Effects of Differing Monomer Compositions on Properties of P(3HB-co-4HB) Synthesized by Aneurinibacillus sp. H1 for Various Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. PHA Production
2.2. Films Production
2.3. Size Exclusion Chromatography
2.4. Atomic Force Microscopy
2.5. Contact Angle Measurements
2.6. Tensile Tests
2.7. X-ray Diffractometry (XRD)
2.8. Active Ingredients Release Kinetics
3. Results and Discussion
3.1. PHA Films Production
3.2. Surface Characteristics
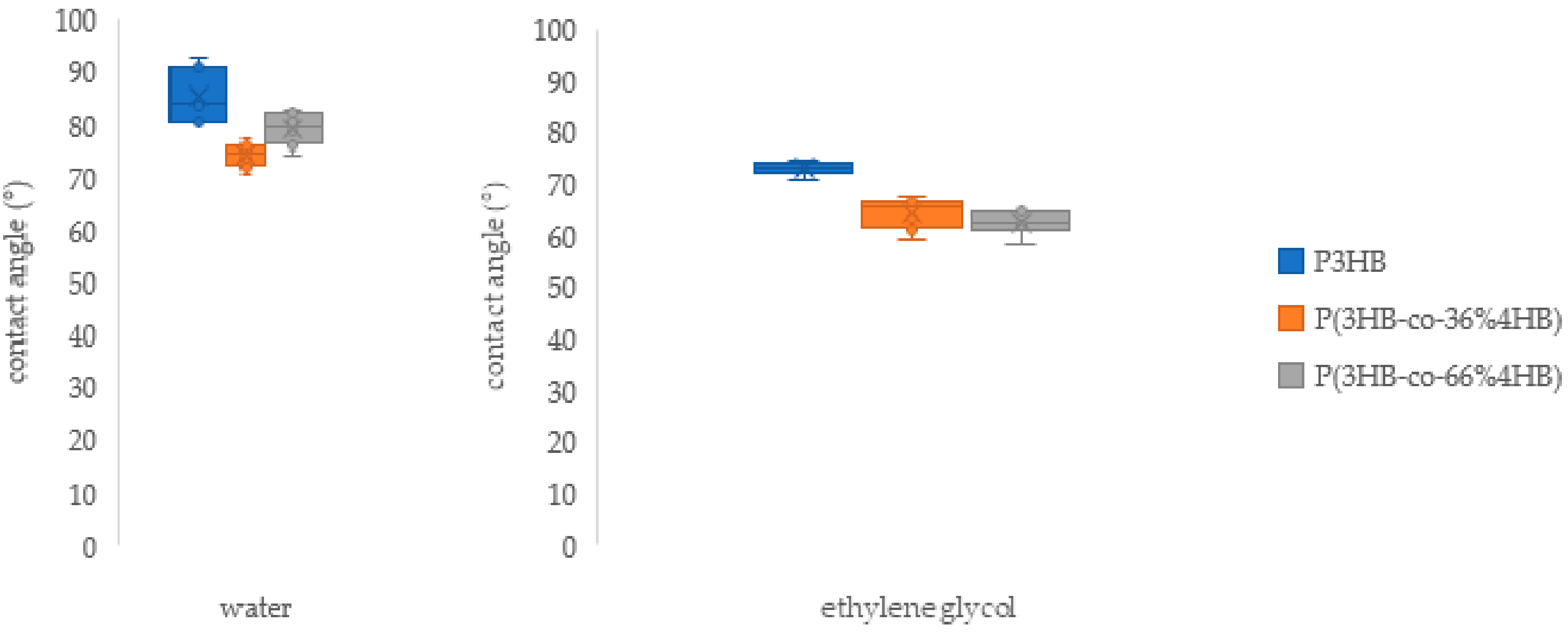
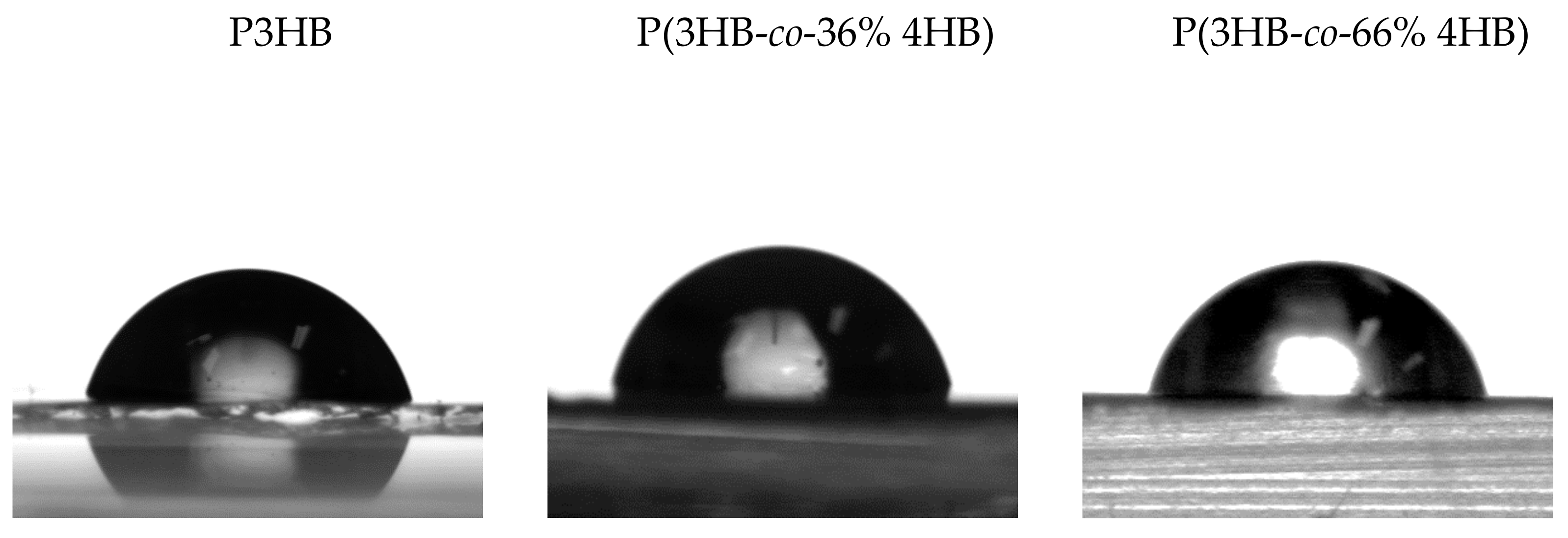
3.3. Contact Angle
3.4. Tensile Test
3.5. X-ray Diffraction Patterns
3.6. Active Ingredients Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Novackova, I.; Sedlacek, P.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the newly isolated bacterium aneurinibacillus sp. H1 as an auspicious thermophilic producer of various polyhydroxyalkanoates (PHA) copolymers-1. Isolation and characterization of the bacterium. Polymers 2020, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Huong, K.H.; Sevakumaran, V.; Amirul, A.A. P(3HB-co-4HB) as high value polyhydroxyalkanoate: Its development over recent decades and current advances. Crit. Rev. Biotechnol. 2021, 41, 474–490. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E. Plastic Recycling. In Handbook of Recycling; Elsevier: Amsterdam, The Netherlands, 2014; pp. 179–190. [Google Scholar]
- Luo, Z.; Wu, Y.-L.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications. Biotechnol. J. 2019, 14, 1900283. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Zavala-Yoe, R.; Bilal, M.; Ramirez-Mendoza, R.A.; Parra-Saldivar, R.; Iqbal, H.M. Poly-3-hydroxybutyrate-based constructs with novel characteristics for drug delivery and tissue engineering applications—A review. Polym. Eng. Sci. 2020, 60, 1760–1772. [Google Scholar] [CrossRef]
- Schmitz, P.; Janocha, S. Films. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Vodicka, J.; Wikarska, M.; Trudicova, M.; Junglova, Z.; Pospisilova, A.; Kalina, M.; Slaninova, E.; Obruca, S.; Sedlacek, P. Degradation of P(3HB-co-4HB) Films in Simulated Body Fluids. Polymers, 2022; Forthcoming. [Google Scholar]
- Obruca, S.; Benesova, P.; Oborna, J.; Marova, I. Application of protease-hydrolyzed whey as a complex nitrogen source to increase poly(3-hydroxybutyrate) production from oils by Cupriavidus necator. Biotechnol. Lett. 2014, 36, 775–781. [Google Scholar] [CrossRef]
- Sedlacek, P.; Pernicova, I.; Novackova, I.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the newly isolated bacterium Aneurinibacillus sp. H1 as an auspicious thermophilic producer of various polyhydroxyalkanoates (PHA) copolymers-2. Material study on the produced copolymers. Polymers 2020, 12, 1235. [Google Scholar] [CrossRef]
- Zhu, C.; Chiu, S.; Nakas, J.P.; Nomura, C.T. Bioplastics from waste glycerol derived from biodiesel industry. J. Appl. Polym. Sci. 2013, 130, 1–13. [Google Scholar] [CrossRef]
- Tsuge, T.; Hyakutake, M.; Mizuno, K. Class IV polyhydroxyalkanoate (PHA) synthases and PHA-producing Bacillus. Appl. Microbiol. Biotechnol. 2015, 99, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Urbieta, M.S.; Donati, E.R.; Chan, K.G.; Shahar, S.; Sin, L.L.; Goh, K.M. Thermophiles in the genomic era: Biodiversity, science, and applications. Biotechnol. Adv. 2015, 33, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Meiron, T.S.; Saguy, I.S. Wetting properties of food packaging. Food Res. Int. 2006, 40, 653–659. [Google Scholar] [CrossRef]
- Karbowiak, T.; Debeaufort, F.; Voilley, A. Importance of surface tension characterization for food, pharmaceutical and packaging products: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Qiu, H.; Chen, Z.; Xu, K. Biodegradable block poly(ester-urethane)s based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymers. Biomaterials 2011, 32, 3178–3188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cheng, S.; Xu, K. Block poly(ester-urethane)s based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(3-hydroxyhexanoate-co-3-hydroxyoctanoate). Biomaterials 2009, 30, 2219–2230. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, A.; Izrayeel, A.M.D.; Samyn, P.; Rastogi, V.K. Physicochemical and thermal characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) films incorporating thyme essential oil for active packaging of white bread. Food Control 2022, 133, 108688. [Google Scholar] [CrossRef]
- Volova, T.G.; Golubev, A.I.; Nemtsev, I.V.; Lukyanenko, A.V.; Dudaev, A.E.; Shishatskaya, E.I. Laser processing of polymer films fabricated from phas differing in their monomer composition. Polymers 2021, 13, 1553. [Google Scholar] [CrossRef]
- Busscher, H.J.; van Pelt, A.W.J.; de Jong, H.P.; Arends, J. Effect of spreading pressure on surface free energy determinations by means of contact angle measurements. J. Colloid Interface Sci. 1983, 95, 23–27. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Segawa, A.; Kunioka, M. Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int. J. Biol. Macromol. 1990, 12, 106–111. [Google Scholar] [CrossRef]
- Chanprateep, S.; Buasri, K.; Muangwong, A.; Utiswannakul, P. Biosynthesis and biocompatibility of biodegradable poly(3-hydroxybutyrate- co-4-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2003–2012. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef]
- Huong, K.H.; Azuraini, M.J.; Aziz, N.A.; Amirul, A.A.A. Pilot scale production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) biopolymers with high molecular weight and elastomeric properties. J. Biosci. Bioeng. 2017, 124, 76–83. [Google Scholar] [CrossRef]
- Huong, K.H.; Elina, K.A.R.; Amirul, A.A. Production of high molecular weight poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer by Cupriavidus malaysiensis USMAA1020 utilising substrate with longer carbon chain. Int. J. Biol. Macromol. 2018, 116, 217–223. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Rajasekaran, G.; Han, E.H.; Chung, Y.H.; Choi, Y.J.; Yang, Y.J.; Lee, J.E.; Kim, H.N.; Lee, K.; Kim, J.S.; et al. Cationic Amphipathic Triazines with Potent Anti-bacterial, Anti-inflammatory and Anti-atopic Dermatitis Properties. Sci. Rep. 2019, 9, 1292. [Google Scholar] [CrossRef]
- Narahashi, T.; Yamada, M.; Frazier, D.T. Cationic Forms of Local Anaesthetics block Action Potentials from Inside the Nerve Membrane. Nature 1969, 223, 748–749. [Google Scholar] [CrossRef]
- Greenspan, P.; Fowler, S.D. Spectrofluorometric studies of the lipid probe, nile red. J. Lipid Res. 1985, 26, 781–789. [Google Scholar] [CrossRef]




| Sample | Substrate | CDM (g/L) | PHA (% per CDM) | Mw (kDa) | Crystallinity + (%) |
|---|---|---|---|---|---|
| P3HB | fructose | 9.06 ± 0.24 | 76.82 ± 12.44 | 481.26 ± 11.62 | 59 |
| P(3HB-co-36% 4HB) * | 1,4-BD:GLY (2:3) | 1.28 ± 0.53 | 29.46 ± 3.67 | 127.14 ± 1.73 | 36 |
| P(3HB-co-66% 4HB) * | 1,4-BD:GLY (2:1) | 1.82 ± 0.18 | 32.09 ± 3.16 | 174.13 ± 4.27 | 43 |
| Sample | Film Thickness (µm) | Roughness Ra (µm) |
|---|---|---|
| P3HB | 10.96 ± 1.11 | 0.35 |
| P(3HB-co-36% 4HB) | 11.12 ± 1.37 | 0.67 |
| P(3HB-co-66% 4HB) | 11.09 ± 0.57 | 0.20 |
| Sample | Et (MPa) | σm (MPa) | εm (%) |
|---|---|---|---|
| P3HB | 2000 ± 167 | 20.4 ± 1.82 | 1.3 ± 0.12 |
| P(3HB-co-36% 4HB) | 161 ± 14.7 | 6.72 ± 0.667 | 5.3 ± 0.91 |
| P(3HB-co-66% 4HB) | 210 ± 35.8 | 13.8 ± 3.63 | 180 ± 52.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pospisilova, A.; Vodicka, J.; Trudicova, M.; Juglova, Z.; Smilek, J.; Mencik, P.; Masilko, J.; Slaninova, E.; Melcova, V.; Kalina, M.; et al. Effects of Differing Monomer Compositions on Properties of P(3HB-co-4HB) Synthesized by Aneurinibacillus sp. H1 for Various Applications. Polymers 2022, 14, 2007. https://doi.org/10.3390/polym14102007
Pospisilova A, Vodicka J, Trudicova M, Juglova Z, Smilek J, Mencik P, Masilko J, Slaninova E, Melcova V, Kalina M, et al. Effects of Differing Monomer Compositions on Properties of P(3HB-co-4HB) Synthesized by Aneurinibacillus sp. H1 for Various Applications. Polymers. 2022; 14(10):2007. https://doi.org/10.3390/polym14102007
Chicago/Turabian StylePospisilova, Aneta, Juraj Vodicka, Monika Trudicova, Zuzana Juglova, Jiri Smilek, Premysl Mencik, Jiri Masilko, Eva Slaninova, Veronika Melcova, Michal Kalina, and et al. 2022. "Effects of Differing Monomer Compositions on Properties of P(3HB-co-4HB) Synthesized by Aneurinibacillus sp. H1 for Various Applications" Polymers 14, no. 10: 2007. https://doi.org/10.3390/polym14102007







