Determination of the Thermal Parameters of Geopolymers Modified with Iron Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Density Results
3.2. Results of Thermal Properties and Their Discussion
4. Conclusions
- The addition of iron to the geopolymers caused significant changes in their thermal properties, such as thermal conductivity, specific heat, and thermal diffusivity.
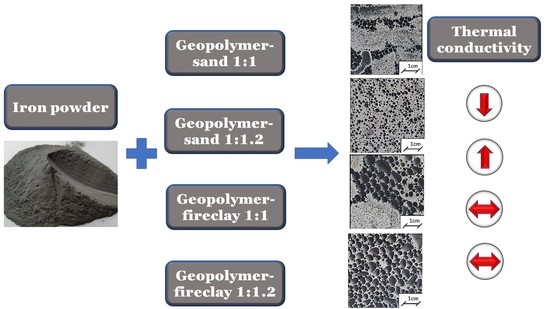
- It was found that the addition of sand or fireclay had a significant influence on the thermal parameters of the obtained composites. The samples after the drying process were characterized by lower values of thermal parameters. The samples with sand were characterized by higher values of thermal conductivity when compared to the samples with fireclay. The highest values of thermal conductivity λ, exceeding 1 W(m·K), were exhibited by the samples containing the geopolymer with sand in the ratio of 1:1.2. The addition of fireclay caused a decrease in the thermal conductivity of the composites by at least 30% when compared to the samples with the addition of sand.
- The lowest value of the thermal conductivity coefficient λ was obtained for the geopolymer with metakaolin and fireclay. When the ratio of these components in the composite was 1:1, the value of thermal conductivity was 0.6413 W/(m·K), while in the case of the ratio of 1:1.2, it was equal to 0.6456 W/(m·K).
- The materials with sand had a thermal conductivity within the range from 0.8865 W/(m·K) to 1.3791 W/(m·K). The thermal conductivity value with the sand in the ratio of 1:1 was 1.3791 W/(m·K) for the non-iron samples. For the samples with the addition of 2.5% Fe, the conductivity decreased to 1.2019 /(m·K). There was a decrease in this value by 12.8%. The non-iron samples containing the geopolymer with sand in the ratio of 1:1.2 had a thermal conductivity equal to 0.9657 W/(m·K), and the samples containing the geopolymer with sand in the ratio of 1:1.2 with the addition of 2.5% Fe had a thermal conductivity equal to 0.9109 W/(m·K). The addition of iron reduced the thermal conductivity by 5.7%.
- In the samples containing fireclay, there was no significant influence of the added iron on the values of thermal conductivity. In turn, in the case of the geopolymer with sand, this influence was noticeable. It was most visible in the samples containing metakaolin and sand in the ratio of 1:1.2. Therefore, it was noticed that the thermal conductivity of the composites increased with the increase in the addition of Fe.
- In the case of specific heat and thermal diffusivity, the samples with sand were also characterized by higher values of these parameters when compared to the samples with fireclay. The samples with fireclay showed very good stability, i.e., there were no changes in thermal diffusivity with regard to the addition of iron.
- For the samples containing geopolymer with sand in the ratio of 1:1.2 and with a 2% addition of iron, the specific heat value was the lowest and amounted to 830 J/(kg·K). It was an almost 15% decrease in the specific heat value when compared to the samples without the addition of iron. The materials with fireclay had a thermal diffusivity within the range from 0.3834 mm2/s to 0.4094 mm2/s. The samples containing sand had a thermal diffusivity of 0.5333–0.7706 mm2/s.
- The calculated densities ρb1 and ρb2 of the obtained geopolymer composites did not differ significantly, despite the use of different calculation methods. In the first case, the density was calculated on the basis of the known weight and volume of the samples. In the second case, the density calculations were based on the known thermal properties of the samples.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- Kalaw, M.E.; Culaba, A.; Hinode, H.; Kurniawan, W.; Gallardo, S.; Promentilla, M.A. Optimizing and Characterizing Geopolymers from Ternary Blend of Philippine Coal Fly Ash, Coal Bottom Ash and Rice Hull Ash. Materials 2016, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Hájková, P. Kaolinite claystone-based geopolymer materials: Effect of chemical composition and curing conditions. Minerals 2018, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Prochon, P.; Zhao, Z.; Courard, L.; Piotrowski, T.; Michel, F.; Garbacz, A. Influence of activators on mechanical properties of modified fly ash based geopolymer mortars. Materials 2020, 13, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samantasinghar, S.; Singh, S.P. Effect of synthesis parameters on compressive strength of fly ash-slag blended geopolymer. Constr. Build. Mater. 2018, 170, 225–234. [Google Scholar] [CrossRef]
- Rocha, S.; Martin-del-Rio, J.J.; Alejandre, F.J.; García-Heras, J.; Jimenez-Aguilar, A. Metakaolin-based geopolymer mortars with different alkaline activators (Na+ and K+). Constr. Build. Mater. 2018, 178, 453–461. [Google Scholar] [CrossRef]
- Glukhovsky, Y.D.; Rostovskaja, G.S.; Rumyna, G.V. High-strength Slag-Alkaline Cements. In Proceedings of the 7th International Congress on the Chemistry of Cement, Geophysics and Space Physics, Paris, France, 30 June–4 July 1980; Volume 13, pp. 64–168. [Google Scholar]
- Vohlídal, J.; Julák, A.; Štulík, K. Chemické a Analytické Tabulky; Grada Publishing: Praha, Czechia, 1999. [Google Scholar]
- Peng, X.; Shuai, Q.; Li, H.; Ding, Q.; Gu, Y.; Cheng, C.; Xu, Z. Fabrication and Fireproofing Performance of the Coal Fly Ash-Metakaolin-Based Geopolymer Foams. Materials 2020, 13, 1750. [Google Scholar] [CrossRef] [Green Version]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2020. [Google Scholar]
- Xie, J.; Zhao, J.; Wang, J.; Wang, C.; Huang, P.; Fang, C. Sulfate resistance of recycled aggregate concrete with GGBS and fly ash-based geopolymer. Materials 2019, 12, 1247. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Xie, L.; Qian, X.; Ruan, S.; Zeng, Q. Compositional Dependence of Pore Structure, Strengthand Freezing-Thawing Resistance of Metakaolin-Based Geopolymers. Materials 2020, 13, 2973. [Google Scholar] [CrossRef]
- Rovnaník, P.; Šafránková, K. Thermal Behaviour of Metakaolin/Fly Ash Geopolymers with Chamotte Aggregate. Materials 2016, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Punurai, W.; Kroehong, W.; Saptamongkol, A.; Chindaprasirt, P. Mechanical properties, microstructure and drying shrinkage of hybrid fly ash-basalt fiber geopolymer paste. Constr. Build. Materi. 2018, 186, 62–70. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and Ordinary Portland Cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Arnoult, M.; Perronnet, M.; Autef, A.; Nait-Ali, B.; Rossignol, S. Understanding the Formation of Geopolymer Foams: Influence of the Additives. Ceram. Mod. Technol. 2019, 1, 163–172. [Google Scholar] [CrossRef]
- Łach, M.; Pławecka, K.; Bąk, A.; Lichocka, K.; Korniejenko, K.; Cheng, A.; Lin, W.-T. Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams. Materials 2021, 14, 5090. [Google Scholar] [CrossRef]
- Van Su, L.; Louda, P.; Tran, H.N.; Nguyen, P.D.; Bakalova, T.; Buczkowska, K.E.; Dufkova, I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers 2020, 12, 2994. [Google Scholar] [CrossRef]
- Łach, M.; Korniejenko, K.; Mikuła, J. Thermal Insulation and Thermally Resistant Materials Made of Geopolymer Foams. Procedia Eng. 2016, 151, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Van Su, L. Thermal Conductivity of Reinforced Geopolymer Foams. Ceram. Silik. 2019, 63, 1–9. [Google Scholar]
- Kamseu, E.; Kaze, C.R.; Fekoua, J.N.N.; Melo, U.C.; Rossignol, S.; Leonelli, C. Ferrisilicates formation during the geopolymerization of natural Fe-rich aluminosilicate precursors. Mater. Chem. Phys. 2020, 240, 122062. [Google Scholar] [CrossRef]
- Poudeu, R.C.; Ekani, C.J.; Djangang, C.N.; Blanchart, P. Role of heat-treated laterite on the strengthening of geopolymer designed with laterite as solid precursor. Ann. Chim. Sci. Des. Mater. 2019, 43, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Kaze, C.R.P.; Venyite, P.; Nana, A.; Juvenal, D.N.; Tchakoute, H.K.; Rahier, H.; Kamseu, E.; Melo, U.C.; Leonelli, C. Meta-halloysite to improve compactness in iron-rich laterite-based alkali activated materials. Mater. Chem. Phys. 2020, 239, 122268. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-); Technical papers #27; Geopolymer Institute Library, Saint-Quentin, France, 2020. Available online: https://www.geopolymer.org/library/technical-papers/(accessed on 15 March 2022). [CrossRef]
- Assi, L.; Ghahari, S.; Deaver, E.E.; Leaphart, D.; Ziehl, P. Improvement of the early and final compressive strength of fly ash-based geopolymer concrete at ambient conditions. Constr. Build. Mater. 2016, 123, 806–813. [Google Scholar] [CrossRef]
- Prałat, K.; Ciemnicka, J.; Grabowski, M.; Jaskulski, R.; Kubissa, W. Application of experimental setup for the thermal conductivity measurement of building materials using the “hot wire” method. Sci. Rev. Eng. Environ. Sci. 2019, 28, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Franco, A. An apparatus for the routine measurement of thermal conductivity of materials for building application based on a transient hot-wire method. Appl. Therm. Eng. 2007, 27, 2495–2504. [Google Scholar] [CrossRef]
- Strzałkowski, J.; Garbalińska, H. Thermal and strength properties of lightweight concretes with the addition of aerogel particles. Adv. Cem. Res. 2016, 28, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Prałat, K.; Ciemnicka, J.; Koper, A.; Buczkowska, K.; Łoś, P. Comparison of the Thermal Properties of Geopolymer and Modified Gypsum. Polymers 2021, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Prałat, K.; Kubissa, W.; Jaskulski, R.; Pilarczyk, S. Wpływ wybranych mikrododatków na przewodnictwo cieplne oraz mikrostrukturę powierzchni modyfikowanych gipsów (Influence of selected micro-additives on thermal conductivity and microstructure of modified gypsum). Acta Sci. Pol. Archit. 2019, 18, 69–75. (In Polish) [Google Scholar] [CrossRef]
- Liu, M.Y.J.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H. Evaluation of thermal conductivity, mechanical and transport properties of lightweight aggregate foamed geopolymer concrete. Energy Build. 2014, 72, 238–245. [Google Scholar] [CrossRef]
- Utami, F.A.R.; Triwiyono, A.; Agustini, N.K.A.; Perdana, I. Thermal conductivity of geopolymer with polypropylene fiber. In IOP Conference Series: Materials Science and Engineering, Bandung, Indonesia, 28–29 November 2019; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 742, p. 012031. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, L.; Pan, Y.; Li, C.; Zhou, T.; Cheng, X. Facile construction of the aerogel/geopolymer composite with ultra-low thermal conductivity and high mechanical performance. RSC Adv. 2018, 8, 2350–2356. [Google Scholar] [CrossRef]
- Nongnuang, T.; Jitsangiam, P.; Rattanasak, U.; Tangchirapat, W.; Suwan, T.; Thongmunee, S. Characteristics of Waste Iron Powder as a Fine Filler in a High-Calcium Fly Ash Geopolymer. Materials 2021, 14, 2515. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.K.; Kumar, S. Influence of iron making slags on strength and microstructure of fly ash geopolymer. Constr. Build. Mater. 2013, 38, 924–930. [Google Scholar] [CrossRef]
- Nkwaju, R.Y.; Djobo, J.; Nouping, J.; Huisken, P.W.M.; Deutou, J.; Courard, L. Iron-rich laterite-bagasse fibers based geopolymer composite: Mechanical, durability and insulating properties. Appl. Clay Sci. 2019, 183, 105333. [Google Scholar] [CrossRef] [Green Version]









| Tested Sample | Metakaolin [g] | Activator [g] | Sand [g] | Fireclay [g] | Fe Powder [g] |
|---|---|---|---|---|---|
| Reference sample (RSS1) | 100 | 90 | 100 | - | 0 |
| Geopolymer: Sand (1:1) + Fe0.5% (GS1Fe0.5) | 100 | 90 | 100 | - | 0.5 |
| Geopolymer: Sand (1:1) + Fe1.0% (GS1Fe1.0) | 100 | 90 | 100 | - | 1.0 |
| Geopolymer: Sand (1:1) + Fe1.5% (GS1Fe1.5) | 100 | 90 | 100 | - | 1.5 |
| Geopolymer: Sand (1:1) + Fe2.0% (GS1Fe2.0) | 100 | 90 | 100 | - | 2.0 |
| Geopolymer: Sand (1:1) + Fe2.5% (GS1Fe2.5) | 100 | 90 | 100 | - | 2.5 |
| Reference sample (RSS1.2) | 100 | 90 | 120 | - | 0 |
| Geopolymer: Sand (1:1.2) + Fe0.5% (GS1.2Fe0.5) | 100 | 90 | 120 | - | 0.5 |
| Geopolymer: Sand (1:1.2) + Fe1.0% (GS1.2Fe1.0) | 100 | 90 | 120 | - | 1.0 |
| Geopolymer: Sand (1:1.2) + Fe1.5% (GS1.2Fe1.5) | 100 | 90 | 120 | - | 1.5 |
| Geopolymer: Sand (1:1.2) + Fe2.0% (GS1.2Fe2.0) | 100 | 90 | 120 | - | 2.0 |
| Geopolymer: Sand (1:1.2) + Fe2.5% (GS1.2Fe2.0) | 100 | 90 | 120 | - | 2.5 |
| Reference sample (RSF1) | 100 | 90 | - | 100 | 0 |
| Geopolymer: Fireclay (1:1) + Fe0.5% (GF1Fe0.5) | 100 | 90 | - | 100 | 0.5 |
| Geopolymer: Fireclay (1:1) + Fe1.0% (GF1Fe1.0) | 100 | 90 | - | 100 | 1.0 |
| Geopolymer: Fireclay (1:1) + Fe1.5% (GF1Fe1.5) | 100 | 90 | - | 100 | 1.5 |
| Geopolymer: Fireclay (1:1) + Fe2.0% (GF1Fe2.0) | 100 | 90 | - | 100 | 2.0 |
| Geopolymer: Fireclay (1:1) + Fe2.5% (GF1Fe2.5) | 100 | 90 | - | 100 | 2.5 |
| Reference sample (RSF1.2) | 100 | 90 | - | 120 | 0 |
| Geopolymer: Fireclay (1:1.2) + Fe0.5% (GF1.2Fe0.5) | 100 | 90 | - | 120 | 0.5 |
| Geopolymer: Fireclay (1:1.2) + Fe1.0% (GF1.2Fe1.0) | 100 | 90 | - | 120 | 1.0 |
| Geopolymer: Fireclay (1:1.2) + Fe1.5% (GF1.2Fe1.5) | 100 | 90 | - | 120 | 1.5 |
| Geopolymer: Fireclay (1:1.2) + Fe2.0% (GF1.2Fe2.0) | 100 | 90 | - | 120 | 2.0 |
| Geopolymer: Fireclay (1:1.2) + Fe2.5% (GF1.2Fe2.5) | 100 | 90 | - | 120 | 2.5 |
| Tested Sample | Before Drying | After Drying | ||
|---|---|---|---|---|
| ρb1 [kg/m3] | ρb2 [kg/m3] | ρb1 [kg/m3] | ρb2 [kg/m3] | |
| RSS1 | 1894 | 1899 | 1854 | 1854 |
| GS1Fe0.5 | 1872 | 1868 | 1840 | 1841 |
| GS1Fe1.0 | 1844 | 1831 | 1818 | 1818 |
| GS1Fe1.5 | 1860 | 1861 | 1842 | 1824 |
| GS1Fe2.0 | 1876 | 1876 | 1768 | 1716 |
| GS1Fe2.5 | 1880 | 1743 | 1854 | 1854 |
| RSS1.2 | 1926 | 1926 | 1846 | 1845 |
| GS1.2Fe0.5 | 1838 | 1845 | 1822 | 1811 |
| GS1.2Fe1.0 | 1882 | 1775 | 1866 | 1866 |
| GS1.2Fe1.5 | 1922 | 1903 | 1860 | 1859 |
| GS1.2Fe2.0 | 1974 | 1959 | 1946 | 1875 |
| GS1.2Fe2.5 | 1997 | 1996 | 1953 | 1953 |
| RSF1 | 1926 | 1857 | 1900 | 1900 |
| GF1Fe0.5 | 1866 | 1888 | 1840 | 1840 |
| GF1Fe1.0 | 1912 | 1904 | 1886 | 1886 |
| GF1Fe1.5 | 1921 | 1913 | 1889 | 1890 |
| GF1Fe2.0 | 1930 | 1990 | 1918 | 1918 |
| GF1Fe2.5 | 1922 | 2132 | 1892 | 1892 |
| RSF1.2 | 1914 | 1911 | 1880 | 1880 |
| GF1.2Fe0.5 | 1976 | 1976 | 1942 | 1942 |
| GF1.2Fe1.0 | 1906 | 1894 | 1876 | 1876 |
| GF1.2Fe1.5 | 1988 | 1988 | 1954 | 1954 |
| GF1.2Fe2.0 | 1940 | 1937 | 1854 | 1859 |
| GF1.2Fe2.5 | 1970 | 1959 | 1940 | 1940 |
| Tested Sample | Designated Confidence Intervals | |
|---|---|---|
| Samples Conditioned in Hygrothermal Conditions | Samples Conditioned in the Dryer | |
| RSS1 | P (1.3787 ≤ λ ≤ 1.7545) = 1 − α | P (0.9622 ≤ λ ≤ 0.9691) = 1 − α |
| P (914.0 ≤ Cp ≤ 999.4) = 1 − α | P (976.0 ≤ Cp ≤ 977.4) = 1 − α | |
| P (0.7951 ≤ a ≤ 0.9298) = 1 − α | P (0.5307 ≤ a ≤ 0.5359) = 1 − α | |
| GS1Fe0.5 | P (1.4302 ≤ λ ≤ 1.5226) = 1 − α | P (0.9416 ≤ λ ≤ 0.9559) = 1 − α |
| P (934.4 ≤ Cp ≤ 1003.1) = 1 − α | P (866.3 ≤ Cp ≤ 866.9) = 1 − α | |
| P (0.8091 ≤ a ≤ 0.8229) = 1 − α | P (0.5902 ≤ a ≤ 0.5997) = 1 − α | |
| GS1Fe1.0 | P (0.9957 ≤ λ ≤ 1.0664) = 1 − α | P (0.9056 ≤ λ ≤ 0.9116) = 1 − α |
| P (817.6 ≤ Cp ≤ 933.4) = 1 − α | P (935.2 ≤ Cp ≤ 936.9) = 1 − α | |
| P (0.6193 ≤ a ≤ 0.6672) = 1 − α | P (0.5326 ≤ a ≤ 0.5353) = 1 − α | |
| GS1Fe1.5 | P (1.1346 ≤ λ ≤ 1.3772) = 1 − α | P (1.0864 ≤ λ ≤ 1.0896) = 1 − α |
| P (885.7 ≤ Cp ≤ 922.0) = 1 − α | P (901.9 ≤ Cp ≤ 930.8) = 1 − α | |
| P (0.6844 ≤ a ≤ 0.8086) = 1 − α | P (0.6489 ≤ a ≤ 0.6533) = 1 − α | |
| GS1Fe2.0 | P (0.9975 ≤ λ ≤ 1.2343) = 1 − α | P (0.8840 ≤ λ ≤ 0.8889) = 1 − α |
| P (915.4 ≤ Cp ≤ 917.8) = 1 − α | P (921.9 ≤ Cp ≤ 924.7) = 1 − α | |
| P (0.5806 ≤ a ≤ 0.7171) = 1 − α | P (0.5167 ≤ a ≤ 0.6023) = 1 − α | |
| GS1Fe2.5 | P (1.0430 ≤ λ ≤ 1.0505) = 1 − α | P (0.9096 ≤ λ ≤ 0.9122) = 1 − α |
| P (868.6 ≤ Cp ≤ 878.1) = 1 − α | P (899.6 ≤ Cp ≤ 902.4) = 1 − α | |
| P (0.5585 ≤ a ≤ 0.8166) = 1 − α | P (0.5438 ≤ a ≤ 0.5466) = 1 − α | |
| RSS1.2 | P (1.9437 ≤ λ ≤ 1.9710) = 1 − α | P (1.3765 ≤ λ ≤ 1.3817) = 1 − α |
| P (969.4 ≤ Cp ≤ 987.3) = 1 − α | P (969.3 ≤ Cp ≤ 970.7) = 1 − α | |
| P (1.0350 ≤ a ≤ 1.0425) = 1 − α | P (0.7684 ≤ a ≤ 0.7727) = 1 − α | |
| GS1.2Fe0.5 | P (1.0935 ≤ λ ≤ 1.4564) = 1 − α | P (1.0202 ≤ λ ≤ 1.0389) = 1 − α |
| P (870.6 ≤ Cp ≤ 984.0) = 1 − α | P (910.8 ≤ Cp ≤ 916.6) = 1 − α | |
| P (0.6843 ≤ a ≤ 0.8062) = 1 − α | P (0.6150 ≤ a ≤ 0.6296) = 1 − α | |
| GS1.2Fe1.0 | P (1.4347 ≤ λ ≤ 1.4384) = 1 − α | P (0.9854 ≤ λ ≤ 1.1019) = 1 − α |
| P (870.9 ≤ Cp ≤ 934.3) = 1 − α | P (829.9 ≤ Cp ≤ 831.6) = 1 − α | |
| P (0.8947 ≤ a ≤ 0.8982) = 1 − α | P (0.6386 ≤ a ≤ 0.7081) = 1 − α | |
| GS1.2Fe1.5 | P (1.7000 ≤ λ ≤ 1.8362) = 1 − α | P (1.0523 ≤ λ ≤ 1.0560) = 1 − α |
| P (896.1 ≤ Cp ≤ 1066.9) = 1 − α | P (895.6 ≤ Cp ≤ 898.2) = 1 − α | |
| P (0.8620 ≤ a ≤ 1.0311) = 1 − α | P (0.6305 ≤ a ≤ 0.6339) = 1 − α | |
| GS1.2Fe2.0 | P (1.2145 ≤ λ ≤ 1.4333) = 1 − α | P (1.1701 ≤ λ ≤ 1.1952) = 1 − α |
| P (797.8 ≤ Cp ≤ 911.4) = 1 − α | P (828.3 ≤ Cp ≤ 832.3) = 1 − α | |
| P (0.6733 ≤ a ≤ 0.9080) = 1 − α | P (0.7148 ≤ a ≤ 0.8045) = 1 − α | |
| GS1.2Fe2.5 | P (1.3518 ≤ λ ≤ 1.5082) = 1 − α | P (1.2001 ≤ λ ≤ 1.2037) = 1 − α |
| P (825.8 ≤ Cp ≤ 845.3) = 1 − α | P (846.6 ≤ Cp ≤ 849.3) = 1 − α | |
| P (0.8007 ≤ a ≤ 0.9141) = 1 − α | P (0.7234 ≤ a ≤ 0.7285) = 1 − α | |
| RSF1 | P (0.8500 ≤ λ ≤ 1.0576) = 1 − α | P (0.6374 ≤ λ ≤ 0.6451) = 1 − α |
| P (887.8 ≤ Cp ≤ 943.2) = 1 − α | P (860.6 ≤ Cp ≤ 861.4) = 1 − α | |
| P (0.5500 ≤ a ≤ 0.5717) = 1 − α | P (0.3891 ≤ a ≤ 0.3950) = 1 − α | |
| GF1Fe0.5 | P (0.8099 ≤ λ ≤ 0.8442) = 1 − α | P (0.6593 ≤ λ ≤ 0.6619) = 1 − α |
| P (909.4 ≤ Cp ≤ 914.0) = 1 − α | P (935.4 ≤ Cp ≤ 936.4) = 1 − α | |
| P (0.4573 ≤ a ≤ 0.5035) = 1 − α | P (0.3819 ≤ a ≤ 0.3853) = 1 − α | |
| GF1Fe1.0 | P (0.9089 ≤ λ ≤ 0.9483) = 1 − α | P (0.6884 ≤ λ ≤ 0.6904) = 1 − α |
| P (824.9 ≤ Cp ≤ 937.8) = 1 − α | P (863.5 ≤ Cp ≤ 864.4) = 1 − α | |
| P (0.5062 ≤ a ≤ 0.6005) = 1 − α | P (0.4223 ≤ a ≤ 0.4239) = 1 − α | |
| GF1Fe1.5 | P (0.7955 ≤ λ ≤ 0.9988) = 1 − α | P (0.7021 ≤ λ ≤ 0.7100) = 1 − α |
| P (834.3 ≤ Cp ≤ 898.8) = 1 − α | P (862.1 ≤ Cp ≤ 863.0) = 1 − α | |
| P (0.4605 ≤ a ≤ 0.6218) = 1 − α | P (0.4306 ≤ a ≤ 0.4356) = 1 − α | |
| GF1Fe2.0 | P (0.8748 ≤ λ ≤ 0.8954) = 1 − α | P (0.6781 ≤ λ ≤ 0.8791) = 1 − α |
| P (902.2 ≤ Cp ≤ 934.6) = 1 − α | P (892.4 ≤ Cp ≤ 896.7) = 1 − α | |
| P (0.4447 ≤ a ≤ 0.5240) = 1 − α | P (0.3930 ≤ a ≤ 0.3980) = 1 − α | |
| GF1Fe2.5 | P (0.9129 ≤ λ ≤ 0.9616) = 1 − α | P (0.6633 ≤ λ ≤ 0.6655) =1 − α |
| P (884.3 ≤ Cp ≤ 945.7) = 1 − α | P (915.2 ≤ Cp ≤ 916.8) = 1 − α | |
| P (0.4548 ≤ a ≤ 0.5061) = 1 − α | P (0.3826 ≤ a ≤ 0.3841) = 1 − α | |
| RSF1.2 | P (0.9425 ≤ λ ≤ 0.9534) = 1 − α | P (0.6427 ≤ λ ≤ 0.6485) = 1 − α |
| P (870.7 ≤ Cp ≤ 952.5) = 1 − α | P (876.0 ≤ Cp ≤ 880.2) = 1 − α | |
| P (0.5198 ≤ a ≤ 0.5684) = 1 − α | P (0.3888 ≤ a ≤ 0.3935) = 1 − α | |
| GF1.2Fe0.5 | P (0.9468 ≤ λ ≤ 0.9556) = 1 − α | P (0.6435 ≤ λ ≤ 0.6448) = 1 − α |
| P (922.6 ≤ Cp ≤ 954.8) = 1 − α | P (844.3 ≤ Cp ≤ 846.4) = 1 − α | |
| P (0.5048 ≤ a ≤ 0.5210) = 1 − α | P (0.3916 ≤ a ≤ 3932) = 1 − α | |
| GF1.2Fe1.0 | P (0.9991 ≤ λ ≤ 1.0046) = 1 − α | P (0.6673 ≤ λ ≤ 0.6728) = 1 − α |
| P (839.7 ≤ Cp ≤ 1009.5) = 1 − α | P (898.8 ≤ Cp ≤ 900.7) = 1 − α | |
| P (0.5195 ≤ a ≤ 0.6248) = 1 − α | P (0.3935 ≤ a ≤ 0.4003) = 1 − α | |
| GF1.2Fe1.5 | P (0.9561 ≤ λ ≤ 0.9639) = 1 − α | P (0.6775 ≤ λ ≤ 0.6793) = 1 − α |
| P (867.4 ≤ Cp ≤ 887.0) = 1 − α | P (854.3 ≤ Cp ≤ 855.2) = 1 − α | |
| P (0.5461 ≤ a ≤ 0.5551) = 1 − α | P (0.4052 ≤ a ≤ 0.4072) = 1 − α | |
| GF1.2Fe2.0 | P (0.8385 ≤ λ ≤ 1.0716) = 1 − α | P (0.6924 ≤ λ ≤ 0.6992) = 1 − α |
| P (807.4 ≤ Cp ≤ 858.4) = 1 − α | P (909.7 ≤ Cp ≤ 918.9) = 1 − α | |
| P (0.5102 ≤ a ≤ 0.6738) = 1 − α | P (0.4048 ≤ a ≤ 0.4139) = 1 − α | |
| GF1.2Fe2.5 | P (0.9380 ≤ λ ≤ 0.9588) = 1 − α | P (0.6691 ≤ λ ≤ 0.6740) = 1 − α |
| P (786.7 ≤ Cp ≤ 944.2) = 1 − α | P (852.0 ≤ Cp ≤ 857.5) = 1 − α | |
| P (0.5145 ≤ a ≤ 0.6041) = 1 − α | P (0.4017 ≤ a ≤ 4083) = 1 − α | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prałat, K.; Ciemnicka, J.; Koper, A.; Szczypiński, M.M.; Łoś, P.; Nguyen, V.V.; Le, V.S.; Rapiejko, C.; Ercoli, R.; Buczkowska, K.E. Determination of the Thermal Parameters of Geopolymers Modified with Iron Powder. Polymers 2022, 14, 2009. https://doi.org/10.3390/polym14102009
Prałat K, Ciemnicka J, Koper A, Szczypiński MM, Łoś P, Nguyen VV, Le VS, Rapiejko C, Ercoli R, Buczkowska KE. Determination of the Thermal Parameters of Geopolymers Modified with Iron Powder. Polymers. 2022; 14(10):2009. https://doi.org/10.3390/polym14102009
Chicago/Turabian StylePrałat, Karol, Justyna Ciemnicka, Artur Koper, Michał Marek Szczypiński, Piotr Łoś, Van Vu Nguyen, Van Su Le, Cezary Rapiejko, Roberto Ercoli, and Katarzyna Ewa Buczkowska. 2022. "Determination of the Thermal Parameters of Geopolymers Modified with Iron Powder" Polymers 14, no. 10: 2009. https://doi.org/10.3390/polym14102009
APA StylePrałat, K., Ciemnicka, J., Koper, A., Szczypiński, M. M., Łoś, P., Nguyen, V. V., Le, V. S., Rapiejko, C., Ercoli, R., & Buczkowska, K. E. (2022). Determination of the Thermal Parameters of Geopolymers Modified with Iron Powder. Polymers, 14(10), 2009. https://doi.org/10.3390/polym14102009