive Techniques—SIGGRAPH’87, Anaheim, CA, USA, 27–31 July 1987; pp. 73–74. [Google Scholar]
- Burey, P.; Bhandari, B.R.; Rutgers, R.P.G.; Halley, P.J.; Torley, P.J. Confectionery gels: A review on formulation, rheological and structural aspects. Int. J. Food Prop. 2009, 12, 176–210. [Google Scholar] [CrossRef] [Green Version]
- Piliugina, I.; Artamonova, M.; Murlykina, N.; Shidakova-Kamenyuka, O. Study of the foaming properties of gelatin with solubilized substances for the production of marshmallows. Food Sci. Technol. 2019, 13, 90–97. [Google Scholar] [CrossRef]
- Mulyani, S.; Pranoto, Y.; Setyabudi, F.M.C.S.; Legowo, A.M.; Santoso, U. The functional properties of buffalo skin gelatin extracted using crude acid protease from cow’s abomasum. J. Appl. Food Technol. 2019, 6, 40–43. [Google Scholar] [CrossRef]
- Yu, W.J.; Xu, D.; Li, D.D.; Guo, L.A.; Su, X.Q.; Zhang, Y.; Wu, F.F.; Xu, X.M. Effect of pigskin-originated gelatin on properties of wheat flour dough and bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
- Manbeck, A.E.; Aldrich, C.G.; Alavi, S.; Zhou, T.; Donadelli, R.A. The effect of gelatin inclusion in high protein extruded pet food on kibble physical properties. Anim. Feed Sci. Technol. 2017, 232, 91–101. [Google Scholar] [CrossRef]
- Serdaroğlu, M.; Nacak, B.; Karabiyikoglu, M.; Keser, G. Effects of partial beef fat replacement with gelled emulsion on functional and quality properties of model system meat emulsions. Korean J. Food Sci. Anim. Resour. 2016, 36, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Mulyani, S.; Priyo Bintoro, V.; Legowo, A.M.; Setiani, B.E. Functional properties comparison of hide buffalo gelatin and commercial bovine gelatin as clarifying agent for the tropical fruit juice. IOP Conf. Ser. Earth Environ. Sci. 2021, 803, 012038. [Google Scholar] [CrossRef]
- Kohjiya, S.; Ikeda, Y. Chemistry, Manufacture and Applications of Natural Rubber; Woodhead Publishing: Cambridge, UK, 2014; p. xxvi. [Google Scholar]
- Ameratunga, R.; Ameratunga, S.; Crooks, C.; Simmons, G. Latex glove use by food handlers: The case for nonlatex gloves. J. Food Prot. 2008, 71, 2334–2338. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Structure | Property | Application |
|---|---|---|---|
| Unmodified | high amylose content | high mechanical strength | strong films |
| or | |||
| high amylopectin content | low mechanical strength, unstable under stress, at high temp. and pH | films require addition of plasticisers | |
| UA | more amylose, less amylopectin | higher strength, higher swelling, better solubility | stronger, more transparent films |
| de-polymerisation | lower viscosity | ||
| more RDS | limited nutritional application |
| Treatment | Structure | Property | Application |
|---|---|---|---|
| Unmodified | basic | water solubility, viscosity, and gelling ability depending on the number of sulphate groups | edible films, active food packaging |
| κ-CG | antimicrobial activity, high water uptake | composite superabsorbent gel | |
| κ-CG, ι-CG | high antioxidant activity | edible films, active food packaging, functional food | |
| κ-CG, ι-CG, λ-CG | high antiviral activity | antiviral drugs | |
| Carboxymethylation | additional carboxymethyl groups | higher water solubility | drug delivery/controlled release |
| Enzyme [Ref.] | Substrate(s) and Reaction | Food-Related Application |
|---|---|---|
| laccase [75] | oxidation of phenols, carbohydrates, unsaturated fatty acids and thiol-containing proteins with a concomitant reduction of oxygen to water | improving the volume, texture, flavour and freshness of bakery products; improving the dough elastic properties of gluten-free flour; stabilising agent preventing the formation of sediments, haze, turbidity; antioxidant synthesis of novel antioxidants for food industry; cross-linking agent used in, e.g., gel or film formation |
| naringinase and α-L-rhamnosidase [76] | conversion of naringin into bitterness products | debittering agents in citrus fruit juices; increases the shelf life of juice |
| pectinase [77] | degradation of pectic molecules | clarification of various fruit juices |
| β-galactosidase TtbGal1 [78] | hydrolysis of lactose and formation of galactooligosaccharides via transgalactosylation | lactose-reduced or lactose-free products; probiotic properties |
| alcalase and flavourzyme [79] | hydrolysis of whole whey proteins | hydrolysates with less sulfhydryl groups; enhanced antioxidant capacity, natural preservatives |
| the thermophilic esterase EST2 [80] | lipolysis of triglycerides | increase in the production of short- and medium-chain fatty acids via lipolysis consequently leading to volatile compounds formation; enhancement of cheese flavour and reduction in the time of ripening |
| L-asparaginase from Melioribacter roseus [81] | conversion of L-asparagine into L-aspartic acid and ammonia | prevention of the acrylamide formation from the conversion of asparagine during some food processing |
| lactose oxidase [82] | oxidation of lactose to lactobionic acid with a concomitant reduction of oxygen to water | control of the outgrowth of L. monocytogenes in milk, cheese and other dairy products increasing their safety |
| novel cold-adapted calcium-activated transglutaminase [83] | cross-linking of lysine and glutamine residues of various polypeptides, e.g., casein, collagen and gelatine | increase in the mechanical stability of meat; can be used in treatments requiring low temperatures |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczoń, P.; Josefsson, H.; Michorowska, S.; Tarnowska, K.; Kowalska, D.; Bartyzel, B.J.; Niemiec, T.; Lipińska, E.; Gruczyńska-Sękowska, E. The Influence of the Structure of Selected Polymers on Their Properties and Food-Related Applications. Polymers 2022, 14, 1962. https://doi.org/10.3390/polym14101962
Koczoń P, Josefsson H, Michorowska S, Tarnowska K, Kowalska D, Bartyzel BJ, Niemiec T, Lipińska E, Gruczyńska-Sękowska E. The Influence of the Structure of Selected Polymers on Their Properties and Food-Related Applications. Polymers. 2022; 14(10):1962. https://doi.org/10.3390/polym14101962
Chicago/Turabian StyleKoczoń, Piotr, Heidi Josefsson, Sylwia Michorowska, Katarzyna Tarnowska, Dorota Kowalska, Bartłomiej J. Bartyzel, Tomasz Niemiec, Edyta Lipińska, and Eliza Gruczyńska-Sękowska. 2022. "The Influence of the Structure of Selected Polymers on Their Properties and Food-Related Applications" Polymers 14, no. 10: 1962. https://doi.org/10.3390/polym14101962