Water Treatment Using High Performance Antifouling Ultrafiltration Polyether Sulfone Membranes Incorporated with Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
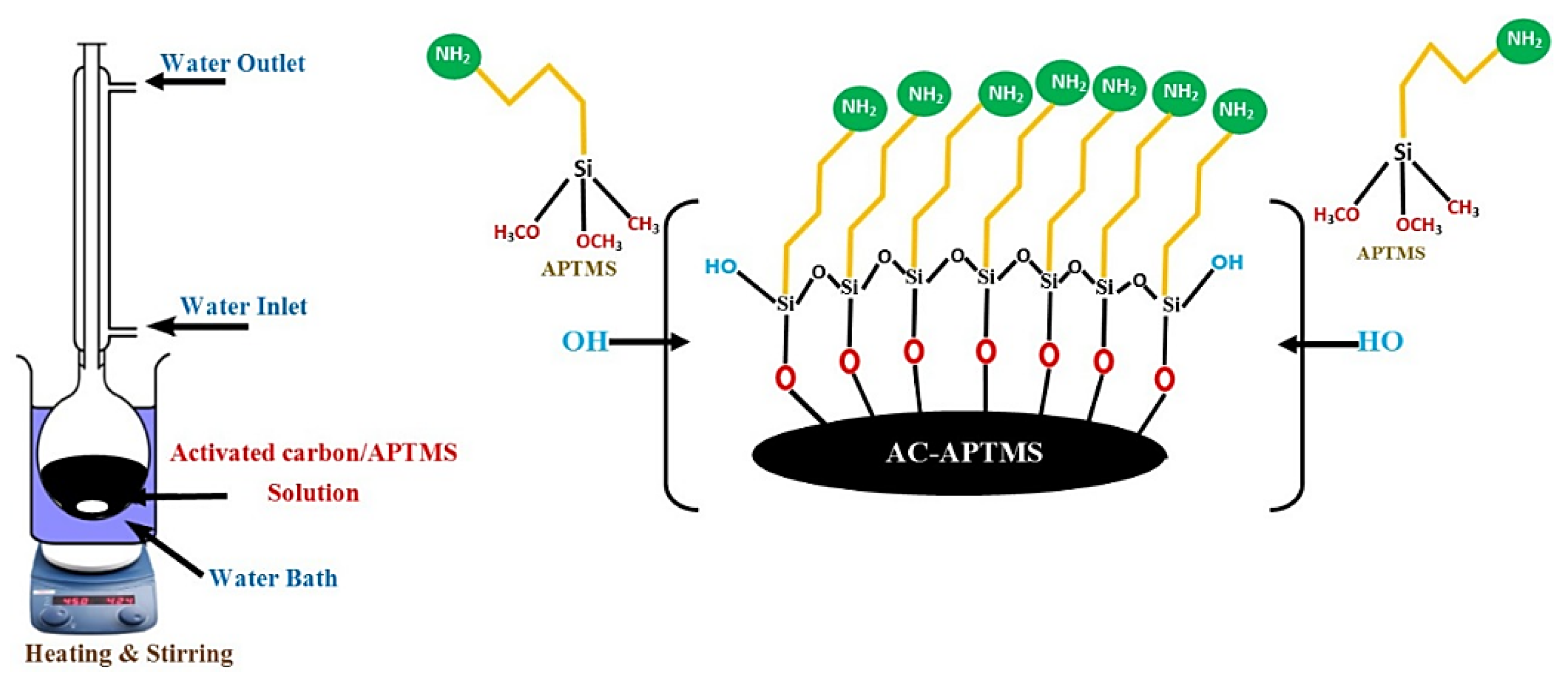
2.2.1. Surface Modification of Activated Carbon (AC)
2.2.2. Membrane Fabrication
2.3. Characterization of Activated Carbon
2.3.1. Ash Determination
2.3.2. N2 Adsorption-Desorption at 77 K
2.3.3. pHPZC and Boehm Titrations
2.4. Characterization of mAC and Fabricated Membranes
2.5. Antibacterial Assay
3. Results
3.1. Characterization of AC
3.1.1. Surface Chemistry
3.1.2. Surface Characteristics Analyses by Brunauer-Emmett-Teller Analysis (BET)
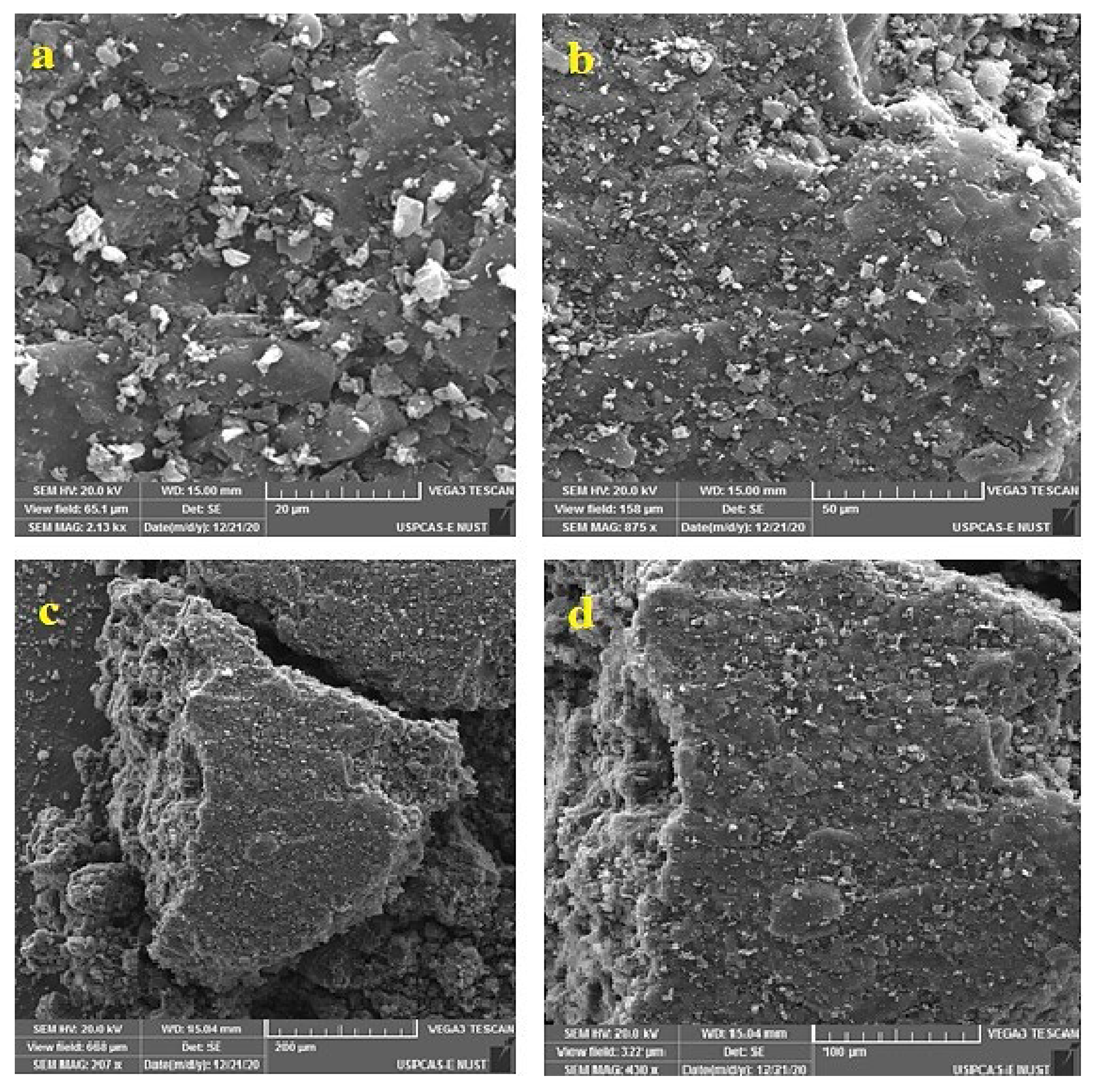
3.1.3. Surface Morphology Analyses by SEM
3.1.4. Afunctional Groups Analyses by FTIR
3.2. Characterization of mAC and Fabricated Membranes
3.2.1. Functional Groups Analyses by FTIR
3.2.2. Surface Morphological Analysis by SEM
3.2.3. Optical Profilometry
3.2.4. Surface Characteristics Analyses by Brunauer-Emmett-Teller Analysis (BET)
3.2.5. Contact Angle and Surface Energy Studies
3.2.6. Water Uptake and Swelling
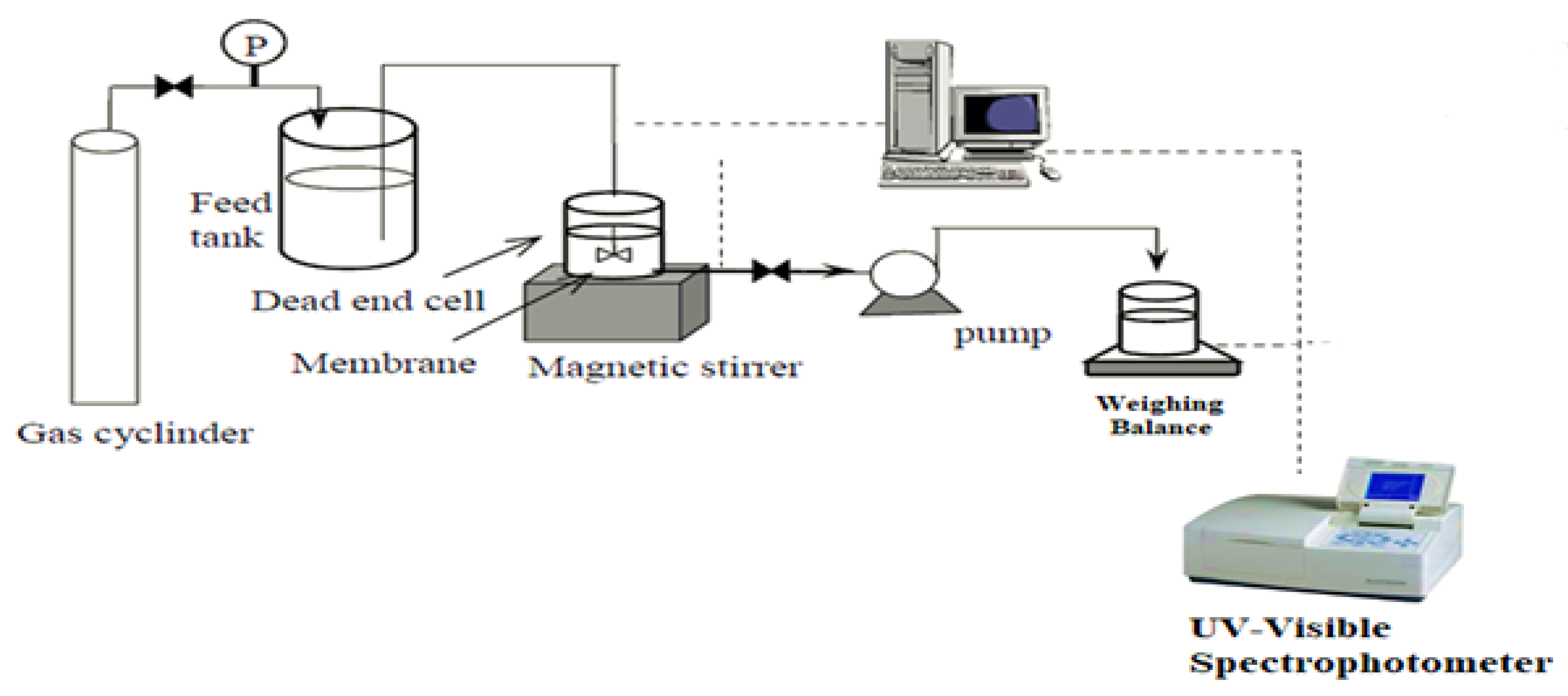
3.2.7. Evaluation of Flux and Fouling Characteristics
3.2.8. Antibacterial Activity of the Fabricated Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and Terpenes as an antibacterial and antibiofouling promotor for PES water filtration membranes. Molecules 2020, 25, 691. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Montgomery, M.A.; Elimelech, M. Water and sanitation in developing countries: Including health in the equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S.; Niknam, Z.; Rombi, E. Adsorption of pentafluorophenol onto powdered, granular, and cloth activated carbons. J. Dispers. Sci. Technol. 2012, 33, 206–212. [Google Scholar] [CrossRef]
- Tong, K.; Ji, G.; Nie, F.; Zhang, M.; Ren, W.; Xie, S. Enhanced removal of organic pollutants from super heavy oil wastewater using specially modified lignite activated coke. Environ. Sci. Water Res. Technol. 2020, 6, 1606–1614. [Google Scholar] [CrossRef]
- László, K.; Szűcs, A. Surface characterization of polyethyleneterephthalate (PET) based activated carbon and the effect of pH on its adsorption capacity from aqueous phenol and 2,3,4-trichlorophenol solutions. Carbon 2001, 39, 1945–1953. [Google Scholar] [CrossRef]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Kasuga, I.; Shimazaki, D.; Kunikane, S. Influence of backwashing on the microbial community in a biofilm developed on biological activated carbon used in a drinking water treatment plant. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2007, 55, 173–180. [Google Scholar] [CrossRef]
- Hassan, M.; Abou-Zeid, R.; Hassan, E.; Berglund, L.; Aitomaki, Y.; Oksman, K. Membranes based on cellulose nanofibers and activated carbon for removal of Escherichia coli bacteria from water. Polymers 2017, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Marshall, K.C. Adsorption and adhesion processes in microbial growth at interfaces. Adv. Colloid Interface Sci. 1986, 25, 59–86. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Pietrowski, P.; Ludwiczak, I.; Tyczkowski, J. Activated carbons modified by Ar and CO2 plasmas—Acetone and cyclohexane adsorption. Mater. Sci. 2012, 18, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; Gomez-Serrano, V.; Alvarez, P.M.; Alvim-Ferraz, M.C.; Dias, J.M. Activated carbon modifications to enhance its water treatment applications. An overview. J. Hazard. Mater. 2011, 187, 1–23. [Google Scholar] [CrossRef]
- Li, Z.; Chang, X.; Zou, X.; Zhu, X.; Nie, R.; Hu, Z.; Li, R. Chemically-modified activated carbon with ethylenediamine for selective solid-phase extraction and preconcentration of metal ions. Anal. Chim. Acta 2009, 632, 272–277. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Lau, W.J.; Ismail, A.F. Inorganic nanomaterials in polymeric ultrafiltration membranes for water treatment. Sep. Purif. Rev. 2014, 44, 216–249. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Alamry, K.A.; Hussein, M.A.; Elfaky, M.A.; Asiri, A.M. Exploring the effect of organic–inorganic additives loaded on modified polyethersulfone membranes. J. Appl. Polym. Sci. 2019, 136, 47686. [Google Scholar] [CrossRef]
- Braeken, L.; Van der Bruggen, B.; Vandecasteele, C. Flux decline in nanofiltration due to adsorption of dissolved organic compounds: Model prediction of time dependency. J. Phys. Chem. B 2006, 110, 2957–2962. [Google Scholar] [CrossRef]
- Tu, S.-C.; Ravindran, V.; Den, W.; Pirbazari, M. Predictive membrane transport model for nanofiltration processes in water treatment. AIChE J. 2001, 47, 1346–1362. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S. Polyethersulfone (PES)/cellulose acetate phthalate (CAP) blend ultrafiltration membranes: Preparation, morphology, performance and antifouling properties. J. Membr. Sci. 2007, 305, 299–312. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Zhang, M.; Field, R.W.; Zhang, K. Biogenic silver nanocomposite polyethersulfone UF membranes with antifouling properties. J. Membr. Sci. 2014, 471, 274–284. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.R.; Hosseini, S.M.; Ray, M.B.; Parvizian, F.; Van der Bruggen, B. Highly hydrophilic and antifouling nanofiltration membrane incorporated with water-dispersible composite activated carbon/chitosan nanoparticles. Chem. Eng. Res. Des. 2018, 132, 812–821. [Google Scholar] [CrossRef]
- Kallem, P.; Ouda, M.; Bharath, G.; Hasan, S.W.; Banat, F. Enhanced water permeability and fouling resistance properties of ultrafiltration membranes incorporated with hydroxyapatite decorated orange-peel-derived activated carbon nanocomposites. Chemosphere 2022, 286, 131799. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.S.; Abbas, M.A.; Mushtaq, S.; Khan Niazi, B.; Batool, M.; Shehnaz, G.; Ahmad, N.; Ahmad, N.M. Anti-foulant ultrafiltration polymer composite membranes incorporated with composite activated carbon/chitosan and activated carbon/thiolated chitosan with enhanced hydrophilicity. Membranes 2021, 11, 827. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Fast removal of organic dyes from aqueous solutions by AC/ferrospinel composite. Desalination 2010, 262, 134–140. [Google Scholar] [CrossRef]
- Hamad, H.; Ezzeddine, Z.; Kanaan, S.; Lakis, F.; Hijazi, A.; Moussawi, M.-A. A novel modification and selective route for the adsorption of Pb2+ by oak charcoal functionalized with glutaraldehyde. Adv. Powder Technol. 2016, 27, 631–637. [Google Scholar] [CrossRef]
- Vatsha, B.; Ngila, J.C.; Moutloali, R.M. Preparation of antifouling polyvinylpyrrolidone (PVP 40K) modified polyethersulfone (PES) ultrafiltration (UF) membrane for water purification. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 125–131. [Google Scholar] [CrossRef]
- Barahimi, V.; Taheri, R.A.; Mazaheri, A.; Moghimi, H. Fabrication of a novel antifouling TiO2/CPTES/metformin-PES nanocomposite membrane for removal of various organic pollutants and heavy metal ions from wastewater. Chem. Pap. 2020, 74, 3545–3556. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1983, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. A simple two-dimensional nldft model of gas adsorption in finite carbon pores. Application to pore structure analysis. J. Phys. Chem. C 2009, 113, 19382–19385. [Google Scholar] [CrossRef]
- Jagiello, J.; Thommes, M. Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distributions. Carbon 2004, 42, 1227–1232. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Terpiłowski, K. Apparent surface free energy of polymer/paper composite material treated by air plasma. Int. J. Polym. Sci. 2017, 2017, 9023197. [Google Scholar] [CrossRef] [Green Version]
- Callow, J.A.; Callow, M.E.; Ista, L.K.; Lopez, G.; Chaudhury, M.K. The influence of surface energy on the wetting behaviour of the spore adhesive of the marine alga Ulva linza (synonym Enteromorpha linza). J. R. Soc. Interface 2005, 2, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Ariadi Lusiana, R.; Muslimah, N.; Riyanati, P.; Sangkota, V.D.A.; Gunawan; Azmiyawati, C. Synthesis and characterization of composite polyethersulfone (PES) membranes with polyethylene glycol (PEG) and heparin-chitosan (Hep-CS). IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012123. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Shanableh, A.; Shahida, S.; Lashari, M.H.; Manzoor, S.; Fernandez, J. SPEEK and SPPO blended membranes for proton exchange membrane fuel cells. Membranes 2022, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Velu, S.; Muruganandam, L.; Arthanareeswaran, G. Preparation and Performance Studies on Polyethersulfone Ultrafiltration Membranes Modified with Gelatin for Treatment of Tannery and Distillery Wastewater. Braz. J. Chem. Eng. 2015, 32, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lu, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. A synergistic antibacterial effect between terbium ions and reduced graphene oxide in a poly(vinyl alcohol)-alginate hydrogel for treating infected chronic wounds. J. Mater. Chem. B 2019, 7, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.; Frigols, B.; Serrano-Aroca, A. Antimicrobial characterization of advanced materials for bioengineering applications. J. Vis. Exp. JoVE 2018, 138, 57710. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.J.; Theydan, S.K. Microporous activated carbon from Siris seed pods by microwave-induced KOH activation for metronidazole adsorption. J. Anal. Appl. Pyrolysis 2013, 99, 101–109. [Google Scholar] [CrossRef]
- Li, L.; Sun, F.; Gao, J.; Wang, L.; Pi, X.; Zhao, G. Broadening the pore size of coal-based activated carbonviaa washing-free chem-physical activation method for high-capacity dye adsorption. RSC Adv. 2018, 8, 14488–14499. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Kang, F. Engineering and applications of carbon materials. In Materials Science and Engineering of Carbon: Fundamentals, 2nd ed.; Elsevier Inc.: Oxford, UK, 2014; pp. 219–525. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Taufiq-Yap, Y.H.; Krishnaiah, D.; Tay, V.V. Modification of commercial activated carbon for the removal of 2,4-dichlorophenol from simulated wastewater. J. King Saud Univ. Sci. 2015, 27, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Jaria, G.; Lourenço, M.A.O.; Silva, C.P.; Ferreira, P.; Otero, M.; Calisto, V.; Esteves, V.I. Effect of the surface functionalization of a waste-derived activated carbon on pharmaceuticals’ adsorption from water. J. Mol. Liq. 2020, 299, 112098. [Google Scholar] [CrossRef]
- Abbas, M.A.; Mushtaq, S.; Cheema, W.A.; Qiblawey, H.; Zhu, S.; Li, Y.; Zhang, R.; Wu, H.; Jiang, Z.; Sadiq, R.; et al. Surface Modification of TFC-PA RO membrane by grafting hydrophilic pH Switchable poly(acrylic acid) brushes. Adv. Polym. Technol. 2020, 2020, 8281058. [Google Scholar] [CrossRef]
- Jain, A.; Hirata, G.A.; Farias, M.H.; Castillon, F.F. Synthesis and characterization of (3-Aminopropyl)trimethoxy-silane (APTMS) functionalized Gd2O3:Eu(3+) red phosphor with enhanced quantum yield. Nanotechnology 2016, 27, 065601. [Google Scholar] [CrossRef]
- Belfer, S. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Sandoval-Olvera, I.G.; Villafaña-López, L.; Reyes-Aguilera, J.A.; Ávila-Rodríguez, M.; Razo-Lazcano, T.A.; González-Muñoz, M.P. Surface modification of polyethersulfone membranes with goethite through self-assembly. Desalin. Water Treat. 2017, 65, 199–207. [Google Scholar] [CrossRef]
- Rahimpour, A. UV photo-grafting of hydrophilic monomers onto the surface of nano-porous PES membranes for improving surface properties. Desalination 2011, 265, 93–101. [Google Scholar] [CrossRef]
- Peyravi, M. Preparation of adsorptive nanoporous membrane using powder activated carbon: Isotherm and thermodynamic studies. Front. Chem. Sci. Eng. 2019, 14, 673–687. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Hołda, A.K.; Vankelecom, I.F.J. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132, 42130. [Google Scholar] [CrossRef]
- Ng, C.A.; Wong, L.Y.; Bashir, M.J.K.; Ng, S.L. Development of hybrid polymeric polyerthersulfone (PES) membrane incorporated with powdered activated carbon (PAC) for palm oil mill effluent (POME) treatment. Int. J. Integr. Eng. 2018, 10, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Basri, H.; Ismail, A.F.; Aziz, M. Polyethersulfone (PES)–silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Giraldo, L.; Vargas, D.P.; Moreno-Pirajan, J.C. Study of CO2 adsorption on chemically modified activated carbon with nitric acid and ammonium aqueous. Front. Chem. 2020, 8, 543452. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arenillas, A.; Rubiera, F.; Pis, J.J. CO2 capture by adsorption with nitrogen enriched carbons. Fuel 2007, 86, 2204–2212. [Google Scholar] [CrossRef] [Green Version]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Arami-Niya, A. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation. Appl. Surf. Sci. 2011, 257, 3936–3942. [Google Scholar] [CrossRef]
- Gohari, B.; Abu-Zahra, N. Polyethersulfone membranes prepared with 3-Aminopropyltriethoxysilane modified alumina nanoparticles for Cu(II) removal from water. ACS Omega 2018, 3, 10154–10162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.; Al-sayaghi, M.A.Q.; Buelke, C.; Alshami, A. Activated carbon in mixed-matrix membranes. Sep. Purif. Rev. 2019, 50, 1–31. [Google Scholar] [CrossRef]
- Astakhov, E.Y.; Kolganov, I.M.; Klinshpont, E.R.; Tsarin, P.G.; Kalacheva, A.A. Influence of polyvinylpyrrolidone on morphology, hydrophilicity, and performance of polyethersulfone microfiltration membranes. Pet. Chem. 2013, 52, 557–564. [Google Scholar] [CrossRef]
- Saranya, R.; Arthanareeswaran, G.; Ismail, A.F. Enhancement of anti-fouling properties during the treatment of paper mill effluent using functionalized zeolite and activated carbon nanomaterials based ultrafiltration. J. Chem. Technol. Biotechnol. 2019, 94, 2805–2815. [Google Scholar] [CrossRef]
- Soria-Sanchez, M.; Maroto-Valiente, A.; Guerrero-Ruiz, A.; Nevskaia, D.M. Adsorption of non-ionic surfactants on hydrophobic and hydrophilic carbon surfaces. J. Colloid Interface Sci. 2010, 343, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.P.; Dubey, N.C.; Stamm, M. Polyethylene glycol cross-linked sulfonated polyethersulfone based filtration membranes with improved antifouling tendency. J. Membr. Sci. 2014, 453, 263–274. [Google Scholar] [CrossRef]
- Bhatti, H.T.; Ahmad, N.M.; Khan Niazi, M.B.; Ur Rehman Alvi, M.A.; Ahmad, N.; Anwar, M.N.; Cheema, W.; Tariq, S.; Batool, M.; Aman, Z.; et al. Graphene oxide-PES-based mixed matrix membranes for controllable antibacterial activity against Salmonella typhi and water treatment. Int. J. Polym. Sci. 2018, 2018, 7842148. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K. Fabrication of Polyamide-6 membranes—The effect of gelation time towards their morphological, physical and transport properties. Membranes 2022, 12, 315. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Amini, S.H.; Khodabakhshi, A.R.; Bagheripour, E.; Van der Bruggen, B. Activated carbon nanoparticles entrapped mixed matrix polyethersulfone based nanofiltration membrane for sulfate and copper removal from water. J. Taiwan Inst. Chem. Eng. 2018, 82, 169–178. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Phan, H.T.; Bartelt-Hunt, S.; Rodenhausen, K.B.; Schubert, M.; Bartz, J.C. Investigation of bovine serum albumin (BSA) attachment onto self-assembled monolayers (SAMs) using combinatorial quartz crystal microbalance with dissipation (QCM-D) and spectroscopic ellipsometry (SE). PLoS ONE 2015, 10, e0141282. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.E.; Latvyte, E.; Greenwood, A.; Emekwuru, N.G. Ultrasonic preparation, stability and thermal conductivity of a capped copper-methanol nanofluid. Ultrason. Sonochem. 2019, 55, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Li, B.; Hu, M.; Zhang, J. Enhanced hydrophilicity and antifouling performance of PES-C/emodin ultrafiltration membrane. High Perform. Polym. 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Wu, X.; Xie, Z.; Wang, H.; Zhao, C.; Ng, D.; Zhang, K. Improved filtration performance and antifouling properties of polyethersulfone ultrafiltration membranes by blending with carboxylic acid functionalized polysulfone. RSC Adv. 2018, 8, 7774–7784. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.; Kochkodan, V.; Mahmoudi, E.; Johnson, D.J.; Mohammad, A.W.; Atieh, M.A. Characterization and separation performance of a novel polyethersulfone membrane blended with acacia gum. Sci. Rep. 2017, 7, 15831. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, P.; Regi, T.; Rahman, M.I.; Arivuoli, D. Low-cost and biodegradable cellulose/PVP/activated carbon composite membrane for brackish water treatment. J. Appl. Polym. Sci. 2019, 137, 48746. [Google Scholar] [CrossRef]
- Hwang, L.-L.; Chen, J.-C.; Wey, M.-Y. The properties and filtration efficiency of activated carbon polymer composite membranes for the removal of humic acid. Desalination 2013, 313, 166–175. [Google Scholar] [CrossRef]
- Seredych, M.; Mikhalovska, L.; Mikhalovsky, S.; Gogotsi, Y. Adsorption of bovine serum albumin on carbon-based materials. C 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Stone, M.T.; Kozlov, M. Separating proteins with activated carbon. Langmuir ACS J. Surf. Colloids 2014, 30, 8046–8055. [Google Scholar] [CrossRef]












| Code | PVP | DMAc | mAC |
|---|---|---|---|
| MP0 | 0.2% | 81.8% | 0% |
| MC1 | 0.2% | 80.8% | 1% |
| MC4 | 0.2% | 77.8% | 4% |
| MC7 | 0.2% | 74.8% | 7% |
| MC10 | 0.2% | 71.8% | 10% |
| Carboxylic Groups (meq.g−1) | Lactonic Groups (meq.g−1) | Phenolic Groups (meq.g−1) | Carbonyl Groups (meq.g−1) | Total Basic Groups (meq.g−1) | pHPZC | Ash Content (%) |
|---|---|---|---|---|---|---|
| 0.00 | 0.20 | 0.20 | 0.48 | 0.53 | 9.5 | 5 |
| BET Surface Area SBET (m2·g−1) | Ultramicropore Volume * (Ø < 8 nm) (cm3·g−1) | Supermicropore Volume # (Ø > 8 nm) (cm3·g−1) | Micropore Volume (cm3·g−1) | Mesoporous Volume (cm3·g−1) | Total Pore Volume $ (cm3·g−1) |
|---|---|---|---|---|---|
| 1044 | 0.26 | 0.21 | 0.47 | 0.07 | 0.53 |
| Properties | MP0 | MC1 | MC4 | MC7 | MC10 |
|---|---|---|---|---|---|
| Contact Angle (θ) | 69 | 62 | 57 | 51 | 58 |
| Water uptake (%) | 63 | 92 | 101 | 134 | 98 |
| Swelling (%) | 39 | 65 | 83 | 102 | 82 |
| Roughness Ra (µm) | 0.212 | 0.423 | 0.619 | 0.814 | 0.981 |
| BET surface area (m2/g) | 0.5173 | 0.5376 | 1.0041 | 1.7126 | 1.1961 |
| Pore volume (cm3/g) | 0.001539 | 0.001718 | 0.003058 | 0.004715 | 0.003432 |
| Water flux (L/m2·h) | 65 | 75 | 94 | 136 | 78 |
| BSA flux (L/m2·h) | 22 | 33 | 62 | 73 | 41 |
| BSA rejection (%) | 70.9 | 55.9 | 49.8 | 8.4 | 15.8 |
| Membrane Material | Method | Contact Angle | Pure Water Flux (L/m2·h) | Pressure (MPAa) | Fouling Characteristics | Reference |
|---|---|---|---|---|---|---|
| PES, APTMS modified AC | Phase inversion | 51 | 136 | 0.1 | BSA rejection 8.4% Zones of inhibition for S. aureus 4.8 mm E. coli 2.99 | Our work |
| PES incorporated Functionalized AC | Phase inversion | 106 | 33 | 0.1 | Reduction in COD, BOD and TDS level | [66] |
| PES-C/emodin ultrafiltration membrane | Phase inversion | - | 350 | 0.2 | S. aureus 3 mm Zone of inhibition | [75] |
| carboxylic acid functionalized polysulfone, PES, PVP | Phase inversion | 74 | 400 | 0.2 | BSA rejection 4% wt | [76] |
| PES, Acacia Gum | Phase inversion | 65 | 70 | 4 | Antibacterial against E. coli, Low BSA rejection | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abid, Z.; Abbas, A.; Mahmood, A.; Rana, N.F.; Khan, S.J.; Duclaux, L.; Deen, K.M.; Ahmad, N.M. Water Treatment Using High Performance Antifouling Ultrafiltration Polyether Sulfone Membranes Incorporated with Activated Carbon. Polymers 2022, 14, 2264. https://doi.org/10.3390/polym14112264
Abid Z, Abbas A, Mahmood A, Rana NF, Khan SJ, Duclaux L, Deen KM, Ahmad NM. Water Treatment Using High Performance Antifouling Ultrafiltration Polyether Sulfone Membranes Incorporated with Activated Carbon. Polymers. 2022; 14(11):2264. https://doi.org/10.3390/polym14112264
Chicago/Turabian StyleAbid, Zubia, Asad Abbas, Azhar Mahmood, Nosheen Fatima Rana, Sher Jamal Khan, Laurent Duclaux, Kashif Mairaj Deen, and Nasir M. Ahmad. 2022. "Water Treatment Using High Performance Antifouling Ultrafiltration Polyether Sulfone Membranes Incorporated with Activated Carbon" Polymers 14, no. 11: 2264. https://doi.org/10.3390/polym14112264
APA StyleAbid, Z., Abbas, A., Mahmood, A., Rana, N. F., Khan, S. J., Duclaux, L., Deen, K. M., & Ahmad, N. M. (2022). Water Treatment Using High Performance Antifouling Ultrafiltration Polyether Sulfone Membranes Incorporated with Activated Carbon. Polymers, 14(11), 2264. https://doi.org/10.3390/polym14112264









